Analysis of the Surface of Historic Fabric from the Auschwitz-Birkenau State Museum after Treatment with Ethanol Mist Used to Eliminate Microorganisms Harmful to Human Health
Abstract
:1. Introduction
2. Materials and Methods
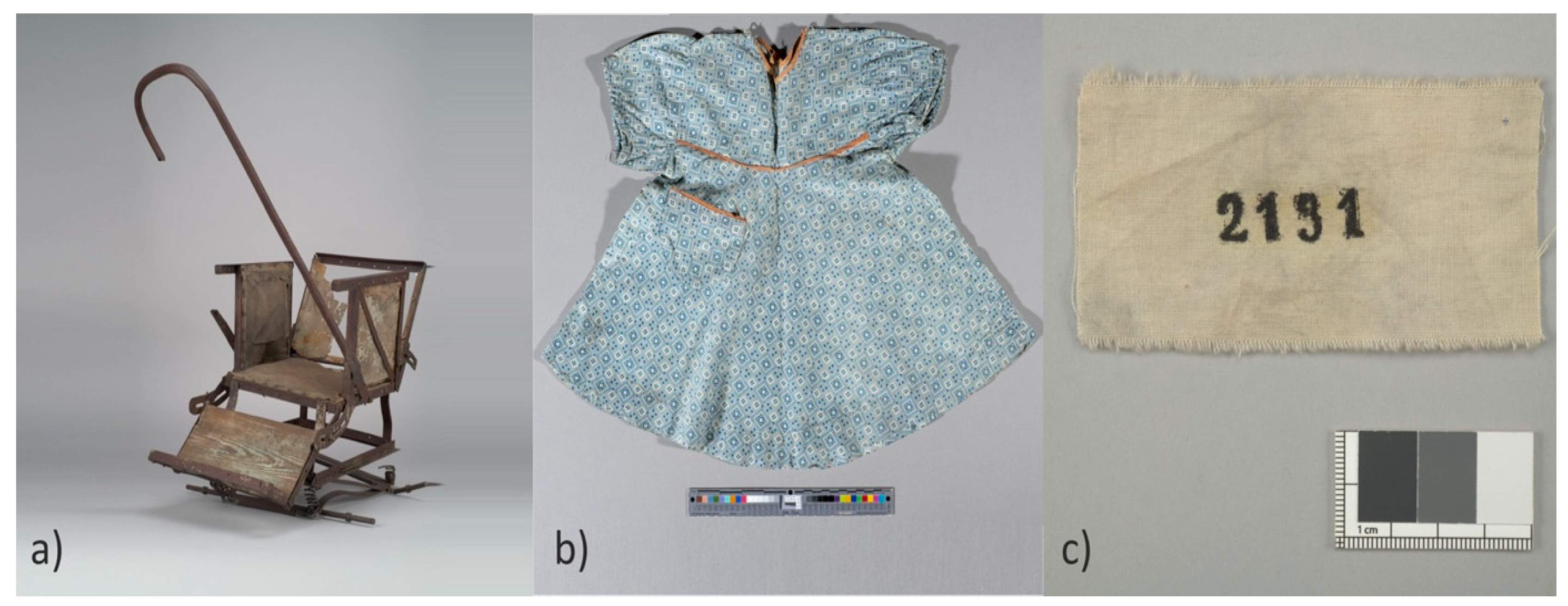
2.1. Research Objects
2.2. Application of 90% Ethanol in Mist Form on Cotton
2.3. Chemical Analysis of the Surfaces
2.3.1. FTIR Analysis of the Chemical Composition of Cotton Surface before and after Decontamination with Ethanol
2.3.2. XPS Analysis of the Chemical Composition of Cotton Surface before and after Decontamination with Ethanol
2.3.3. Quality Assurance in Research
3. Results
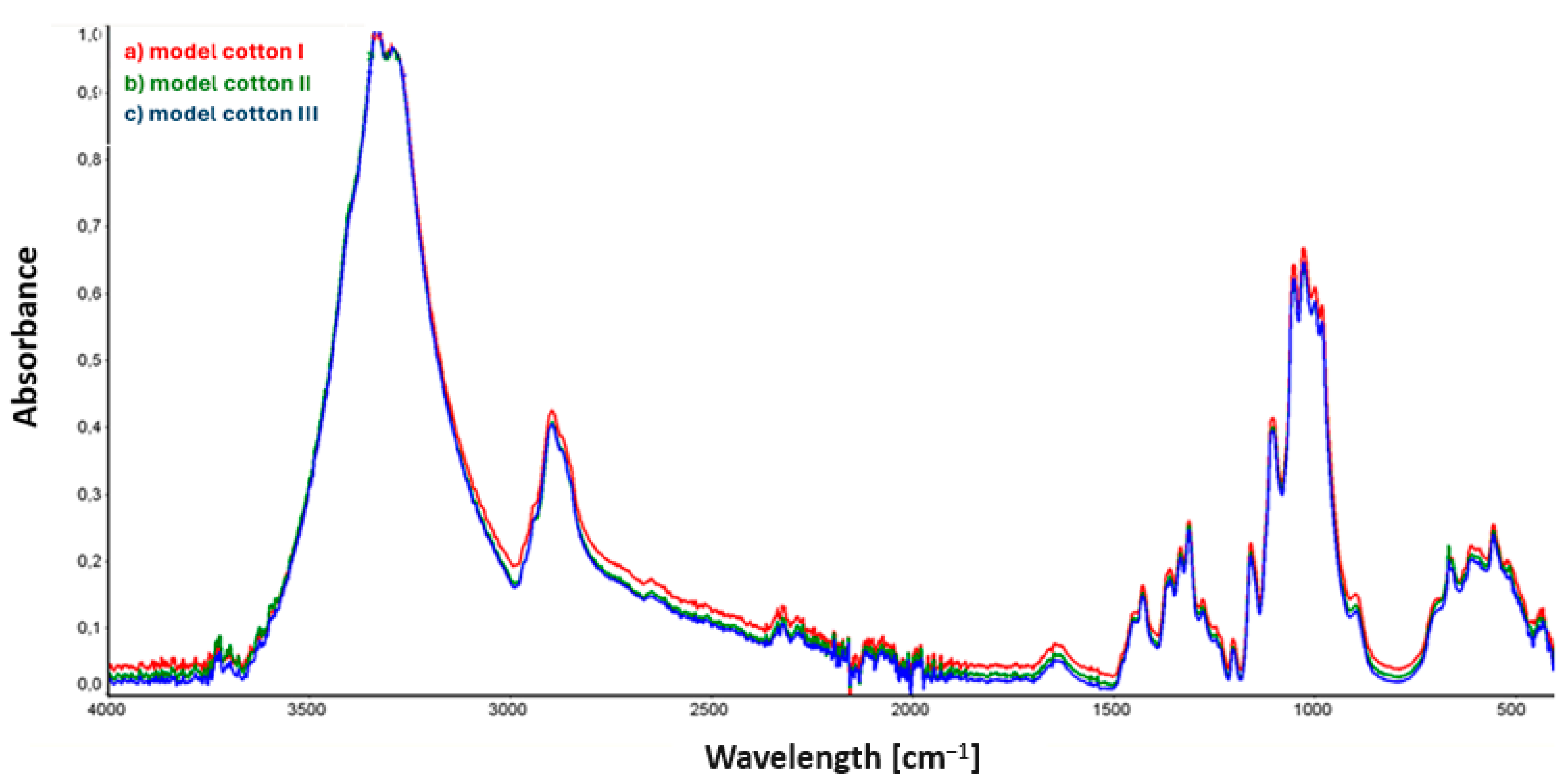
3.1. Fourier-Transform Infrared Spectroscopy (FTIR) Analysis of the Surface of Disinfected Model and Historical Cotton
3.1.1. Fourier-Transform Infrared Spectroscopy (FTIR) Analysis of the Surface of Disinfected Model Cotton
3.1.2. Fourier-Transform Infrared Spectroscopy (FTIR) Analysis of the Surface of Disinfected Historical Cotton
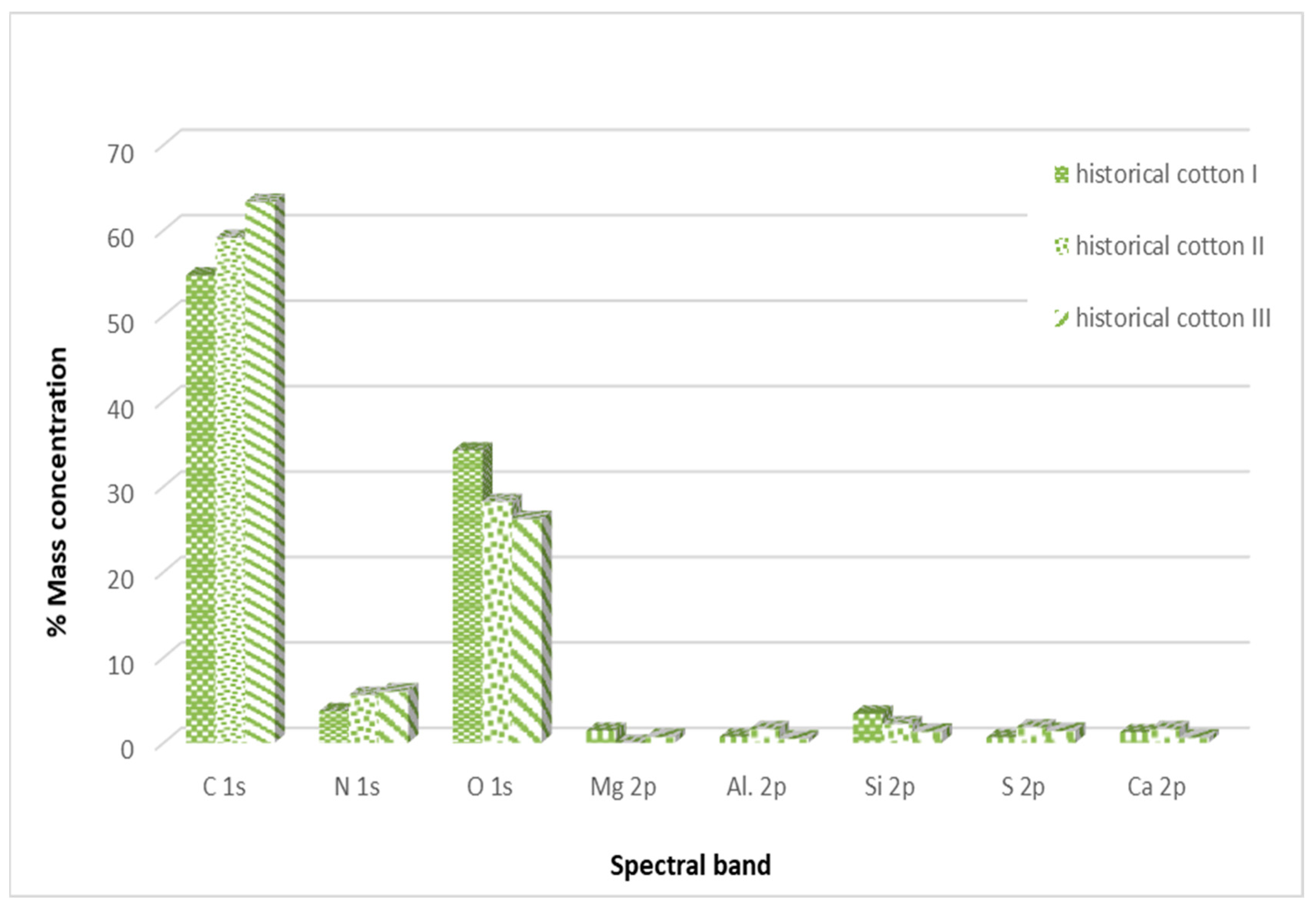
3.2. X-ray Photoelectron Spectroscopy (XPS) Analysis of the Surface of Decontaminated Model and Historical Cotton
3.2.1. X-ray Photoelectron Spectroscopy (XPS) Analysis of the Surface of Decontaminated Model Cotton
3.2.2. X-ray Photoelectron Spectroscopy (XPS) Analysis of the Surface of Decontaminated Historical Cotton
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cywiński, P.A.; Lachendro, J.; Setkiewicz, P. Auschwitz from A to Z; Auschwitz-Birkenau State Museum: Oświęcim, Poland, 2013; p. 11. [Google Scholar]
- Szostak-Kotowa, J. Biodeterioration of textiles. Int. Biodeterior. Biodegrad. 2004, 53, 165–170. [Google Scholar] [CrossRef]
- Abdel-Kareem, O. Evaluating the combined efficacy of polymers with fungicides for protection of museum textiles against fungal deterioration in Egypt. Pol. J. Microbiol. 2010, 59, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Arshad, K.; Mujahid, M. Biodegradation of Textile Materials; University of Borås/Swedish School of Textiles: Boras, Sweden, 2011. [Google Scholar]
- Gutarowska, B.; Pietrzak, K.; Machnowski, W.; Milczarek, J.M. Historical textiles–a review of microbial deterioration analysis and disinfection methods. Text. Res. J. 2016, 87, 2388–2406. [Google Scholar] [CrossRef]
- Wawrzyk, A.; Rahnama, M.; Rybitwa, D.; Wieczorek, K.; Michalczewski, G.; Łobacz, M. Decontamination of microbiologically contaminated abiotic porous surfaces in an oral surgery clinic using vaporised hydrogen peroxide (VHP). J. Environ. Health Sci. Eng. 2020, 18, 639–653. [Google Scholar] [CrossRef]
- Mazzoli, R.; Giuffrida, M.G.; Pessione, E. Back to the past: “find the guilty bug-microorganisms involved in the biodeterioration of archeological and historical artifacts”. Appl. Microbiol. Biotechnol. 2018, 102, 6393–6407. [Google Scholar] [CrossRef] [PubMed]
- Safarov, B.; Indrie, L.; Costea, M.; Turza, A.; Avazov, K.; Baias, S.; ILIEȘ, D.C.; Zdrinca, M.; Pantea, E.; ILIEȘ, G.; et al. Non-invasive analytical methods applied in the study of cultural heritage artefacts. Ind. Text. 2023, 74, 321–331. [Google Scholar] [CrossRef]
- Tewari, A.; Abdullah, S. Bacillus cereus food poisoning: International and Indian perspective. J. Food Sci. Technol. 2015, 52, 2500–2511. [Google Scholar] [CrossRef]
- Kamar, R.; Gohar, M.; Jéhanno, I.; Réjasse, A.; Kallassy, M.; Lereclus, D.; Sanchis, V.; Ramarao, N. Pathogenic potential of Bacillus cereus strains as revealed by phenotypic analysis. J. Clin. Microbiol. 2013, 51, 320–323. [Google Scholar] [CrossRef] [PubMed]
- Wawrzyk, A.; Gutarowska, B.; Rybitwa, D.; Pietrzak, K.; Machnowski, W.; Wrzosek, H.; Papis, A.; Walawska, A.; Otlewska, A.; Szulc, J.; et al. Vapourised hydrogen peroxide (VHP) and ethylene oxide (EtO) methods for disinfecting historical. Int. Biodeterior. Biodegrad. 2018, 133, 42–51. [Google Scholar] [CrossRef]
- Rudramurthy, S.M.; Paul, R.A.; Chakrabarti, A.; Mouton, J.W.; Meis, J.F. Invasive Aspergillosis by Aspergillus flavus: Epidemiology, Diagnosis, Antifungal Resistance, and Management. J. Fungi 2019, 5, 55. [Google Scholar] [CrossRef]
- Amaike, S.; Keller, N.P. Aspergillus flavus. Annu. Rev. Phytopathol. 2011, 49, 107–133. [Google Scholar] [CrossRef]
- Klich, M.A. Aspergillus flavus: The major producer of aflatoxin. Mol. Plant Pathol. 2007, 8, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Paulussen, C.; Hallsworth, J.E.; Álvarez-Pérez, S.; Nierman, W.C.; Hamill, P.G.; Blain, D.; Rediers, H.; Lievens, B. Ecology of aspergillosis: Insights into the pathogenic potency of Aspergillus fumigatus and some other Aspergillus species. Microb. Biotechnol. 2017, 10, 296–322. [Google Scholar] [CrossRef]
- Workum, J.D.; de Jong, S.W.; Gresnigt, M.S.; Becker, K.L.; Pickkers, P.; van de Veerdonk, F.L.; Heijdra, Y.F.; Kolwijck, E. Microbiological and immunological characteristics of a lethal pulmonary Aspergillus niger infection in a non-neutropenic patient. Med. Mycol. Case Rep. 2018, 21, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Frisvad, J.C.; Møller, L.L.; Larsen, T.O.; Kumar, R.; Arnau, J. Safety of the fungal workhorses of industrial biotechnology: Update on the mycotoxin and secondary metabolite potential of Aspergillus niger, Aspergillus oryzae, and Trichoderma reesei. Appl. Microbiol. Biotechnol. 2018, 102, 9481–9515. [Google Scholar] [CrossRef]
- Rybitwa, D.; Wawrzyk, A.; Wilczyński, S.; Łobacz, M. Irradiation with medical diode laser as a new method of spot-elimination of microorganisms to preserve historical cellulosic objects and human health. Int. Biodeterior. Biodegrad. 2020, 154, 105055. [Google Scholar] [CrossRef]
- Wawrzyk, A.; Dymel, M.; Guzińska, K.; Cywiński, P.; Papis, A.; Konka, A.; Wawrzyk-Bochenek, I.; Wilczyński, S. Optimization of the Process of Eliminating Microorganisms Harmful to Human Health and Threatening Objects Isolated from Historical Materials from the Auschwitz-Birkenau State Museum in Poland (A-BSM) Collection with the Use of Ethanol in the Form of Mist. Materials 2023, 16, 2700. [Google Scholar] [CrossRef]
- Peets, P.; Kaupmees, K.; Vahur, S.; Leito, I. Reflectance FT-IR spectroscopy as a viable option for textile fiber identification. Herit Sci. 2019, 7, 93. [Google Scholar] [CrossRef]
- Yan, Y.; Wen, C.; Jin, M.; Duan, L.; Zhang, R.; Luo, C.; Xiao, J.; Ye, Z.; Gao, B.; Liu, P.; et al. FTIR spectroscopy in cultural heritage studies: Non-destructive analysis of Chinese Handmade Papers. Chem. Res. Chin. Univ. 2019, 35, 586–591. [Google Scholar] [CrossRef]
- Margariti, C. The application of FTIR microspectroscopy in a non-invasive and non-destructive way to the study and conservation of mineralised excavated textiles. Herit. Sci. 2019, 7, 63. [Google Scholar] [CrossRef]
- Rybitwa, D.; Wawrzyk, A.; Rahnama, M. Application of a medical diode laser (810 nm) for disinfecting small microbiologically contaminated spots on degraded collagenous materials for improved biosafety in objects of exceptional historical value from the Auschwitz-Birkenau State Museum and Protection of Human Health. Front. Microbiol. 2020, 11, 596852. [Google Scholar] [CrossRef] [PubMed]
- Topalovic, T.; Nierstrasz, V.A.; Bautista, L.; Jocic, D.; Navarro, A.; Warmoeskerken, M. XPS and contact angle study of cotton surface oxidation by catalytic bleaching. Colloids Surf. A Physicochem. Eng. Asp. 2007, 296, 76–85. [Google Scholar] [CrossRef]
- Willneff, E.A.; Ormsby, B.A.; Stevens, J.S.; Jaye, C.; Fischer, D.A.; Schroeder, S. Conservation of artists' acrylic emulsion paints: XPS, NEXAFS and ATR-FTIR studies of wet cleaning methods. Surf. Interface Anal. 2014, 46, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Benkhaya, S.; M'rabet, S.; Elharfi, A. A review on classifications, recent synthesis and applications of textile dyes. Inorg. Chem. Commun. 2020, 115, 107891. [Google Scholar] [CrossRef]
- Cortella, L.; Tran, Q.K.; Gluszewski, W.; Moise, I.; Ponta, C. Nuclear Techniques for Preservation of Cultural Heritage Artifacts; Report No. RER 8015; International Atomic Energy Agency (AT): Vienna, Austria, 2011; 44p. [Google Scholar]
- Drabkova, K.; Durovic, M.; Kucerova, I. Influence of gamma radiation on properties of paper and textile fibres during disinfection. Radiat. Phys. Chem. 2018, 152, 75–80. [Google Scholar] [CrossRef]
- Vadrucci, M.; Bellis, G.; Mazzuca, C.; Mercuri, F.; Borgognoni, F.; Schifano, E.; Uccelletti, D.; Cicero, C. Effects of the ionizing radiation disinfection treatment on historical leather. Front. Mater. 2020, 7, 21. [Google Scholar] [CrossRef]
- Szulc, J.; Urbaniak-Domagala, W.; Machnowski, W.; Wrzosek, H.; Lacka, K.; Gutarowska, B. Low temperature plasma for textiles disinfection. Int. Biodeter. Biodegr. 2017, 131, 97–106. [Google Scholar] [CrossRef]
- Palla, F.; Bruno, M.; Mercurio, F.; Tantillo, A.; Rotolo, V. Essential oils as natural biocides in conservation of cultural heritage. Molecules 2020, 25, 730. [Google Scholar] [CrossRef]
- Gutarowska, B.; Pietrzak, K.; Machnowski, W.; Danielewicz, D.; Szynkowska, M.; Konca, P.; Surma-Slusarska, B. Application of silver nanoparticles for disinfection of materials to protect historical objects. Curr. Nanosci. 2014, 10, 277–286. [Google Scholar] [CrossRef]
- Wawrzyk, A.; Rybitwa, D.; Rahnama, M.; Wilczynski, S. Microorganisms colonising historical cardboard objects from the Auschwitz-Birkenau State Museum in Oswięcim, Poland and their disinfection with vaporised hydrogen peroxide (VHP). Int. Biodeter. Biodegr. 2020, 152, 104997. [Google Scholar] [CrossRef]
- Wawrzyk, A.; Rahnama, M.; Sofińska-Chmiel, W.; Wilczyński, S.; Łobacz, M. The Use of the Diode Laser against the Microbiome on Composites Closing the Screw Access Hall (Sah) in the Reconstruction of Dental Implants: Ex Vivo Studies. Int. J. Environ. Res. Public Health 2022, 19, 7494. [Google Scholar] [CrossRef] [PubMed]
- Wawrzyk, A.; Łobacz, M.; Adamczuk, A.; Sofińska-Chmiel, W.; Rahnama, M. The Use of a Diode Laser for Removal of Microorganisms from the Surfaces of Zirconia and Porcelain Applied to Superstructure Dental Implants. Microorganisms 2021, 9, 2359. [Google Scholar] [CrossRef] [PubMed]
- Wawrzyk, A.; Rahnama, M.; Sofińska-Chmiel, W.; Wilczyński, S.; Gutarowska, B.; Konka, A.; Zeljas, D.; Łobacz, M. Analysis of the Microbiome on the Surface of Corroded Titanium Dental Implants in Patients with Periimplantitis and Diode Laser Irradiation as an Aid in the Implant Prosthetic Treatment: An Ex Vivo Study. Materials 2022, 15, 5890. [Google Scholar] [CrossRef] [PubMed]
- Matusiak, K.; Machnowski, W.; Wrzosek, H.; Polak, J.; Rajkowska, K.; Smigielski, K.; Kunicka-Styczynska, A.; Gutarowska, B. Application of Cinnamomum zeylanicum essential oil in vapour phase for heritage textiles disinfection. Int. Biodeter. Biodegr. 2018, 131, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Pironti, C.; Motta, O.; Proto, A. Development of a new vapour phase methodology for textiles disinfection. Clean. Eng. Technol. 2021, 4, 100170. [Google Scholar] [CrossRef]
- Sequeira, S.; Phillips, A.; Cabrita, E.; Macedo, M. Ethanol as an antifungal treatment for paper: Short-term and long-term effects. Stud. Conserv. 2017, 62, 33–42. [Google Scholar] [CrossRef]
- Karbowska-Berent, J.; Gorniak, B.; Czajkowska-Wagner, L.; Rafalska, K.; Jarmiłko, J.; Kozielec, T. The initial disinfection of paper-based historic items e Observations on some simple suggested methods. Int. Biodeterior. Biodegrad. 2018, 131, 60–66. [Google Scholar] [CrossRef]
- Nabil, A.; Basma, M.; El-Aziz, E.; Tarek, M.; Elmaaty, A.; Ramadanc, S. Multifunctional cellulose-containing fabrics using modified finishing formulations. RSC Adv. 2017, 7, 33219–33230. [Google Scholar] [CrossRef]
- Costantini, R.; Balliana, E.; Dalla Torre, D.; Aricò, F.; Zendri, E. Evaluating the Impacts of Alcohol-Based Solutions on Silk: Chemical, Mechanical and Wettability Changes before and after Artificial Ageing. Heritage 2022, 5, 3588–3604. [Google Scholar] [CrossRef]
- Chung, C.; Lee, M.; Choe, E.K. Characterization of cotton fabric scouring by FT-IR ATR spectroscopy. Carbohydr. Polym. 2004, 58, 417–420. [Google Scholar] [CrossRef]
- Abdel-Kareem, O. The long-term effect of selected conservation materials used in the treatment of museum artefacts on some properties of textiles. Polym. Degrad. Stab. 2005, 87, 121–130. [Google Scholar] [CrossRef]
- Kavkler, K.; Gunde-Cimerman, N.; Zalar, P. FTIR spectroscopy of biodegraded historical textiles. Polym. Degrad. Stab. 2011, 96, 574–580. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wawrzyk, A.; Poskrobko, J.; Guzińska, K.; Kaźmierczak, D.; Papis, A.; Jastrzębiowska, N.; Uroda, N.; Szymankiewicz, M.; Zeljaś, D.; Wawrzyk-Bochenek, I.; et al. Analysis of the Surface of Historic Fabric from the Auschwitz-Birkenau State Museum after Treatment with Ethanol Mist Used to Eliminate Microorganisms Harmful to Human Health. Materials 2024, 17, 2323. https://doi.org/10.3390/ma17102323
Wawrzyk A, Poskrobko J, Guzińska K, Kaźmierczak D, Papis A, Jastrzębiowska N, Uroda N, Szymankiewicz M, Zeljaś D, Wawrzyk-Bochenek I, et al. Analysis of the Surface of Historic Fabric from the Auschwitz-Birkenau State Museum after Treatment with Ethanol Mist Used to Eliminate Microorganisms Harmful to Human Health. Materials. 2024; 17(10):2323. https://doi.org/10.3390/ma17102323
Chicago/Turabian StyleWawrzyk, Anna, Janina Poskrobko, Krystyna Guzińska, Dorota Kaźmierczak, Aleksandra Papis, Nel Jastrzębiowska, Natalia Uroda, Maria Szymankiewicz, Dagmara Zeljaś, Iga Wawrzyk-Bochenek, and et al. 2024. "Analysis of the Surface of Historic Fabric from the Auschwitz-Birkenau State Museum after Treatment with Ethanol Mist Used to Eliminate Microorganisms Harmful to Human Health" Materials 17, no. 10: 2323. https://doi.org/10.3390/ma17102323




