Theoretical Study of Single-Atom Catalysts for Hydrogen Evolution Reaction Based on BiTeBr Monolayer
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Structural Model and Hydrogen Adsorption Ability of Janus BiTeBr Monolayer
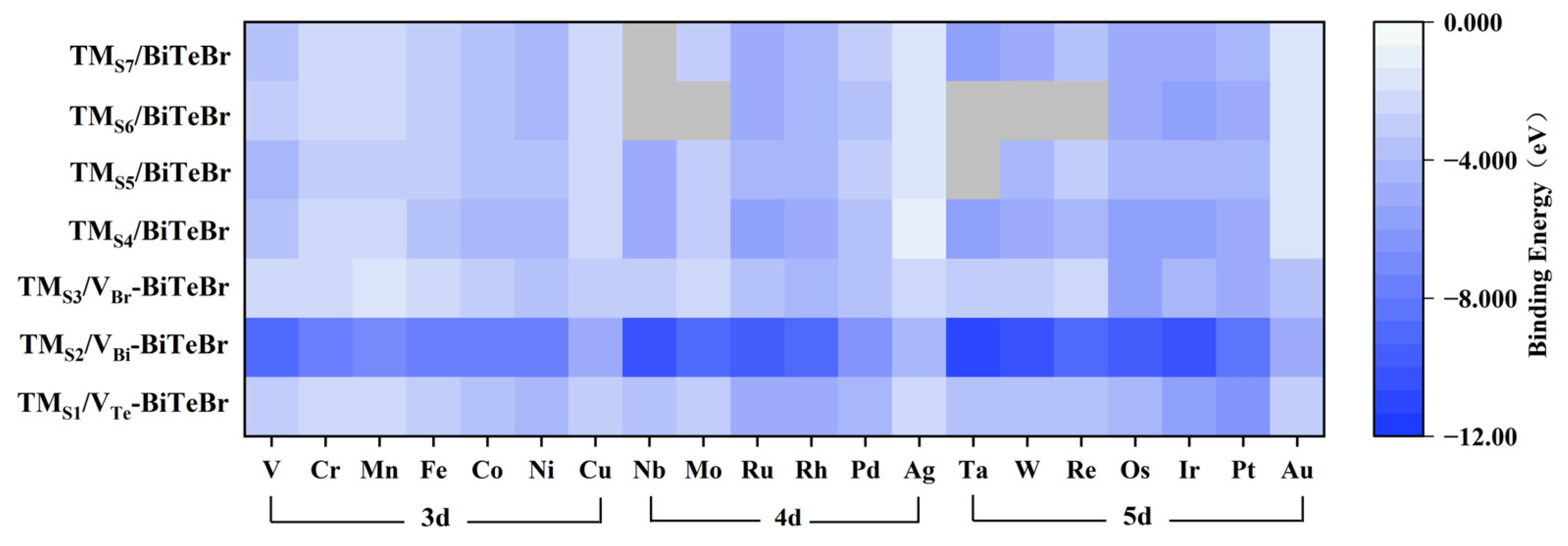
3.2. The Stability of SACs Based on BiTeBr Monolayer
3.3. Catalytic Activity of SACs
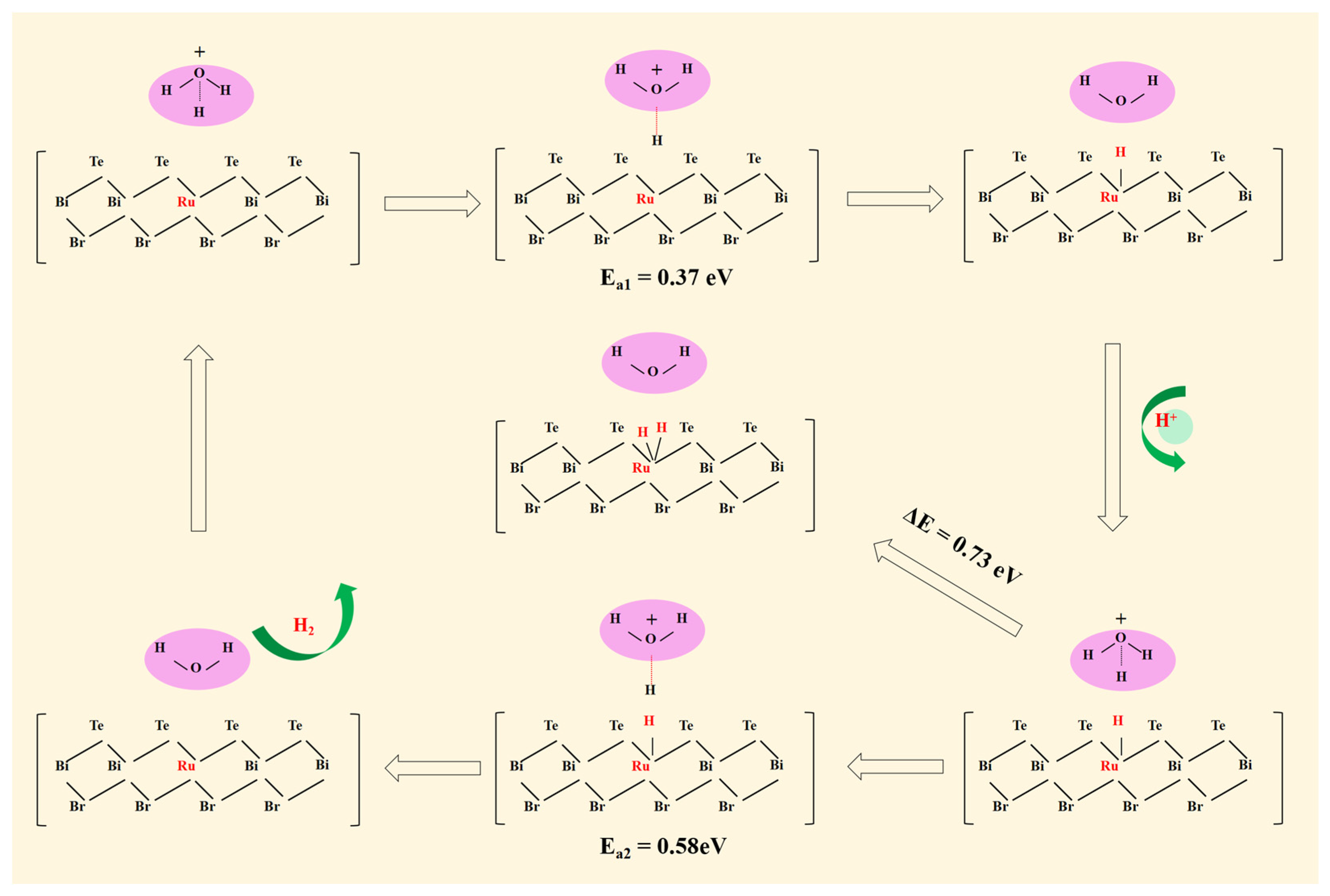
3.4. Reaction Mechanism for RuS2/VBi-BiTeBr
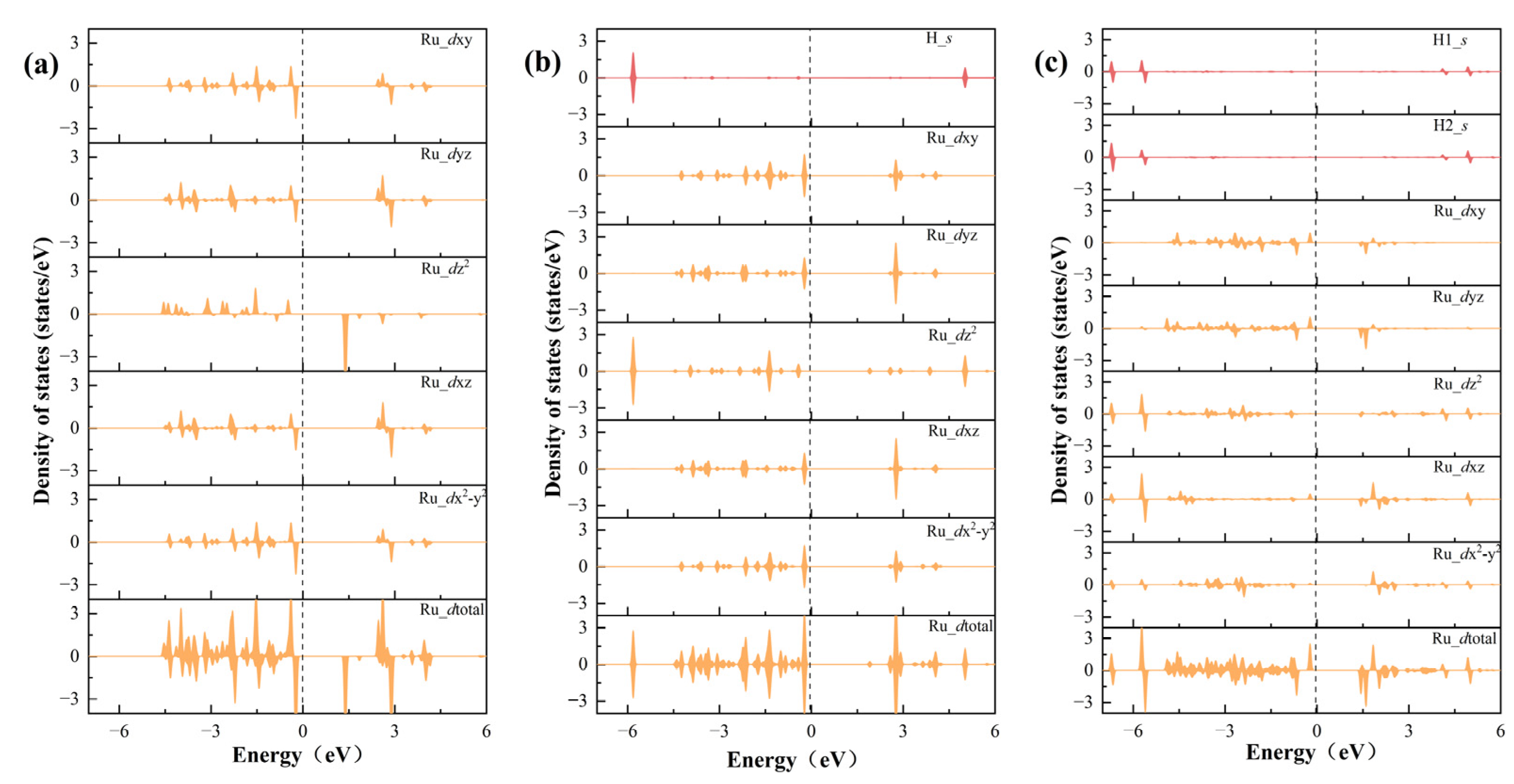
3.5. Analysis of Electronic Structures
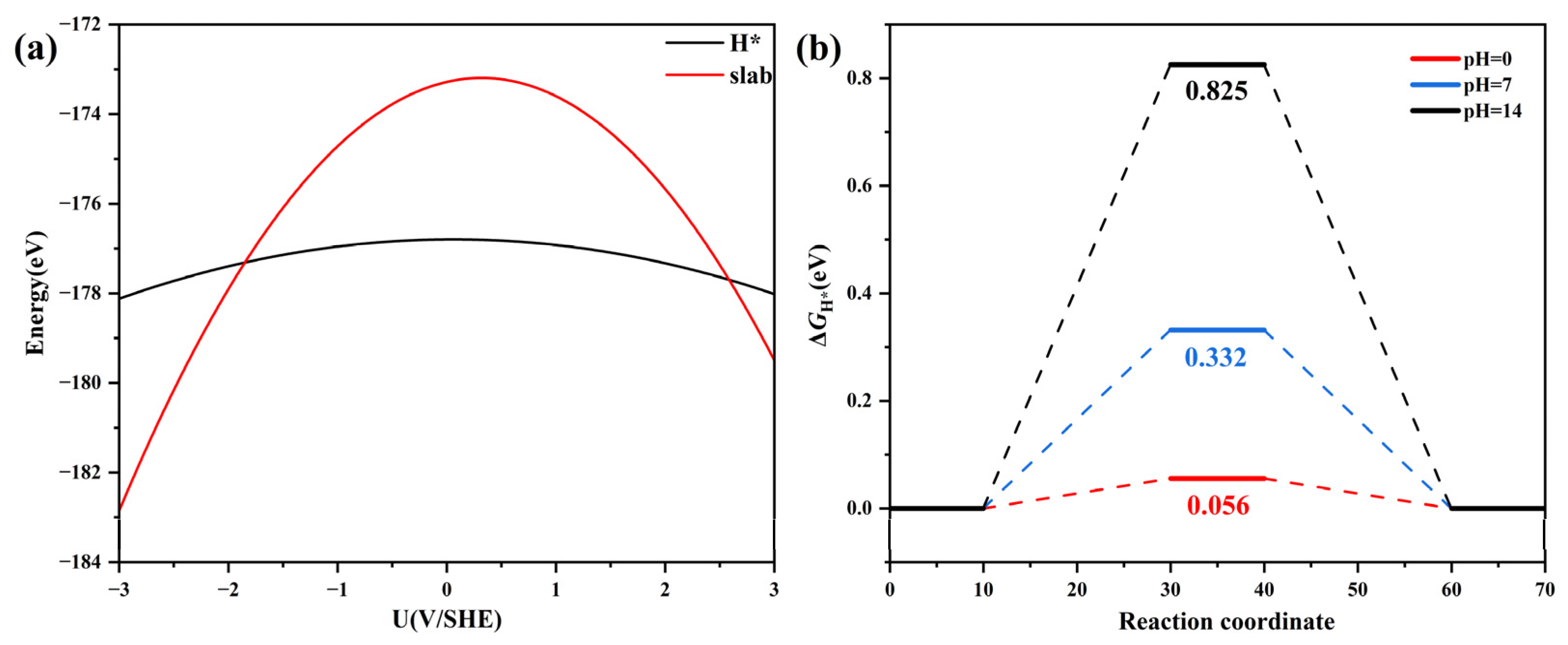
3.6. Constant Potential
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, R.; Singh, M.; Gautam, S. Hydrogen economy, energy, and liquid organic carriers for its mobility. Mater. Today Proc. 2021, 46, 5420–5427. [Google Scholar] [CrossRef]
- Hassan, I.A.; Ramadan, H.S.; Saleh, M.A.; Hissel, D. Hydrogen storage technologies for stationary and mobile applications: Review, analysis and perspectives. Renew. Sustain. Energy Rev. 2021, 149, 111311. [Google Scholar] [CrossRef]
- Esapour, K.; Abbasian, M.; Saghafi, H. Intelligent energy management in hybrid microgrids considering tidal, wind, solar and battery. Int. J. Electr. Power Energy Syst. 2021, 127, 106615. [Google Scholar] [CrossRef]
- Yoo, J.J.; Seo, G.; Chua, M.R.; Park, T.G.; Lu, Y.; Rotermund, F.; Kim, Y.-K.; Moon, C.S.; Jeon, N.J.; Correa-Baena, J.-P.; et al. Efficient perovskite solar cells via improved carrier management. Nature 2021, 590, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Subbaraman, R.; Tripkovic, D.; Strmcnik, D.; Chang, K.-C.; Uchimura, M.; Paulikas, A.P.; Stamenkovic, V.; Markovic, N.M. Enhancing Hydrogen Evolution Activity in Water Splitting by Tailoring Li+-Ni(OH)2-Pt Interfaces. Science 2011, 334, 1256–1260. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Xu, X.; Xia, Y.; Huang, W.; Li, Z.; Teng, B. Insights into electrocatalytic hydrogen evolution reaction in acidic medium at in-situ dispersed Pt atoms on nanoporous gold films. J. Catal. 2018, 368, 379–388. [Google Scholar] [CrossRef]
- Liang, S.; Hu, H.; Liu, J.; Shen, H.; Li, Q.; Qiu, N.; Guo, H.; Guo, X.; Du, S.; Zhu, Y.; et al. Nickel-nitride composite: An eco-friendly and efficient alternative to platinum for electrocatalytic hydrogen production. Appl. Catal. B Environ. 2023, 337, 123008. [Google Scholar] [CrossRef]
- Serafin, J.; Kusiak-Nejman, E.; Wanag, A.; Morawski, A.W.; Llorca, J. Hydrogen photoproduction on TiO2-reduced graphene oxide hybrid materials from water-ethanol mixture. J. Photochem. Photobiol. A Chem. 2021, 418, 113406. [Google Scholar] [CrossRef]
- Zhu, C.; Shi, Q.; Feng, S.; Du, D.; Lin, Y. Single-Atom Catalysts for Electrochemical Water Splitting. ACS Energy Lett. 2018, 3, 1713–1721. [Google Scholar] [CrossRef]
- Gao, X.; Zhou, Y.; Liu, S.; Cheng, Z.; Tan, Y.; Shen, Z. Single cobalt atom anchored on N-doped graphyne for boosting the overall water splitting. Appl. Surf. Sci. 2020, 502, 144155. [Google Scholar] [CrossRef]
- Gu, Y.; Wei, B.; Legut, D.; Fu, Z.; Du, S.; Zhang, H.; Francisco, J.S.; Zhang, R. Single Atom-Modified Hybrid Transition Metal Carbides as Efficient Hydrogen Evolution Reaction Catalysts. Adv. Funct. Mater. 2021, 31, 2104285. [Google Scholar] [CrossRef]
- Cheng, Y.-W.; Dai, J.-H.; Zhang, Y.-M.; Song, Y. Transition metal modification and carbon vacancy promoted Cr2CO2 (MXenes): A new opportunity for a highly active catalyst for the hydrogen evolution reaction. J. Mater. Chem. A 2018, 6, 20956–20965. [Google Scholar] [CrossRef]
- Zhao, D.; Chen, Z.; Yang, W.; Liu, S.; Zhang, X.; Yu, Y.; Cheong, W.-C.; Zheng, L.; Ren, F.; Ying, G.; et al. MXene (Ti3C2) Vacancy-Confined Single-Atom Catalyst for Efficient Functionalization of CO2. J. Am. Chem. Soc. 2019, 141, 4086–4093. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Zhou, J.; Chen, J.; Zhang, T.; Sun, Z. Cu single atoms on Ti2CO2 as a highly efficient oxygen reduction catalyst in a proton exchange membrane fuel cell. J. Mater. Chem. A 2019, 7, 26062–26070. [Google Scholar] [CrossRef]
- Lu, A.-Y.; Zhu, H.; Xiao, J.; Chuu, C.-P.; Han, Y.; Chiu, M.-H.; Cheng, C.-C.; Yang, C.-W.; Wei, K.-H.; Yang, Y.; et al. Janus monolayers of transition metal dichalcogenides. Nat. Nanotechnol. 2017, 12, 744–749. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jia, S.; Kholmanov, I.; Dong, L.; Er, D.; Chen, W.; Guo, H.; Jin, Z.; Shenoy, V.B.; Shi, L.; et al. Janus Monolayer Transition-Metal Dichalcogenides. ACS Nano 2017, 11, 8192–8198. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Lou, J.; Shenoy, V.B. Large In-Plane and Vertical Piezoelectricity in Janus Transition Metal Dichalchogenides. ACS Nano 2017, 11, 8242–8248. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Jia, F.; Zhao, G.; Wu, J.; Stroppa, A.; Ren, W. Intrinsic and anisotropic Rashba spin splitting in Janus transition-metal dichalcogenide monolayers. Phys. Rev. B 2018, 97, 235404. [Google Scholar] [CrossRef]
- Ma, Y.; Kou, L.; Huang, B.; Dai, Y.; Heine, T. Two-dimensional ferroelastic topological insulators in single-layer Janus transition metal dichalcogenides MSSe (M = Mo, W). Phys. Rev. B 2018, 98, 085420. [Google Scholar] [CrossRef]
- Er, D.; Ye, H.; Frey, N.C.; Kumar, H.; Lou, J.; Shenoy, V.B. Prediction of Enhanced Catalytic Activity for Hydrogen Evolution Reaction in Janus Transition Metal Dichalcogenides. Nano Lett. 2018, 18, 3943–3949. [Google Scholar] [CrossRef]
- Xia, C.; Xiong, W.; Du, J.; Wang, T.; Peng, Y.; Li, J. Universality of electronic characteristics and photocatalyst applications in the two-dimensional Janus transition metal dichalcogenides. Phys. Rev. B 2018, 98, 165424. [Google Scholar] [CrossRef]
- Ge, L.; Fu, Z.; Lu, Y. Activating basal plane of Janus VSSe for efficient hydrogen evolution reaction by non-noble metal element doping: A first-principal study. Int. J. Hydrogen Energy 2022, 47, 34924–34931. [Google Scholar] [CrossRef]
- Wang, M.; Wang, X.; Zheng, M.; Zhou, X. Improving Catalytic Activity of “Janus” MoSSe Based on Surface Interface Regulation. Molecules 2022, 27, 6038. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Sa, R.; Ma, Z.; Cui, Z.; Du, W.; Sun, X.; Li, Q.; Deng, H. High-throughput screening of transition metal single-atom catalyst anchored on Janus MoSSe basal plane for hydrogen evolution reaction. Int. J. Hydrogen Energy 2021, 46, 10337–10345. [Google Scholar] [CrossRef]
- Chen, Y.L.; Kanou, M.; Liu, Z.K.; Zhang, H.J.; Sobota, J.A.; Leuenberger, D.; Mo, S.K.; Zhou, B.; Yang, S.L.; Kirchmann, P.S.; et al. Discovery of a single topological Dirac fermion in the strong inversion asymmetric compound BiTeCl. Nat. Phys. 2013, 9, 704–708. [Google Scholar] [CrossRef]
- Yagmurcukardes, M.; Qin, Y.; Ozen, S.; Sayyad, M.; Peeters, F.M.; Tongay, S.; Sahin, H. Quantum properties and applications of 2D Janus crystals and their superlattices. Appl. Phys. Rev. 2020, 7, 011311. [Google Scholar] [CrossRef]
- Li, H.; Qin, Y.; Ko, B.; Trivedi, D.B.; Hajra, D.; Sayyad, M.Y.; Liu, L.; Shim, S.-H.; Zhuang, H.; Tongay, S. Anomalous Behavior of 2D Janus Excitonic Layers under Extreme Pressures. Adv. Mater. 2020, 32, 2002401. [Google Scholar] [CrossRef] [PubMed]
- Hajra, D.; Sailus, R.; Blei, M.; Yumigeta, K.; Shen, Y.; Tongay, S. Epitaxial Synthesis of Highly Oriented 2D Janus Rashba Semiconductor BiTeCl and BiTeBr Layers. ACS Nano 2020, 14, 15626–15632. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Tang, Q.; Jiang, D. Theoretical Advances in Understanding and Designing the Active Sites for Hydrogen Evolution Reaction. ACS Catal. 2022, 12, 8404–8433. [Google Scholar] [CrossRef]
- Tang, Q.; Jiang, D. Mechanism of Hydrogen Evolution Reaction on 1T-MoS2 from First Principles. ACS Catal. 2016, 6, 4953–4961. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Bafekry, A.; Karbasizadeh, S.; Stampfl, C.; Faraji, M.; Hoat, D.M.; Sarsari, I.A.; Feghhi, S.A.H.; Ghergherehchi, M. Two-dimensional Janus semiconductor BiTeCl and BiTeBr monolayers: A first-principles study on their tunable electronic properties via an electric field and mechanical strain. Phys. Chem. Chem. Phys. 2021, 23, 15216–15223. [Google Scholar] [CrossRef]
- Baroni, S.; de Gironcoli, S.; Dal Corso, A.; Giannozzi, P. Phonons and related crystal properties from density-functional perturbation theory. Rev. Mod. Phys. 2001, 73, 515–562. [Google Scholar] [CrossRef]
- Nørskov, J.K.; Bligaard, T.; Logadottir, A.; Kitchin, J.R.; Chen, J.G.; Pandelov, S.; Stimming, U. Trends in the Exchange Current for Hydrogen Evolution. J. Electrochem. Soc. 2005, 152, J23. [Google Scholar] [CrossRef]
- Mathew, K.; Sundararaman, R.; Letchworth-Weaver, K.; Arias, T.A.; Hennig, R.G. Implicit solvation model for density-functional study of nanocrystal surfaces and reaction pathways. J. Chem. Phys. 2014, 140, 084106. [Google Scholar] [CrossRef] [PubMed]
- Sakano, M.; Bahramy, M.S.; Katayama, A.; Shimojima, T.; Murakawa, H.; Kaneko, Y.; Malaeb, W.; Shin, S.; Ono, K.; Kumigashira, H.; et al. Strongly Spin-Orbit Coupled Two-Dimensional Electron Gas Emerging near the Surface of Polar Semiconductors. Phys. Rev. Lett. 2013, 110, 107204. [Google Scholar] [CrossRef]
- Kim, J.; Rabe, K.M.; Vanderbilt, D. Negative piezoelectric response of van der Waals layered bismuth tellurohalides. Phys. Rev. B 2019, 100, 104115. [Google Scholar] [CrossRef]
- Banhart, F.; Kotakoski, J.; Krasheninnikov, A.V. Structural Defects in Graphene. ACS Nano 2011, 5, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Skowron, S.T.; Lebedeva, I.V.; Popov, A.M.; Bichoutskaia, E. Energetics of atomic scale structure changes in graphene. Chem. Soc. Rev. 2015, 44, 3143–3176. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Huynh, H.L.; Lou, F.; Guo, K.; Yu, Z. Single transition metal atom embedded antimonene monolayers as efficient trifunctional electrocatalysts for the HER, OER and ORR: A density functional theory study. Nanoscale 2021, 13, 12885–12895. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Huang, Z.; Gao, Z.; Perez-Aguilar, J.M.; Gu, Z.; Tu, Y. Single Atom Catalysis for Hydrogen Evolution Reaction using Transition-metal Atoms Doped g-C3N3: A Density Functional Theory Study. ChemistrySelect 2023, 8, e202203475. [Google Scholar] [CrossRef]
- Ye, J.; Feng, Z.; Li, H.; Dai, X. DFT+U study on the magnetic properties of 3d transition metal doped β12 borophene. Phys. E Low-Dimens. Syst. Nanostruct. 2023, 147, 115576. [Google Scholar] [CrossRef]
- Zhou, Y.; Sheng, L.; Luo, Q.; Zhang, W.; Yang, J. Improving the Activity of Electrocatalysts toward the Hydrogen Evolution Reaction, the Oxygen Evolution Reaction, and the Oxygen Reduction Reaction via Modification of Metal and Ligand of Conductive Two-Dimensional Metal–Organic Frameworks. J. Phys. Chem. Lett. 2021, 12, 11652–11658. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, X.; Qin, J.; Liu, R. Transition metal atom doped Ni3S2 as efficient bifunctional electrocatalysts for overall water splitting: Design strategy from DFT studies. Mol. Catal. 2021, 516, 111955. [Google Scholar] [CrossRef]
- Li, L.; Martirez, J.M.P.; Carter, E.A. Prediction of Highly Selective Electrocatalytic Nitrogen Reduction at Low Overpotential on a Mo-Doped g-GaN Monolayer. ACS Catal. 2020, 10, 12841–12857. [Google Scholar] [CrossRef]
- Yan, P.; Yang, T.; Lin, M.; Guo, Y.; Qi, Z.; Luo, Q.; Yu, X.-Y. “One Stone Five Birds” Plasma Activation Strategy Synergistic with Ru Single Atoms Doping Boosting the Hydrogen Evolution Performance of Metal Hydroxide. Adv. Funct. Mater. 2023, 33, 2301343. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, Y.-J.; Zhou, K. Self-Adjusting Activity Induced by Intrinsic Reaction Intermediate in Fe–N–C Single-Atom Catalysts. J. Am. Chem. Soc. 2019, 141, 14115–14119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Asthagiri, A. Solvation effects on DFT predictions of ORR activity on metal surfaces. Catal. Today 2019, 323, 35–43. [Google Scholar] [CrossRef]
- Ru, S.; He, M.; Zhou, Y.; Xu, C.; Luo, Q.; Yang, J. Theoretical and Comparative Analysis of Graphdiyne and Confined Flexible Nitrogen-Doped Graphdiyne-Supported Single-Atom Catalysts for Electrochemical Nitrogen Reduction. J. Phys. Chem. C 2022, 126, 18282–18291. [Google Scholar] [CrossRef]
- Skúlason, E.; Tripkovic, V.; Björketun, M.E.; Gudmundsdóttir, S.; Karlberg, G.; Rossmeisl, J.; Bligaard, T.; Jónsson, H.; Nørskov, J.K. Modeling the Electrochemical Hydrogen Oxidation and Evolution Reactions on the Basis of Density Functional Theory Calculations. J. Phys. Chem. C 2010, 114, 18182–18197. [Google Scholar] [CrossRef]
- Duan, Z.; Henkelman, G. Identification of Active Sites of Pure and Nitrogen-Doped Carbon Materials for Oxygen Reduction Reaction Using Constant-Potential Calculations. J. Phys. Chem. C 2020, 124, 12016–12023. [Google Scholar] [CrossRef]
- Mehdipour, H.; Kratzer, P. Structural defects in a Janus MoSSe monolayer: A density functional theory study. Phys. Rev. B 2022, 106, 235414. [Google Scholar] [CrossRef]
- Pan, H.-X.; Feng, L.-P.; Zeng, W.; Zhang, Q.-C.; Zhang, X.-D.; Liu, Z.-T. Active Sites in Single-Layer BiOX (X = Cl, Br, and I) Catalysts for the Hydrogen Evolution Reaction. Inorg. Chem. 2019, 58, 13195–13202. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-J.; Zhang, S.; Chai, Y.-M.; Dong, B. Ligand Modulation of Active Sites to Promote Cobalt-Doped 1T-MoS2 Electrocatalytic Hydrogen Evolution in Alkaline Media. Angew. Chem. Int. Ed. 2023, 62, e202313845. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, T.; Li, J.; Zhang, Q.; Li, B.; Gao, M. Construction of Ru, O Co-Doping MoS2 for Hydrogen Evolution Reaction Electrocatalyst and Surface-Enhanced Raman Scattering Substrate: High-Performance, Recyclable, and Durability Improvement. Adv. Funct. Mater. 2023, 33, 2210939. [Google Scholar] [CrossRef]


| Sites | t1 | b1 | h1 | h2 | t2 | b2 | h3 | h4 |
|---|---|---|---|---|---|---|---|---|
| ΔGH* (eV) | 1.97 | 1.77 | 2.12 | 1.67 | 1.76 | 1.86 | 1.86 | 2.32 |
| Models | ΔE | |
|---|---|---|
| CHE model | −0.24 | |
| Explicit models | H3O+ | −0.51 |
| H3O+ + (H2O)4 | 0.34 | |
| H3O+ + (H2O)5 | 0.89 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, T.; Luo, Q. Theoretical Study of Single-Atom Catalysts for Hydrogen Evolution Reaction Based on BiTeBr Monolayer. Materials 2024, 17, 2377. https://doi.org/10.3390/ma17102377
Yang T, Luo Q. Theoretical Study of Single-Atom Catalysts for Hydrogen Evolution Reaction Based on BiTeBr Monolayer. Materials. 2024; 17(10):2377. https://doi.org/10.3390/ma17102377
Chicago/Turabian StyleYang, Tao, and Qiquan Luo. 2024. "Theoretical Study of Single-Atom Catalysts for Hydrogen Evolution Reaction Based on BiTeBr Monolayer" Materials 17, no. 10: 2377. https://doi.org/10.3390/ma17102377




