Stable Near-Infrared Photoluminescence of Hexagonal-Shaped PbS Nanoparticles with 1-Dodecanethiol Ligands
Abstract
1. Introduction
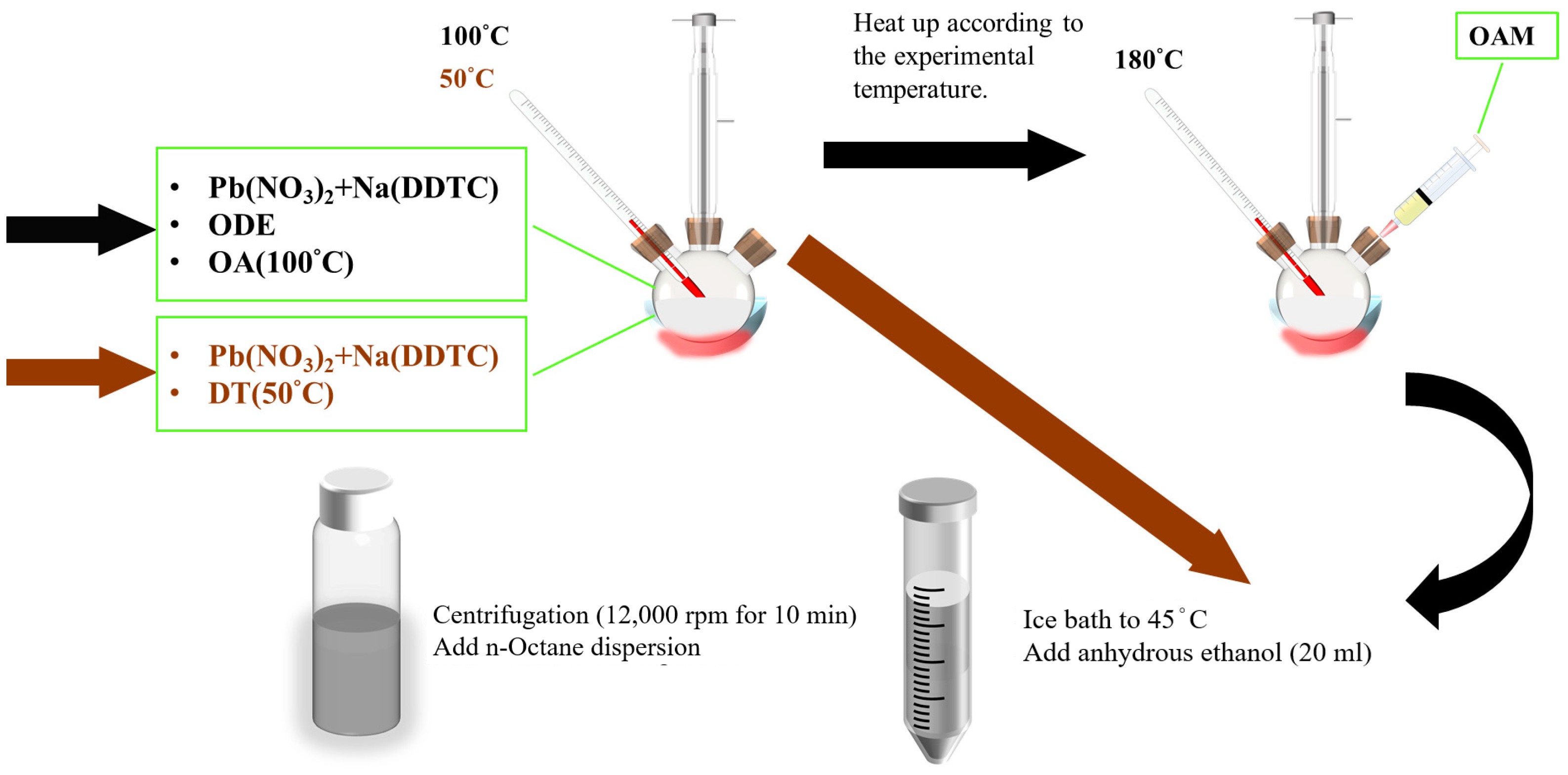
2. Materials and Method
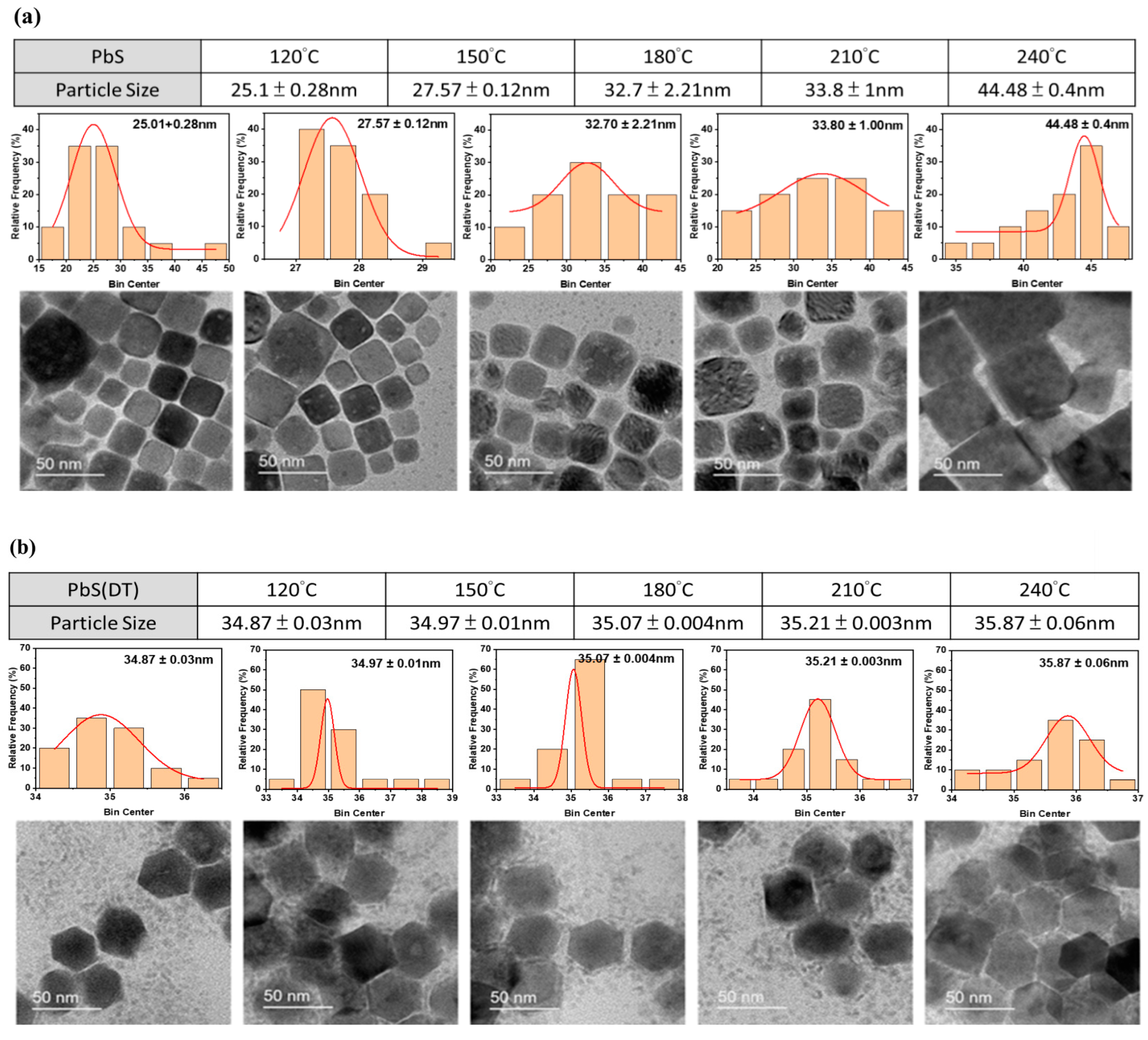
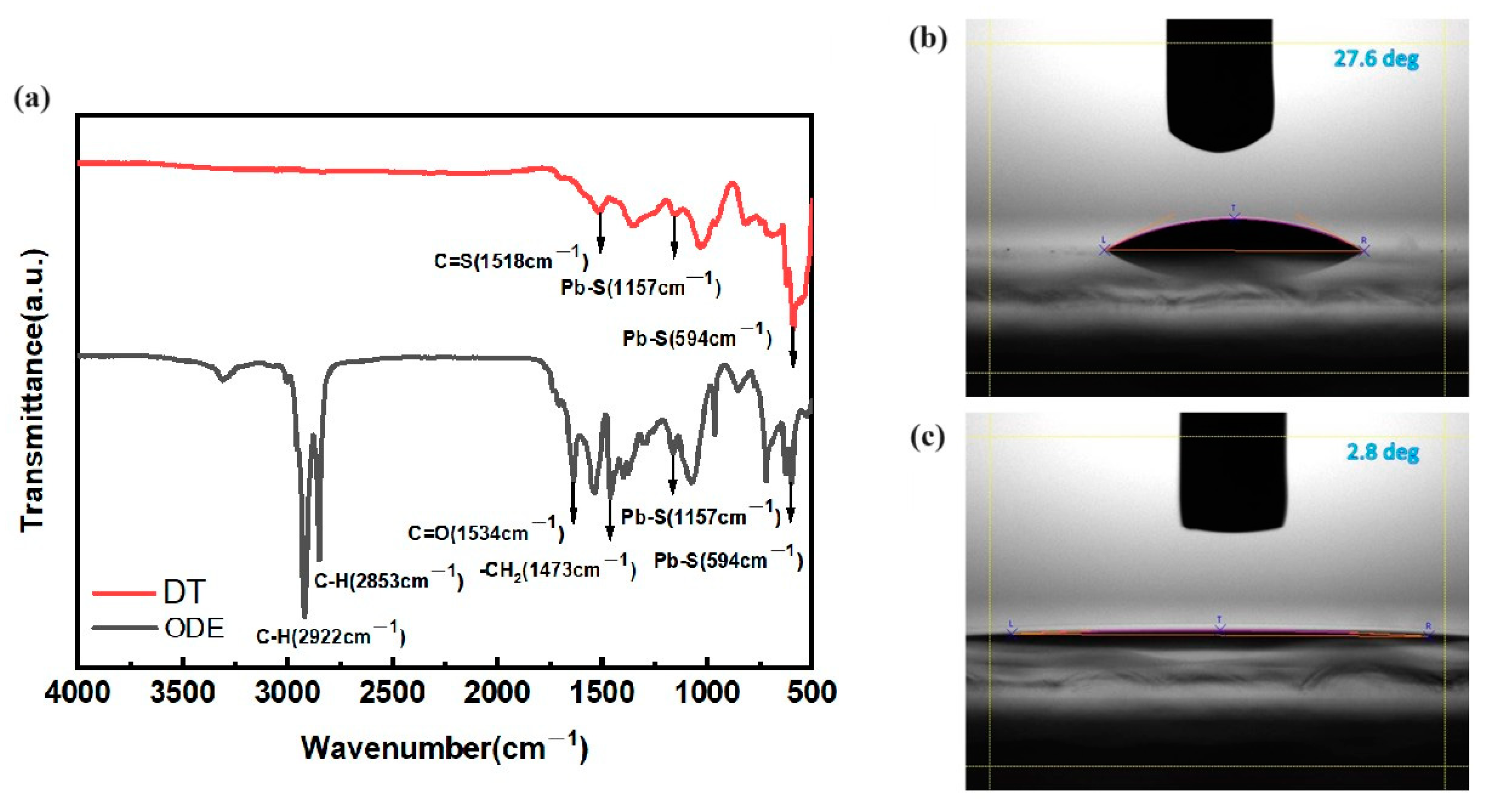
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Su, G.; Liu, C.; Deng, Z.; Zhao, X.; Zhou, X. Size-Dependent Photoluminescence of PbS QDs Embedded in Silicate Glasses. Opt. Mater. Express 2017, 7, 2194. [Google Scholar] [CrossRef]
- Gao, W.; Zhai, G.; Zhang, C.; Shao, Z.; Zheng, L.; Zhang, Y.; Yang, Y.; Li, X.; Liu, X.; Xu, B. Towards Understanding the Initial Performance Improvement of PbS Quantum Dot Solar Cells upon Short-Term Air Exposure. RSC Adv. 2018, 8, 15149–15157. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Bharti, P.; Pradhan, B. Performance Optimization of Efficient PbS Quantum Dots Solar Cells through Numerical Simulation. Sci. Rep. 2023, 13, 1–9. [Google Scholar] [CrossRef]
- Yin, X.; Zhang, C.; Guo, Y.; Yang, Y.; Xing, Y.; Que, W. PbS QD-Based Photodetectors: Future-Oriented near-Infrared Detection Technology. J. Mater. Chem. C 2021, 9, 417–438. [Google Scholar] [CrossRef]
- Georgitzikis, E.; Malinowski, P.E.; Li, Y.; Maes, J.; Hagelsieb, L.M.; Guerrieri, S.; Hens, Z.; Heremans, P.; Cheyns, D. Integration of PbS Quantum Dot Photodiodes on Silicon for NIR Imaging. IEEE Sens. J. 2020, 20, 6841–6848. [Google Scholar] [CrossRef]
- Li, F.; Liu, J.J.; Xu, Q.; Chang, R.; Wang, L.; Wu, Z.; Shen, H.; Du, Z. High-Radiance Shortwave Infrared Light-Emitting Diodes Based on Highly Stable PbS Colloidal Quantum Dots. J. Phys. Chem. Lett. 2023, 14, 4252–4258. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.; Yuan, W.; Wu, Z.; Wang, X.; Xu, W.; Wang, L.; Zhang, Q.; Zhang, C.; Wang, J.; Song, Q. Mechanochemical Synthesis of Lead Sulfide (PbS) Nanocrystals from Lead Oxide. Powder Technol. 2019, 347, 130–135. [Google Scholar] [CrossRef]
- Raja, V.R.; Rosaline, D.R.; Suganthi, A.; Rajarajan, M. Facile Fabrication of PbS/MoS2 Nanocomposite Photocatalyst with Efficient Photocatalytic Activity under Visible Light. Solid State Sci. 2017, 67, 99–108. [Google Scholar] [CrossRef]
- Liu, S.; Han, L.; Liu, H. Synthesis, Characterization and Photocatalytic Performance of PbS/Ni2P Flowers. Appl. Surf. Sci. 2016, 387, 393–398. [Google Scholar] [CrossRef]
- Hedayati, K.; Kord, M.; Goodarzi, M.; Ghanbari, D.; Gharigh, S. Photo-Catalyst and Magnetic Nanocomposites: Hydrothermal Preparation of Core–Shell Fe3O4@PbS for Photo-Degradation of Toxic Dyes. J. Mater. Sci. Mater. Electron. 2017, 28, 1577–1589. [Google Scholar] [CrossRef]
- Saran, R.; Curry, R.J. Https://Doi.Org/10.1038/Nmat1299Lead Sulphide Nanocrystal Photodetector Technologies. Nat. Photonics 2016, 10, 81–92. [Google Scholar] [CrossRef]
- Mcdonald, S.A.; Konstantatos, G.; Zhang, S.; Cyr, P.W.; Klem, E.J.D.; Levina, L.; Sargent, E.H. Solution-Processed PbS Quantum Dot Infrared Photodetectors and Photovoltaics. Nat. Mater. 2005, 4, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Huang, X.; Kong, T.; Liu, X.; Qin, Q.; Li, Z. Synthesis and Characterization of PbS Crystals via a Solvothermal Route. J. Alloys Compd. 2009, 485, 554–560. [Google Scholar] [CrossRef]
- Popov, G.; Bačić, G.; Mattinen, M.; Manner, T.; Lindström, H.; Seppänen, H.; Suihkonen, S.; Vehkamäki, M.; Kemell, M.; Jalkanen, P.; et al. Atomic Layer Deposition of PbS Thin Films at Low Temperatures. Chem. Mater. 2020, 32, 8216–8228. [Google Scholar] [CrossRef]
- Yin, Y.; Alivisatos, A.P. Colloidal Nanocrystal Synthesis and the Organic-Inorganic Interface. Nature 2005, 437, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Green, M. The Nature of Quantum Dot Capping Ligands. J. Mater. Chem. 2010, 20, 5797–5809. [Google Scholar] [CrossRef]
- Angeloski, A.; Gentle, A.R.; Scott, J.A.; Cortie, M.B.; Hook, J.M.; Westerhausen, M.T.; Bhadbhade, M.; Baker, A.T.; McDonagh, A.M. From Lead(II) Dithiocarbamate Precursors to a Fast Response PbS Positive Temperature Coefficient Thermistor. Inorg. Chem. 2018, 57, 2132–2140. [Google Scholar] [CrossRef]
- Duan, X.; Ma, J.; Shen, Y.; Zheng, W. A Novel PbS Hierarchical Superstructure Guided by the Balance between Thermodynamic and Kinetic Control via a Single-Source Precursor Route. Inorg. Chem. 2012, 51, 914–919. [Google Scholar] [CrossRef]
- Saah, S.A.; Boadi, N.O.; Adu-Poku, D.; Wilkins, C. Lead Ethyl Dithiocarbamates: Efficient Single-Source Precursors to PbS Nanocubes. R. Soc. Open Sci. 2019, 6, 190943. [Google Scholar] [CrossRef]
- Giansante, C.; Infante, I.; Fabiano, E.; Grisorio, R.; Suranna, G.P.; Gigli, G. “darker-than-Black” PbS Quantum Dots: Enhancing Optical Absorption of Colloidal Semiconductor Nanocrystals via Short Conjugated Ligands. J. Am. Chem. Soc. 2015, 137, 1875–1886. [Google Scholar] [CrossRef]
- Anderson, N.C.; Hendricks, M.P.; Choi, J.J.; Owen, J.S. Ligand Exchange and the Stoichiometry of Metal Chalcogenide Nanocrystals: Spectroscopic Observation of Facile Metal-Carboxylate Displacement and Binding. J. Am. Chem. Soc. 2013, 135, 18536–18548. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Kemp, K.W.; Hoogland, S.; Jeong, K.S.; Liu, H.; Levina, L.; Furukawa, M.; Wang, X.; Debnath, R.; Cha, D.; et al. Colloidal-Quantum-Dot Photovoltaics Using Atomic-Ligand Passivation. Nat. Mater. 2011, 10, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Fafarman, A.T.; Koh, W.K.; Diroll, B.T.; Kim, D.K.; Ko, D.K.; Oh, S.J.; Ye, X.; Doan-Nguyen, V.; Crump, M.R.; Reifsnyder, D.C.; et al. Thiocyanate-Capped Nanocrystal Colloids: Vibrational Reporter of Surface Chemistry and Solution-Based Route to Enhanced Coupling in Nanocrystal Solids. J. Am. Chem. Soc. 2011, 133, 15753–15761. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, K.R.; Sahoo, Y.; Jang, S.; Prasad, P.N. Efficient Photosensitization and High Optical Gain in a Novel Quantum-Dot-Sensitized Hybrid Photorefractive Nanocomposite at a Telecommunications Wavelength. Adv. Funct. Mater. 2005, 15, 751–756. [Google Scholar] [CrossRef]
- Baek, G.W.; Kim, Y.J.; Lee, M.; Kwon, Y.; Chun, B.; Park, G.; Seo, H.; Yang, H.; Kwak, J. Progress in the Development of Active-Matrix Quantum-Dot Light-Emitting Diodes Driven by Non-Si Thin-Film Transistors. Materials 2022, 15, 8511. [Google Scholar] [CrossRef] [PubMed]
- Suganya, M.; Balu, A.R. PbS Nanopowder-Synthesis, Characterization and Antimicrobial Activity. Mater. Sci. Pol. 2017, 35, 322–328. [Google Scholar] [CrossRef]
- Masikane, S.C.; Mlowe, S.; Gervas, C.; Revaprasadu, N.; Pawar, A.S.; Garje, S.S. Lead (II) Halide Cinnamaldehyde Thiosemicarbazone Complexes as Single Source Precursors for Oleylamine-Capped Lead Sulfide Nanoparticles. J. Mater. Sci. Mater. Electron. 2018, 29, 1479–1488. [Google Scholar] [CrossRef]
- Kind, H.; Bonard, J.M.; Emmenegger, C.; Nilsson, L.O.; Hernadi, K.; Maillard-Schaller, E.; Schlapbach, L.; Forró, L.; Kern, K. Patterned Films of Nanotubes Using Microcontact Printing of Catalysts; Wiley Online Library: Hoboken, NJ, USA, 1999; Volume 11, ISBN 2172440809. [Google Scholar]
- Heuer-jungemann, A.; Feliu, N.; Bakaimi, I.; Hamaly, M.; Alkilany, A.; Chakraborty, I.; Masood, A.; Casula, M.F.; Kostopoulou, A.; Oh, E.; et al. The Role of Ligands in the Chemical Synthesis and Applications of Inorganic Nanoparticles. Chem. Rev. 2019, 19, 4819–4880. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Im, S.H.; Kang, M.; Heo, J.H.; Seok, S., II; Kim, S.W.; Mora-Seró, I.; Bisquert, J. Air-Stable and Efficient Inorganic-Organic Heterojunction Solar Cells Using PbS Colloidal Quantum Dots Co-Capped by 1-Dodecanethiol and Oleic Acid. Phys. Chem. Chem. Phys. 2012, 14, 14999–15002. [Google Scholar] [CrossRef]
- Klem, E.J.D.; MacNeil, D.D.; Levina, L.; Sargent, E.H. Solution Processed Photovoltaic Devices with 2% Infrared Monochromatic Power Conversion Efficiency: Performance Optimization and Oxide Formation. Adv. Mater. 2008, 20, 3433–3439. [Google Scholar] [CrossRef]
- Tavakoli, M.M.; Tayyebi, A.; Simchi, A.; Aashuri, H.; Outokesh, M.; Fan, Z. Physicochemical Properties of Hybrid Graphene–Lead Sulfide Quantum Dots Prepared by Supercritical Ethanol. J. Nanoparticle Res. 2015, 17, 9. [Google Scholar] [CrossRef]
- Ai, Y.; Liu, X.; Yang, B.; Li, Q.; Liu, S. Materials Science in Semiconductor Processing Lead Sulfide Quantum Dot Assembly with Biocompatible Mechanical Property and Tunable Hydrophilicity. Mater. Sci. Semicond. Process. 2022, 140, 106374. [Google Scholar] [CrossRef]
- Dagher, S.; Haik, Y.; Tit, N.; Ayesh, A. PbS/CdS Heterojunction Quantum Dot Solar Cells. J. Mater. Sci. Mater. Electron. 2016, 27, 3328–3340. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, T.-C.; Su, H.-Y.; Uma, K.; Chen, S.-A.; Deng, Z.-C.; Kao, T.-T.; Lin, C.-C.; Chen, L.-C. Stable Near-Infrared Photoluminescence of Hexagonal-Shaped PbS Nanoparticles with 1-Dodecanethiol Ligands. Materials 2024, 17, 2380. https://doi.org/10.3390/ma17102380
Liang T-C, Su H-Y, Uma K, Chen S-A, Deng Z-C, Kao T-T, Lin C-C, Chen L-C. Stable Near-Infrared Photoluminescence of Hexagonal-Shaped PbS Nanoparticles with 1-Dodecanethiol Ligands. Materials. 2024; 17(10):2380. https://doi.org/10.3390/ma17102380
Chicago/Turabian StyleLiang, Tsair-Chun, Hsin-Yu Su, Kasimayan Uma, Sih-An Chen, Zhi-Chi Deng, Tzung-Ta Kao, Chun-Cheng Lin, and Lung-Chien Chen. 2024. "Stable Near-Infrared Photoluminescence of Hexagonal-Shaped PbS Nanoparticles with 1-Dodecanethiol Ligands" Materials 17, no. 10: 2380. https://doi.org/10.3390/ma17102380
APA StyleLiang, T.-C., Su, H.-Y., Uma, K., Chen, S.-A., Deng, Z.-C., Kao, T.-T., Lin, C.-C., & Chen, L.-C. (2024). Stable Near-Infrared Photoluminescence of Hexagonal-Shaped PbS Nanoparticles with 1-Dodecanethiol Ligands. Materials, 17(10), 2380. https://doi.org/10.3390/ma17102380






