Nanocomposite Coatings for Anti-Corrosion Properties of Metallic Substrates
Abstract
1. Introduction
2. Matrices
2.1. Metals
2.2. Polymers
2.2.1. Biopolymers
2.2.2. Synthetic Polymers
2.3. Ceramics
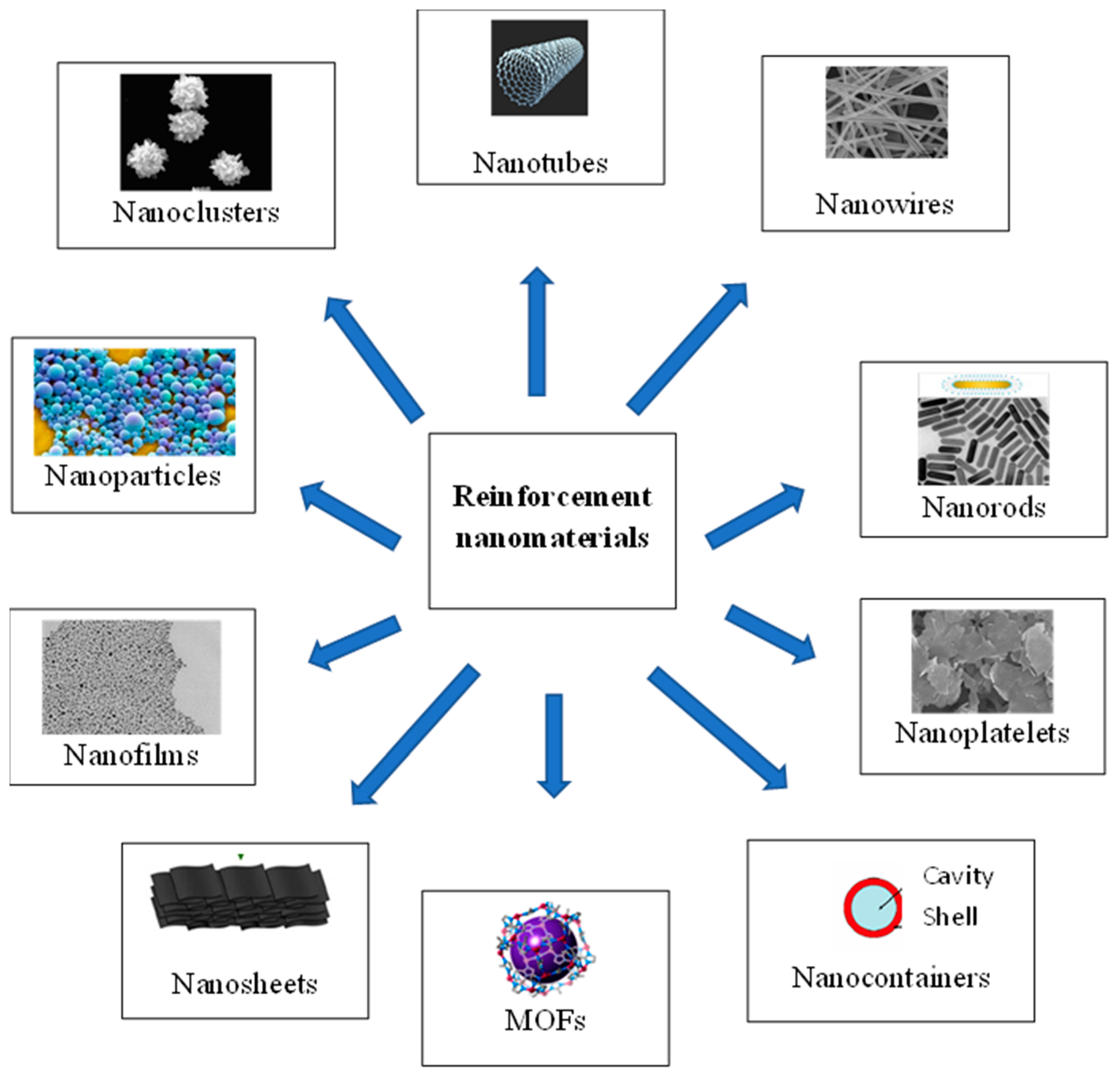
3. Reinforcement Materials/Fillers
3.1. Nanoparticles (NPs)
3.2. Nanotubes
3.3. Nanocontainers
3.4. Clays and Zeolites
3.5. Metal–Organic Frameworks (MOFs)
4. Metal Matrix Nanocomposites (MMNCs)
5. Polymer Matrix Nanocomposites (PMNCs)
6. Ceramic Matrix Nanocomposites (CMNCs)
7. Conclusions and Future Prospects
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abdeen, D.H.; El Hachach, M.; Koc, M.; Atieh, M.A. A Review on the Corrosion Behaviour of Nanocoatings on Metallic Substrates. Materials 2019, 12, 210. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Tri, P.; Nguyen, T.A.; Carriere, P.; Xuan, C.N. Nanocomposite Coatings: Preparation, Characterization, Properties, and Applications. Int. J. Corros. 2018, 2018, 4749501. [Google Scholar] [CrossRef]
- Muresan, L.M. Electrodeposited Zn-nanoparticles composite coatings for corrosion protection of steel. In Handbook of Nanoelectrochemistry; Aliofkhazraei, M., Makhlouf, A.S.H., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; Chapter 9; pp. 333–353. ISBN 978-3-319-15265-3. [Google Scholar]
- Kamboj, A.; Raghupathy, Y.; Rekha, M.Y.; Srivastava, C. Morphology, Texture and Corrosion Behavior of Nanocrystalline Copper–Graphene Composite Coatings. JOM 2017, 69, 1149–1154. [Google Scholar] [CrossRef]
- Radhamani, A.V.; Lau, H.C.; Ramakrishna, S. Nanocomposite coatings on steel for enhancing the corrosion resistance: A review. J. Compos. Mater. 2020, 54, 681–701. [Google Scholar] [CrossRef]
- Sadreddini, S.; Afshar, A. Corrosion resistance enhancement of Ni-P-nano SiO2 composite coatings on aluminum. Appl. Surf. Sci. 2014, 303, 125–130. [Google Scholar] [CrossRef]
- Li, N.; Zhang, L.; Xu, M.; Xia, T.; Ruan, X.; Song, S.; Ma, H. Preparation and mechanical property of electrodeposited Al-graphene composite coating. Mater. Des. 2016, 111, 522–527. [Google Scholar] [CrossRef]
- Li, X.; Yin, S.; Luo, H. Fabrication of robust superhydrophobic Ni–SiO2 composite coatings on aluminum alloy surfaces. Vacuum 2020, 181, 109674. [Google Scholar] [CrossRef]
- Riquelme, A.; Rodrigo, P.; Escalera-Rodríguez, M.D.; Rams, J. Characterisation and mechanical properties of Al/SiC metal matrix composite coatings formed on ZE41 magnesium alloys by laser cladding. Results Phys. 2019, 13, 102160. [Google Scholar] [CrossRef]
- Camargo, P.H.C.; Satyanarayana, K.G.; Wypych, F. Nanocomposites: Synthesis, Structure, Properties and New Application Opportunities. Mater. Res. 2009, 12, 1–39. [Google Scholar] [CrossRef]
- Conde, A.; Durán, A.; de Damborenea, J. Polymeric sol–gel coatings as protective layers of aluminium alloys. Prog. Org. Coat. 2003, 46, 288–296. [Google Scholar] [CrossRef]
- Pathak, S.S.; Khanna, A.S. Sol–gel nanocoatings for corrosion protection. In Corrosion Protection and Control Using Nanomaterials; Woodhead Publishing Ltd.: Sawton, UK, 2012; Chapter 12; pp. 304–329. [Google Scholar]
- Mashtalyar, D.; Nadaraia, K.; Sinebryukhov, S.; Gnedenkov, S. Polymer-Containing Layers Formed by PEO and Spray-Coating Method. Mater. Today Proc. 2019, 11, 150–154. [Google Scholar] [CrossRef]
- Luo, X.; Cui, X.T. Electrochemical deposition of conducting polymer coatings on magnesium surfaces in ionic liquid. Acta Biomater. 2011, 7, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Sun, J. Layer-by-Layer Codeposition of Polyelectrolyte Complexes and Free Polyelectrolytes for the Fabrication of Polymeric Coatings. Macromolecules 2010, 43, 2413–2420. [Google Scholar] [CrossRef]
- Shahini, M.H.; Ramezanzadeh, B.; Mohammadloo, H.E. Recent advances in biopolymers/carbohydrate polymers as effective corrosion inhibitive macro-molecules: A review study from experimental and theoretical views. J. Mol. Liq. 2020, 325, 115110. [Google Scholar] [CrossRef]
- Muthulakshmi, L.; Kumar, B.A.; Rajasekar, A.; Annaraj, J.; Pruncu, C.I. The benefits of k-Carrageenan-gelatin hybrid composite coating on the medical grade stainless steel (SS304) used as anticorrosive barrier. Mater. Chem. Phys. 2020, 258, 123909. [Google Scholar] [CrossRef]
- Gowraraju, N.D.; Jagadeesan, S.; Ayyasamy, K.; Olasunkanmi, L.O.; Ebenso, E.E.; Subramanian, C. Adsorption characteristics of Iota-carrageenan and Inulin biopolymers as potential corrosion inhibitors at mild steel/sulphuric acid interface. J. Mol. Liq. 2017, 232, 9–19. [Google Scholar] [CrossRef]
- Obot, I.B.; BOnyeachu, A.I.; Kumar, M. Sodium alginate: A promising biopoly mer for corrosion protection of API X60 high strength carbon steel in saline medium. Carbohydr. Polym. 2017, 178, 200–208. [Google Scholar] [CrossRef]
- Nathanael, A.J.; Oh, T.H. Biopolymer Coatings for Biomedical Applications. Polymers 2020, 12, 3061. [Google Scholar] [CrossRef]
- Christopher, G.; Kulandainathan, M.A.; Harichandran, G. Biopolymers nanocomposite for material protection: Enhancement of corrosion protection using waterborne polyurethane nanocomposite coatings. Prog. Org. Coat. 2016, 99, 91–102. [Google Scholar] [CrossRef]
- Vathsala, K.; Venkatesha, T.V.; Praveen, B.M.; Nayana, K.O. Electrochemical Generation of Zn-Chitosan Composite Coating on Mild Steel and its Corrosion Studies. Engineering 2010, 2, 580–584. [Google Scholar] [CrossRef]
- Carneiro, J.; Tedim, J.; Fernandes, S.C.M.; Freire, C.S.R.; Gandini, A.; Ferreira, M.G.S.; Zheludkevich, M.L. Functionalized chitosan-based coatings for active corrosion protection. Surf. Coat. Technol. 2013, 226, 51–59. [Google Scholar] [CrossRef]
- Szőke, Á.F.; Szabó, G.S.; Hórvölgyi, Z.; Albert, E.; Muresan, L.M. Eco-friendly Indigo Carmine-loaded Chitosan coatings for improved anti-corrosion properties of zinc substrates. Carbohydr. Polym. 2019, 215, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Luckachan, G.E.; Mittal, V. Anti-corrosion behavior of layer by layer coatings of crosslinked chitosan and poly(vinyl butyral) on carbon steel. Cellulose 2015, 22, 3275–3290. [Google Scholar] [CrossRef]
- Mathew, Z.P.; Shamnamol, G.K.; Greeshma, K.P.; John, S. Insight on the corrosion inhibition of nanocomposite chitosan/boron nitride integrated epoxy coating system against mild steel. Corros. Commun. 2023, 9, 36–43. [Google Scholar] [CrossRef]
- Raj, R.M.; Priya, P.; Raj, V. Gentamicin-loaded ceramic-biopolymer dual layer coatings on the Ti with improved bioactive and corrosion resistance properties for orthopedic applications. J. Mech. Behav. Biomed. Mater. 2018, 82, 299–309. [Google Scholar]
- Papageorgiou, S.K.; Kouvelos, E.P.; Favvas, E.P.; Sapalidis, A.A.; Romanos, G.E.; Katsaros, F.K. Metal–carboxylate interactions in metal–alginate complexes studied with FTIR spectroscopy. Carbohydr. Res. 2010, 345, 469–473. [Google Scholar] [CrossRef]
- Vakili, N.; Asefnejad, A. Titanium coating: Introducing an antibacterial and bioactive chitosan-alginate film on titanium by spin coating. Biomed. Eng. Biomed. Technol. 2020, 65, 621–630. [Google Scholar] [CrossRef]
- He, X.; Liu, Y.; Huang, J.; Chen, X.; Ren, K.; Li, H. Adsorption of alginate and albumin on aluminum coatings inhibits adhesion of Escherichia coli and enhances the anti-corrosion performances of the coatings. Appl. Surf. Sci. 2015, 332, 89–96. [Google Scholar] [CrossRef]
- Kabeb, S.M.; Hassan, A.; Mohamad, Z.; Sharer, Z.; Mokhtar, M.; Ahmad, F. Exploring the Effects of Nanofillers of Epoxy Nanocomposite Coating for Sustainable Corrosion Protection. Chem. Eng. Trans. 2019, 72, 121–126. [Google Scholar]
- Yu, Y.-H.; Lin, Y.-Y.; Lin, C.-H.; Chan, C.-C.; Huang, Y.-C. High-performance polystyrene/graphene-based nanocomposites with excellent anti-corrosion properties. Polym. Chem. 2014, 5, 535–550. [Google Scholar] [CrossRef]
- Wang, M.; Yun, H.; Tan, K.; Guo, A.; Ling, J.; Jiang, F.; Shen, X.; Xu, Q. One-step electrochemical synthesis of poly(vinyl pyrrolidone) modified polyaniline coating on stainless steel for high corrosion protection performance. Prog. Org. Coat. 2020, 149, 105908. [Google Scholar] [CrossRef]
- Zhang, Y.; Shao, Y.; Liu, X.; Shi, C.; Wang, Y.; Meng, G.; Zeng, X.; Yang, Y. A study on corrosion protection of different polyaniline coatings for mild steel. Prog. Org. Coat. 2017, 111, 240–247. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, W.; Yin, X.; Chen, Y.; Liu, Y.; Xu, B. Corrosion protection of 304 stainless steel from a smart conducting polypyrrole coating doped with pH-sensitive molybdate-loaded TiO2 nanocontainers. Prog. Org. Coat. 2020, 146, 105750. [Google Scholar] [CrossRef]
- Nautiyal, A.; Qiao, M.; Cook, J.E.; Zhang, X.; Huang, T.-S. High performance polypyrrole coating for corrosion protection and biocidal applications. Appl. Surf. Sci. 2018, 427, 922–930. [Google Scholar] [CrossRef]
- Golgoon, A.; Aliofkhazraei, M.; Toorani, M.; Moradi, M.H.; Sabour Rouhaghdam, A. Corrosion and Wear Properties of Nanoclay- Polyester Nanocomposite Coatings Fabricated by Electrostatic Method. Procedia Mater. Sci. 2015, 11, 536–541. [Google Scholar] [CrossRef]
- Abu-Thabit, N.Y.; Makhlouf, A.S.H. Recent Advances in Nanocomposite Coatings for Corrosion Protection Applications. In Handbook of Nanoceramic and Nanocomposite Coatings and Materials; Elsevier: Amsterdam, The Netherlands, 2015; Chapter 24; pp. 515–548. [Google Scholar]
- Wu, D.Y.; Meure, S.; Solomon, D. Self-healing polymeric materials: A review of recent developments. Prog. Polym. Sci. 2008, 33, 479–522. [Google Scholar] [CrossRef]
- Zechel, S.; Geitner, R.; Abend, M.; Siegmann, M.; Enke, M.; Kuhl, N.; Klein, M.; Vitz, J.; Gräfe, S.; Dietzek, B.; et al. Intrinsic self-healing polymers with a high E-modulus based on dynamic reversible urea bonds. NPG Asia Mater. 2017, 9, e420. [Google Scholar] [CrossRef]
- Willocq, B.; Odent, J.; Dubois, P.; Raquez, J.-M. Advances in intrinsic self-healing polyurethanes and related composites. RSC Adv. 2020, 10, 13766–13782. [Google Scholar] [CrossRef]
- Paolillo, S.; Bose, R.K.; Santana, M.H.; Grande, A.M. Intrinsic Self-Healing Epoxies in Polymer Matrix Composites (PMCs) for Aerospace Applications. Polymers 2021, 13, 201. [Google Scholar] [CrossRef]
- Ecco, L.G.; Fedel, M.; Deflorian, F.; Becker, J.; Iversen, B.B.; Mamakhel, A. Waterborne acrylic paint system based on nanoceria for corrosion protection of steel. Prog. Org. Coat. 2016, 96, 19–25. [Google Scholar] [CrossRef]
- Yang, M.; Wu, J.; Fang, D.; Li, B.; Yang, Y. Corrosion protection of waterborne epoxy coatings containing mussel-inspired adhesive polymers based on polyaspartamide derivatives on carbon steel. J. Mater. Sci. Technol. 2018, 34, 2464–2471. [Google Scholar] [CrossRef]
- Yao, M.; Tang, E.; Guo, C.; Liu, S.; Tian, H.; Gao, H. Synthesis of waterborne epoxy/polyacrylate composites via miniemulsion polymerization and corrosion resistance of coatings. Prog. Org. Coat. 2017, 113, 143–150. [Google Scholar] [CrossRef]
- Kirubaharan, A.M.K.; Kuppusami, P. Corrosion behavior of ceramic nanocomposite coatings at nanoscale. In Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2020; Chapter 16; pp. 295–314. [Google Scholar]
- Rűdinger, A.; Nöth, A.; Pritzkov, W. Oxide Ceramic Matrix Composites—Manufacturing, Machining, Properties and Industrial Applications. Ceram. Appl. 2015, 3, 48–54. [Google Scholar]
- Demetrescu, I.; Pirvu, C.; Mitran, V. Effect of nano-topographical features of Ti/TiO2 electrode surface on cell response and electrochemical stability in artificial saliva. Bioelectrochemistry 2010, 79, 122–129. [Google Scholar] [CrossRef]
- Dey, T.; Roy, P.; Fabry, B.; Schmuki, P. Anodic mesoporous TiO2 layer on Ti for enhanced formation of biomimetic hydroxyapatite. Acta Biomater. 2011, 7, 1873–1879. [Google Scholar] [CrossRef]
- Kunze, J.; Müller, L.; Macak, J.M.; Greil, P.; Schmuki, P.; Müller, F.A. Time-dependent growth of biomimetic apatite on anodic TiO2 nanotubes. Electrochim. Acta 2008, 53, 6995–7003. [Google Scholar] [CrossRef]
- An, Q.; Chen, J.; Ming, W.; Chen, M. Machining of SiC ceramic matrix composites: A review. Chin. J. Aeronaut. 2020, 34, 540–567. [Google Scholar] [CrossRef]
- Huang, J.-L.; Nayak, P.K. Effect of Nano-TiN on Mechanical Behavior of Si3N4 Based Nanocomposites by Spark Plasma Sintering (SPS). In Nanocomposites—New Trends and Developments; Ebrahimi, F., Ed.; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Xie, X.; Hosni, B.; Chen, C.; Wu, H.; Li, Y.; Chen, Z.; Verdy, C.; Kedim, O.E.; Zhong, Q.; Addad, A.; et al. Corrosion behavior of cold sprayed 7075Al composite coating reinforced with TiB2 nanoparticles. Surf. Coat. Technol. 2020, 404, 126460. [Google Scholar] [CrossRef]
- Parsapour, A.; Khorasani, S.N.; Fathi, M.H. Corrosion behavior and biocompatibility of hydroxyapatite coating on H2SO4 passivated 316L SS for human body implant. Acta Met. Sin. 2013, 26, 409–415. [Google Scholar] [CrossRef]
- Keyvani, A.; Zamani, M.; Bahamirian, M.; Nikoomanzari, E.; Fattah-Alhosseini, A.; Sina, H. Role of incorporation of ZnO nanoparticles on corrosion behavior of ceramic coatings developed on AZ31 magnesium alloy by plasma electrolytic oxidation technique. Surf. Interfaces 2021, 22, 100728. [Google Scholar] [CrossRef]
- Arunnellaiappan, T.; Arun, S.; Hariprasad, S.; Gowtham, S.; Ravisankar, B.; Krishna, L.R.; Rameshbabu, N. Fabrication of corrosion resistant hydrophobic ceramic nanocomposite coatings on PEO treated AA7075. Ceram. Int. 2018, 44, 874–884. [Google Scholar]
- Raj, V.; Mubarak Ali, M. Formation of ceramic alumina nanocomposite coatings on aluminium for enhanced corrosion resistance. J. Mater. Process. Technol. 2009, 209, 5341–5352. [Google Scholar] [CrossRef]
- Lv, G.; Gu, W.; Chen, H.; Feng, W.; Khosa, M.L.; Li, L.; Niu, E.; Zhang, G.; Yang, S.-Z. Characteristic of ceramic coatings on aluminum by plasma electrolytic oxidation in silicate and phosphate electrolyte. Appl. Surf. Sci. 2006, 253, 2947–2952. [Google Scholar] [CrossRef]
- Çomaklı, O.; Yazic, M.; Yetim, T.; Yetim, A.F.; Çelik, A. Effect of Ti amount on wear and corrosion properties of Ti-doped Al2O3 nanocomposite ceramic coated CP titanium implant material. Ceram. Int. 2018, 44, 7421–7428. [Google Scholar] [CrossRef]
- Emarati, S.M.; Mozammel, M. Efficient one-step fabrication of superhydrophobic nano-TiO2/TMPSi ceramic composite coating with enhanced corrosion resistance on 316L. Ceram. Int. 2020, 46, 1652–1661. [Google Scholar] [CrossRef]
- Gadow, R.; Kern, F.; Killinger, A. Manufacturing technologies for nanocomposite ceramic structural materials and coatings. Mater. Sci. Eng. B 2008, 148, 58–64. [Google Scholar] [CrossRef]
- Gao, X.; Yan, R.; Xu, L.; Ma, H. Effect of amorphous phytic acid nanoparticles on the corrosion mitigation performance and stability of sol-gel coatings on cold-rolled steel substrates. J. Alloys Compd. 2018, 747, 747–754. [Google Scholar] [CrossRef]
- Ying, L.; Li, L. Understanding the corrosion resistance of nanocrystalline materials: Electrochemical influences. In Corrosion Protection and Control Using Nanomaterials; Saji, V.S., Cook, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; Chapter 4; pp. 59–85. [Google Scholar]
- Chen, F.; Liu, P. Conducting Polyaniline Nanoparticles and Their Dispersion for Waterborne Corrosion Protection Coatings. ACS Appl. Mater. Interfaces 2011, 3, 2694–2702. [Google Scholar] [CrossRef]
- Khodair, Z.T.; Khadom, A.A.; Jasim, H.A. Corrosion protection of mild steel in different aqueous media via epoxy/nanomaterial coating: Preparation, characterization and mathematical views. J. Mater. Res. Technol. 2019, 8, 424–435. [Google Scholar] [CrossRef]
- Indira, K.; Mudali, U.K.; Nishimura, T.; Rajendran, N. A Review on TiO2 Nanotubes: Influence of Anodization Parameters, Formation Mechanism, Properties, Corrosion Behavior, and Biomedical Applications. J. Bio- Tribo-Corros. 2015, 1, 28–30. [Google Scholar] [CrossRef]
- Živković, L.j.S.; Jegdić, B.V.; Andrić, V.; Rhee, K.Y.; Bajat, J.B.; Mišković-Stanković, V.B. The effect of ceria and zirconia nanoparticles on the corrosion behaviour of cataphoretic epoxy coatings on AA6060 alloy. Prog. Org. Coat. 2019, 136, 105219. [Google Scholar] [CrossRef]
- Nikoomanzari, E.; Fattah-alhosseini, A.; Pajohi Alamoti, M.R.; Keshavarz, M.K. Effect of ZrO2 nanoparticles addition to PEO coatings on Ti–6Al–4Vsubstrate: Microstructural analysis, corrosion behavior and antibacterial effect of coatings in Hank’s physiological solution. Ceram. Int. 2020, 46, 13114–13124. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Liu, Z.; Peng, W.-X.; Yoysefi, N. Amino acid and TiO2 nanoparticles mixture inserted into sol-gel coatings: An efficient corrosion protection system for AZ91 magnesium alloy. Prog. Org. Coat. 2019, 136, 10529. [Google Scholar] [CrossRef]
- Hoseini, A.; Yarmand, B.; Kolahi, A. Inhibitory effects of hematite nanoparticles on corrosion protection function of TiO2 coating prepared by plasma electrolytic oxidation. Surf. Coat. Technol. 2021, 409, 126938. [Google Scholar] [CrossRef]
- Selegård, L.; Poot, T.; Eriksson, P.; Palisaitis, J.; Persson, P.O.Å.; Hu, Z.; Uvdal, K. In-situ growth of cerium nanoparticles for chrome-free, corrosion resistant anodic coatings. Surf. Coat. Technol. 2021, 410, 126958. [Google Scholar] [CrossRef]
- Kumar, H.K.; Venkatesh, N.; Bhowmik, H.; Kuila, A. Metallic Nanoparticle: A Review. Biomed. J. Sci. Technol. Res. 2018, 4, 3765–3774. [Google Scholar]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities Arabian. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Zhaodi, R.; Meng, N.; Shehzad, K.; Xu, Y.; Qu, S.; Yu, B.; Luo, J.K. Mechanical properties of nickel-graphene composites synthesized by electrochemical deposition. Nanotechnology 2015, 26, 065706. [Google Scholar]
- Liu, C.; Su, F.; Liang, J. Producing cobalt–graphene composite coating by pulse electrodeposition with excellent wear and corrosion resistance. Appl. Surf. Sci. 2015, 351, 889–896. [Google Scholar] [CrossRef]
- Tavandashti, N.P.; Almas, S.M.; Esmaeilzadeh, E. Corrosion protection performance of epoxy coating containing alumina/PANI nanoparticles doped with cerium nitrate inhibitor on Al-2024 substrates. Prog. Org. Coat. 2021, 152, 106133. [Google Scholar] [CrossRef]
- Ngo, N.K.; Shao, S.; Conrad, H.; Sanders, S.F.; D’Souza, F.; Golden, T.D. Synthesis, characterization, and the effects of organo-grafted nanoparticles in nickel coatings for enhanced corrosion protection. Mater. Today Commun. 2020, 25, 101628. [Google Scholar] [CrossRef]
- Shi, X.; Nguyen, T.A.; Suo, Z.; Liu, Y.; Avci, R. Effect of nanoparticles on the anticorrosion and mechanical properties of epoxy coating. Surf. Coat. Technol. 2009, 204, 237–245. [Google Scholar] [CrossRef]
- Kamburova, K.; Boshkova, N.; Boshkov, N.; Radeva, T. Composite coatings with polymeric modified ZnO nanoparticles and nanocontainers with inhibitor for corrosion protection of low carbon steel. Colloids Surf. A Physicochem. Eng. Asp. 2021, 609, 125741. [Google Scholar] [CrossRef]
- Samad, U.A.; Alam, M.A.; Anis, A.; Sherif El-Sayed, M.; Al-Mayman, S.I.; Al-Zahrani, S.M. Effect of Incorporated ZnO Nanoparticles on the Corrosion Performance of SiO2 Nanoparticle-Based Mechanically Robust Epoxy Coatings. Materials 2020, 13, 3767. [Google Scholar] [CrossRef]
- Zamblau, I.; Varvara, S.; Bulea, C.; Muresan, L.M. Corrosion Behavior of Composite Coatings Obtained by ElectrolyticCodeposition of Copper with Al2O3 Nanoparticles. Chem. Biochem. Eng. Q. 2009, 23, 43–52. [Google Scholar]
- Nemes, P.I.; Zaharescu, M.; Muresan, L.M. Initial corrosion behavior of composite coatings obtained by electrolytic codeposition of zinc with nanoparticles of Ti and.Ce oxides. J. Solid State Electrochem. 2013, 17, 511–518. [Google Scholar] [CrossRef]
- Haddadi, S.A.; Mehmandar, E.; Jabari, H.; ARamazani, S.A.; Mohammadkhani, R.; Yan, N.; Arjmand, M. Zinc-doped silica/polyaniline core/shell nanoparticles towards corrosion protection epoxy nanocomposite coatings. Compos. Part B Eng. 2021, 212, 108713. [Google Scholar] [CrossRef]
- Najjar, R.; Katourani, S.A.; Hosseini, M.G. Self-healing and corrosion protection performance of organic polysulfide@urea-formaldehyde resin core-shell nanoparticles in epoxy/PANI/ZnO nanocomposite coatings on anodized aluminum alloy. Prog. Org. Coat. 2018, 124, 110–121. [Google Scholar] [CrossRef]
- Gu, B.-E.; Huang, C.-Y.; Shen, T.-H.; Lee, Y.-L. Effects of multiwall carbon nanotube addition on the corrosion resistance and underwater acoustic absorption properties of polyurethane coatings. Prog. Org. Coat. 2018, 121, 226–235. [Google Scholar] [CrossRef]
- Kumar, M.K.P.; Singh, M.P.; Srivastava, C. Electrochemical behavior of Zn–graphene composite coatings. RSC Adv. 2015, 5, 25603–25608. [Google Scholar] [CrossRef]
- Bai, Z.; Zhang, B. Fabrication of superhydrophobic reduced-graphene oxide/nickel coating with mechanical durability, self-cleaning and anticorrosion performance. Nano Mater. Sci. 2020, 2, 151–158. [Google Scholar] [CrossRef]
- Selim, M.S.; El-Safty, S.A.; Abbas, M.A.; Shenashen, M.A. Facile design of graphene oxide-ZnO nanorod-based ternary nanocomposite as a superhydrophobic and corrosion-barrier coating. Colloids Surf. A Physicochem. Eng. Asp. 2020, 611, 125793. [Google Scholar] [CrossRef]
- Rajitha, K.; Mohana, K.N.S. Synthesis of graphene oxide-based nanofillers and their influence on the anticorrosion performance of epoxy coating in saline medium. Diam. Relat. Mater. 2020, 108, 107974. [Google Scholar] [CrossRef]
- Shi, S.; Zhao, Y.; Zhang, Z.; Yu, L. Corrosion protection of a novel SiO2@PANI coating for Q235 carbon steel. Prog. Org. Coat. 2019, 132, 227–234. [Google Scholar] [CrossRef]
- Wang, T.; Ge, H.; Zhang, K. A novel core-shell silica@graphene straticulate structured antistatic anticorrosion composite coating. J. Alloys Compd. 2018, 745, 705–715. [Google Scholar] [CrossRef]
- Daneshvar, F. Carbon nanotube/metal corrosion issues for nanotube coatings and inclusions in a matrix. Appl. Phys. 2018, 119212468. [Google Scholar] [CrossRef]
- Ganguli, S.; Bhuyan, M.; Allie, L.; Aglan, H. Effect of multi-walled carbon nanotube reinforcement on the fracture behavior of a tetrafunctional epoxy. J. Mater. Sci. 2005, 40, 3593–3595. [Google Scholar] [CrossRef]
- Deyab, M.A.; Awadallah, A.E. Advanced anticorrosive coatings based on epoxy/functionalized multiwall carbon nanotubes composites. Prog. Org. Coat. 2020, 139, 105423. [Google Scholar] [CrossRef]
- Yousefi, A.; Javadian, S.; Sharifi, M.; Dalir, N.; Motaee, A. An Experimental and Theoretical Study of Biodegradable Gemini Surfactants and Surfactant/Carbon Nanotubes (CNTs) Mixtures as New Corrosion Inhibitor. J. Bio-Tribo-Corros. 2019, 5, 82. [Google Scholar] [CrossRef]
- Hong, M.-S.; Park, Y.; Kim, T.; Kim, K.; Kim, J.-G. Polydopamine/carbon nanotube nanocomposite coating for corrosion Resistance. J. Mater. 2020, 6, 158–166. [Google Scholar] [CrossRef]
- Rui, M.; Zhu, A. The synthesis and corrosion protection mechanisms of PANI/CNT nanocomposite doped with organic phosphoric acid. Prog. Org. Coat. 2021, 153, 106134. [Google Scholar] [CrossRef]
- Montemor, M.F.; Ferreira, M.G.S. Analytical characterisation and corrosion behaviour of bis-aminosilane coatings modified with carbon nanotubes activated with rare-earth salts applied on AZ31 Magnesium alloy. Surf. Coat. Technol. 2008, 202, 4766–4774. [Google Scholar] [CrossRef]
- Muresan, L.M. Corrosion Protective Coatings for Ti and Ti Alloys Used for Biomedical Implants. In Intelligent Coatings for Corrosion Control; Tiwari, A., Rawlins, J., Hihara, L.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Chapter 17; pp. 585–603. ISBN 978-0-12-411467-8. [Google Scholar]
- Gunputh, U.F.; Le, H.; Besinis, A.; Tredwin, C.; Handy, R.D. Multilayered composite coatings of titanium dioxide nanotubes decorated with zinc oxide and hydroxyapatite nanoparticles: Controlled release of Zn and antimicrobial properties against Staphylococcus aureus. Int. J. Nanomed. 2019, 14, 3583–3600. [Google Scholar] [CrossRef] [PubMed]
- Da Cunha, A.B.M.; Leal, D.A.; Santos, L.R.L.; Riegel-Vidotti, I.C.; Marino, C.E.B. pH-sensitive microcapsules based on biopolymers for active corrosion protection of carbon steel at different pH. Surf. Coat. Technol. 2020, 402, 126338. [Google Scholar] [CrossRef]
- Amiri, S.; Rahimi, A. Anticorrosion behavior of cyclodextrins/inhibitor nanocapsule-based self-healing coatings. J. Coat. Technol. Res. 2016, 13, 1095–1102. [Google Scholar] [CrossRef]
- Wei, H.; Wang, Y.; Guo, J.; Shen, N.Z.; Jiang, D.; Zhang, X.; Yan, X.; Zhu, J.; Wang, Q.; Shao, L.; et al. Advanced micro/nanocapsules for self-healing smart anticorrosion coatings. J. Mater. Chem. A 2015, 3, 469–480. [Google Scholar] [CrossRef]
- Bagale, U.D.; Sonawane, S.H.; Bhanvase, B.A.; Kulkarni, R.D.; Gogate, P.R. Green synthesis of nanocapsules for self-healing anticorrosion coating using ultrasound-assisted approach. Green Process. Synth. 2018, 7, 147–159. [Google Scholar] [CrossRef]
- Malekkhouyan, R.; Khorasani, S.N.; Neisiany, R.E.; Torkaman, R.; Koochaki, M.S.; Das, O. Preparation and Characterization of Electrosprayed Nanocapsules Containing Coconut-Oil-Based Alkyd Resin for the Fabrication of Self-Healing Epoxy Coatings. Appl. Sci. 2020, 10, 3171. [Google Scholar] [CrossRef]
- Zea, C.; Barranco-García, R.; Chico, B.; Díaz, I.; Morcillo, M.; de la Fuente, D. Smart Mesoporous Silica Nanocapsules as Environmentally Friendly Anticorrosive Pigments. Int. J. Corros. 2015, 2015, 426397. [Google Scholar] [CrossRef]
- Telegdi, J.; Shaban, A.; Vastag, G. Micro/nano-capsules for anticorrosion coatings. In Fundamentals of Nanoparticles: Classifications, Synthesis Methods, Properties and Characterization-Micro and Nano Technologies, 1st ed.; Butterworth-Heinemann: Oxford, UK; Elsevier: Amsterdam, The Netherlands, 2018; Chapter 17. [Google Scholar]
- Jadhav, R.S.; Mane, V.; Bagle, A.V.; Hundiwale, D.G.; Mahulikar, P.P.; Waghoo, G. Synthesis of multicore phenol formaldehyde microcapsules and their application in polyurethane paint formulation for self-healing anticorrosive coating. Int. J. Ind. Chem. 2013, 4, 31–39. [Google Scholar] [CrossRef]
- Li, G.L.; Zheng, Z.; Mohwald, H.; Shchukin, D.G. Silica/Polymer Double-Walled Hybrid Nanotubes: Synthesis and Application as Stimuli-Responsive Nanocontainers in Self-Healing Coatings. ACS Nano 2013, 7, 2470–2478. [Google Scholar] [CrossRef] [PubMed]
- Madhup, M.K.; Shah, N.K.; Parekh, N.R. Investigation and improvement of abrasion resistance, water vapor barrier and anticorrosion properties of mixed clay epoxy nanocomposite coating. Prog. Org. Coat. 2017, 102, 186–193. [Google Scholar] [CrossRef]
- Hikasa, A.; Sekino, T.; Hayashi, Y.; Rajagopalan, R.; Niihara, K. Preparation and Corrosion Studies of Self-Healing Multi-Layered Nano Coatings of Silica And Swelling Clay. Mat. Res. Innovat. 2004, 8, 84–88. [Google Scholar] [CrossRef]
- Calabrese, L.; Bonaccorsi, L.; Caprì, A.; Proverbio, E. Adhesion aspects of hydrophobic silane zeolite coatings for corrosion protection of aluminium substrate. Prog. Org. Coat. 2014, 77, 1341–1350. [Google Scholar] [CrossRef]
- Rotella, G.; Candamano, S. Fabrication and characterization of zeolite coatings on aluminum and magnesium alloys. Eng. Sci. Technol. Int. J. 2020, 23, 1273–1278. [Google Scholar] [CrossRef]
- Calabrese, L.; Bonaccorsi, L.; Caprì, A.; Proverbio, E. Enhancement of the hydrophobic and anti-corrosion properties of a composite zeolite coating on Al6061 substrate by modification of silane matrix. Corros. Eng. Sci. Technol. 2017, 52, 61–72. [Google Scholar] [CrossRef]
- Rassouli, L.; Naderi, R.; Mahdavian, M. Study of the active corrosion protection properties of epoxy ester coating with zeolite nanoparticles doped with organic and inorganic inhibitors. J. Taiwan Inst. Chem. Eng. 2018, 85, 207–220. [Google Scholar] [CrossRef]
- Calabrese, L.; Proverbio, E. A Brief Overview on the Anticorrosion Performances of Sol-Gel Zeolite Coatings. Coatings 2019, 9, 409. [Google Scholar] [CrossRef]
- Fayyad, E.M.; Jlassi, K.; Sliem, M.H.; Nabhan, F.; Abdullah, A.M. Design of highly anti-corrosive electroless plated Ni–P/modified halloysite nanotubes nanocomposite coating. J. Mater. Res. Technol. 2023, 24, 8014–8034. [Google Scholar] [CrossRef]
- Saheban, M.; Bakhsheshi-Rad, H.R.; Kasiri-Asgarani, M.; Hamzah, E.; Ismail, A.F.; Aziz, M.; Dayaghi, E. Effect of zeolite on the corrosion behavior, biocompatibility and antibacterial activity of porous magnesium/zeolite composite scaffolds. Mater. Technol. 2019, 34, 258–269. [Google Scholar] [CrossRef]
- Iqbal, N.; Iqbal, S.; Iqbal, T.; Bakhsheshi-Rad, H.R.; Alsakkaf, A.; Kamil, A.; Raghav, H.B. Zinc-doped hydroxyapatite—Zeolite/polycaprolactone composites coating on magnesium substrate for enhancing in-vitro corrosion and antibacterial performance. Trans. Nonferr. Met. Soc. China 2020, 30, 123–133. [Google Scholar] [CrossRef]
- Rowsell, J.L.C.; Yaghi, O.M. Metal-organic frameworks: A new class of porous materials. Microporous Mesoporous Mater. 2004, 73, 3–14. [Google Scholar] [CrossRef]
- Seidi, F.; Jouyandeh, M.; Taghizadeh, M.; Taghizadeh, A.; Vahabi, H.; Habibzadeh, S.; Formela, K.; Saeb, M.R. Metal-Organic Framework (MOF)/Epoxy Coatings: A Review. Materials 2020, 13, 2881. [Google Scholar] [CrossRef] [PubMed]
- Valizadeh, B.; Nguyen, T.N.; Stylianou, K.C. Shape engineering of metal–organic frameworks. Polyhedron 2018, 145, 1–15. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, L.; Wang, L.; Sun, Y.; Liu, Y. Insights into the Use of Metal−Organic Framework As High- Performance Anticorrosion Coatings. ACS Appl. Mater. Interfaces 2018, 10, 2259–2263. [Google Scholar] [CrossRef]
- Wei, W.; Liu, Z.; Wei, R.; Han, G.-C.; Liang, C. Synthesis of MOFs/GO composite for corrosion resistance application on carbon steel. RSC Adv. 2020, 10, 29923. [Google Scholar] [CrossRef]
- Romanov, I.; Chernyshov, E.; Romanov, A. Assessment of the possibility for substituting cast iron in vehicle brake disc with Aluminum-Based Metal-matrix composite material produced by internal oxidation. Mater. Today Proc. 2019, 19, 2125–2128. [Google Scholar] [CrossRef]
- Deepa, M.J.; Arunima, S.R.; Ramesh, H.; Sumi, V.S.; Sha, M.A.; Riyas, A.H.; Shibli, S.M.A. Tuning of electrochemical and topographical characteristics for enhancement of anti-corrosion performance of hot-dip TiO2–Zn–(1%)Al composite coatings. Corros. Sci. 2020, 177, 108944. [Google Scholar] [CrossRef]
- Doğan, F.; Uysal, M.; Algül, H.; Duru, E.; Akbulut, H.; Aslan, S. Optimization of pulsed electro co-deposition for Ni-B-TiN composites and the variation of tribological and corrosion behaviors. Surf. Coat. Technol. 2020, 400, 126209. [Google Scholar] [CrossRef]
- Ranganatha, S.; Venkatesha, T.V.; Vathsala, K.; Kumar, M.K.P. Electrochemical studies on Zn/nano-CeO2 electrodeposited composite coatings. Surf. Coat. Technol. 2012, 208, 64–72. [Google Scholar] [CrossRef]
- Shibli, S.M.A.; Chacko, F. Development of nano CeO2-incorporated high performance hot-dip zinc coating. Surf. Coat. Technol. 2008, 202, 4971–4975. [Google Scholar] [CrossRef]
- Praveen, B.M.; Venkatesha, T.V. Electrodeposition and properties of Zn-nanosized TiO2 composite coatings. Appl. Surf. Sci. 2008, 254, 2418–2424. [Google Scholar] [CrossRef]
- Vlasa, A.; Varvara, S.; Pop, A.; Bulea, C.; Muresan, L.M. Muresan, Electrodeposited Zn–TiO2 nanocomposite coatings and their corrosion behavior. J. Appl. Electrochem. 2010, 40, 1519–1527. [Google Scholar] [CrossRef]
- Al-Dhire, T.M.; Zuhailawati, H.; Anasyida, A.S. Effect of current density on corrosion and mechanical properties of Zn-SiC composite coating. Mater. Today Proc. 2019, 17, 664–671. [Google Scholar] [CrossRef]
- Winnicki, M.; Kozerski, S.; Małachowska, A.; Pawłowski, L.; Rutkowska-Gorczyca, M. Optimization of ceramic content in nickel–alumina composite coatings obtained by low pressure cold spraying. Surf. Coat. Technol. 2020, 405, 126732. [Google Scholar] [CrossRef]
- Kumar, C.M.P.; Venkatesha, T.V.; Shabadi, R. Preparation and corrosion behavior of Ni and Ni–graphene composite coatings. Mater. Res. Bull. 2013, 48, 1477–1483. [Google Scholar] [CrossRef]
- Munday, G.; Hogan, J.; McDonald, A. On the Microstructure -dependency of Mechanical Properties and Failure of Low-Pressure Cold-Sprayed Tungsten Carbide-Nickel Metal Matrix Composite Coatings. Surf. Coat. Technol. 2020, 396, 125947. [Google Scholar] [CrossRef]
- Bartkowski, D.; Bartkowska, A.; Jurči, P. Laser cladding process of Fe/WC metal matrix composite coatings on low carbon steel using Yb: YAG disk laser. Opt. Laser Technol. 2021, 136, 106784. [Google Scholar] [CrossRef]
- Xie, X.; Tan, Z.; Chen, C.; Xie, Y.; Wu, H.; Yan, X.; Gao, S.; Li, Z.; Ji, G.; Liao, H. Synthesis of carbon nanotube reinforced Al matrix composite coatings via cold spray deposition. Surf. Coat. Technol. 2020, 405, 126676. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, F.; Han, E.-H.; Xu, L.; Uzoma, P.C. Effects of Al2O3 on the microstructures and corrosion behavior of low pressure cold gas sprayed Al 2024-Al2O3 composite coatings on AA 2024-T3 substrate. Surf. Coat. Technol. 2019, 370, 53–68. [Google Scholar] [CrossRef]
- Memar, S.; Azadi, M.; Abdoos, H. Microstructure and corrosion evaluations for Al2O3 –leaded brass matrix nanocomposites in marine solution. Mater. Chem. Phys. 2023, 295, 127124. [Google Scholar] [CrossRef]
- Omanović-Mikličanin, E.; Badnjević, A.; Kazlagić, A.; Hajlovac, M. Nanocomposites: A brief review. Health Technol. 2020, 10, 51–59. [Google Scholar] [CrossRef]
- Wei, H.; Ding, D.; Wei, S.; Guo, Z. Anticorrosive conductive polyurethane multiwalled carbon nanotube nanocomposites. J. Mater. Chem. A 2013, 1, 10805–10813. [Google Scholar] [CrossRef]
- Blaiszik, B.J.; Kramer, S.L.B.; Olugebefola, S.C.; Moore, J.S.; Sottos, N.R.; White, S.R. Self-Healing Polymers and Composites. Annu. Rev. Mater. Res. 2010, 40, 179–211. [Google Scholar] [CrossRef]
- Muresan, L.M. Self-healing coatings for corrosion protection of steel. In Majid Hosseini; Industrial Application of Intelligent Polymers and Coatings; Makhlouf, A.S.H., Ed.; Springer: Berlin/Heidelberg, Germany, 2016; Chapter 22; Volume 1, pp. 479–492. ISBN 978-3-319-26891-0. [Google Scholar]
- Khajouei, A.; Jamalizadeh, E.; Hosseini, S.M.A. Corrosion protection of coatings doped with inhibitor-loaded nanocapsules, Anti-Corros. Method. Mater. 2015, 62, 88–94. [Google Scholar]
- Vijayan, P.; Al-Maadeed, M. Self-Repairing Composites for Corrosion Protection: A Review on Recent Strategies and Evaluation Methods. Materials 2019, 12, 2754. [Google Scholar]
- Deyab, M.A. Anticorrosion properties of nanocomposites coatings: A critical review. J. Mol. Liq. 2020, 313, 113533. [Google Scholar] [CrossRef]
- Show, Y.; Nakashima, T.; Fukami, Y. Anticorrosion Coating of Carbon Nanotube/Polytetrafluoroethylene Composite Film on the Stainless Steel Bipolar Plate for Proton Exchange Membrane Fuel Cells. J. Nanomater. 2013, 2013, 378752. [Google Scholar] [CrossRef]
- Ma, I.A.W.; Shafammri, A.; Kasi, R.; Zaini, F.N.; Balakrishnan, V.; Subramaniam, R.; Arof, A.K. Anticorrosion properties of Epoxy/nanocellulose nanocomposite coating. Bioresources 2017, 12, 2912–2929. [Google Scholar]
- Pang, X.; Zhitomirsky, I. Electrophoretic Deposition of Composite Hydroxyapatite-Chitosan Coatings. Mater. Charact. 2007, 58, 339–348. [Google Scholar] [CrossRef]
- Bahari, H.S.; Ye, F.; Carrillo, E.A.T.; Leliopoulos, C.; Savaloni, H.; Dutta, J. Chitosan nanocomposite coatings with enhanced corrosion inhibition effects for copper. Int. J. Biol. Macromol. 2020, 162, 1566–1577. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, W.M.; Al-Mhyawi, S.R.; Albukhari, S.M. Chitosan-Clay Nanocomposite as a Green Corrosion Inhibitor for Mild Steel in Hydrochloric Acid Solution. Int. J. Electrochem. Sci. 2022, 17, 220848. [Google Scholar] [CrossRef]
- Sumi, V.S.; Arunima, S.R.; Deepa, M.J.; Sha, M.A.; Riyas, A.H.; Meera, M.S.; Saji, V.S.; Shibli, S.M.A. PANI-Fe2O3 composite for enhancement of active life of alkyd resin coating for corrosion protection of steel. Mater. Chem. Phys. 2020, 2471, 122881. [Google Scholar] [CrossRef]
- TabkhPaz, M.; Park, D.-Y.; Lee, P.C.; Hugo, R.; Park, S.S. Development of nanocomposite coatings with improved mechanical, thermal, and corrosion protection properties. J. Compos. Mater. 2017, 52, 1045–1060. [Google Scholar] [CrossRef]
- Jlassi, K.; Radwan, A.B.; Sadasivuni, K.K.; Mrlik, M.; Abdullah, A.M.; Chehimi, M.M.; Krupa, I. Anti-corrosive and oil sensitive coatings based on epoxy/polyaniline/magnetite-clay composites through diazonium interfacial chemistry. Sci. Rep. 2018, 8, 13369. [Google Scholar] [CrossRef]
- Behera, A.; Swain, B.; Sahoo, D.K. Fiber-reinforced ceramic matrix nanocomposites. In Fiber-Reinforced Nanocomposites: Fundamentals and Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 359–368. [Google Scholar]
- Lee, J.-H.; Kim, H.-E.; Koh, Y.-H. Highly porous titanium (Ti) scaffolds with bioactive microporous hydroxyapatite/TiO2 hybrid coating layer. Mater. Lett. 2009, 63, 1995–1998. [Google Scholar] [CrossRef]
- Lamon, J. Properties and Characteristics of SiC and SiC/SiC Composites. Compr. Nucl. Mater. 2012, 2, 323–338. [Google Scholar]
- Kalisz, M.; Grobelny, M.; Świniarski, M.; Firek, P. Comparison of the structural and corrosion properties of the graphene/SiN(200) coating system deposited on titanium alloy surfaces covered with SiN transition layers. Surf. Coat. Technol. 2016, 299, 65–70. [Google Scholar] [CrossRef]
- Balázsi, K.; Furkó, M.; Balázsi, C. Ceramic Matrix Graphene and Carbon Nanotube Composites. In Encyclopedia of Materials: Technical Ceramics and Glasses; Elsevier: Amsterdam, The Netherlands, 2021; ISBN 9780128035818. [Google Scholar] [CrossRef]
- Ye, K.; Li, F.; Zhang, J.; Chen, J.; Li, Z.; Cui, G.; Liu, J.; Xing, X. Effect of SiO2 on microstructure and mechanical properties of composite ceramic coatings prepared by centrifugal-SHS process. Ceram. Int. 2021, 47, 12833–12842. [Google Scholar] [CrossRef]
- Shokouhfar, M.; Allahkaram, S.R. Effect of incorporation of nanoparticles with different composition on wear and corrosion behavior of ceramic coatings developed on pure titanium by micro arc oxidation. Surf. Coat. Technol. 2017, 309, 767–778. [Google Scholar] [CrossRef]
- Sobieszczyk, S. Hydroxiapatite coatings on porous Ti and Ti alloys. Adv. Mater. Sci. 2010, 10, 19–28. [Google Scholar]
- Dezfuli, S.M.; Sabzi, M. Deposition of ceramic nanocomposite coatings by electroplating process: A review of layer-deposition mechanisms and effective parameters on the formation of the coating. Ceram. Int. 2019, 45, 21835–21842. [Google Scholar] [CrossRef]
- Yan, D.-R.; Dai, X.-R.; Yang, Y.; Dong, Y.-C.; Chen, X.-G.; Chu, Z.-H.; Zhang, J.-X. Microstructure and properties of in-situ ceramic matrix eutectic nanocomposite coating prepared by plasma spraying Al-Cr2O3-Al2O3 powder. J. Alloys Compd. 2018, 748, 230–235. [Google Scholar] [CrossRef]
- Sirajunisha, H.; Balakrishnan, T.; Sakthivel, P.; Krishnaveni, A. Enhanced corrosion resistance, antibacterial and biological properties of sol-gel derived Ti-rGO-HAp nanocomposites. Chem. Phys. Impact 2023, 6, 100159. [Google Scholar] [CrossRef]
- Ramachandran, K.; Gnanasagaran, C.L.; Pazhani, A.; Kumar, V.H.; Xavior, M.A.; Subramani, R.R.; Arunkumar, T. Impact behaviour of MWCNTs reinforced YSZ and Al2O3 ceramic-nanocomposites prepared via vacuum hot-pressing technique. J. Mater. Res. Technol. 2023, 24, 6595–6603. [Google Scholar] [CrossRef]
- Dash, T.; Rani, S.T.; Dash, B.; Biswal, S.K. Development of an advanced flexible ceramic material from graphene-incorporated alumina nanocomposite. In Advanced Ceramic Materials, Advanced Flexible Ceramics; Gupta, R., Behera, A., Farhad, S., Anh Nguyen, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 541–550. [Google Scholar]
- Mekeridis, E.; Kartsonakis, I.; Kordas, G. Electro-deposited Sol-gel Coatings Containing Ceramic Nanocontainers Loaded with Inhibitors for the Corrosion Protection of AA2024-T3. Adv. Ceram. Sci. Eng. 2012, 1, 1–10. [Google Scholar]
- Amiri, S.; Rahimi, A. Anticorrosion hybrid nanocomposite coatings with encapsulated organic corrosion inhibitors. J. Coat. Technol. Res. 2015, 12, 587–593. [Google Scholar]
| Nanoparticles | Particles Concentration in the Composite Preparation Step | Advantages | Limitations/Drawbacks | References |
|---|---|---|---|---|
| Carbon nanotubes | 0.4–1% CNT |
|
| [73,85] |
| Graphene | 100 mg L−1 |
|
| [4,86] |
| Graphene oxide | 0.1 gL−1 GO 0.5–5 wt.% GO |
|
| [87,88] |
| Metals | In situ growth |
|
| [71] |
| Oxides | 5 mM CeO2 1% TiO2 1, 3 and 5 g/L ZrO2 0.1 g/L ZnO 1, 2, 3 wt.% ZnO 3 gL−1 hematite |
|
| [67,68,69,70,79,80] |
| Organo-grafted nanoparticles | 10 gL−1 Fc-CeO2 GO-Aminothiazole (AT) and GO-2-amino-4-(1-Naphthyl)Thiazole (ANT) |
|
| [77,89] |
| Core-shell particles | 3 wt.% PANI-MSNs 20 mg SiO2@PANI/1g silicone 5% (f-SiO2@RGO) |
|
| [83,90,91] |
| No. | Matrix | Reinforcement | Substrate | Obtaining Method | Reference |
|---|---|---|---|---|---|
| 1 | Zn | CeO2 | Mild steel | Electrodeposition | [128] |
| 2 | Zn | CeO2 | Mild steel | Hot-dip galvanization | [129] |
| 3 | Zn | TiO2 | Steel | Electrodeposition | [130,131] |
| 4 | Zn | SiC | Steel | Electrodeposition | [132] |
| 5 | Zn | Graphene | Steel | Electrodeposition | [86] |
| 6 | Ni | Al2O3 | Steel S235JR | Low-pressure cold spraying | [133] |
| 7 | Ni | Graphene | Steel | Electrodeposition | [134] |
| 8 | Ni-P | SiO2 | Aluminum | Electrodeposition | [6] |
| 9 | Ni | WC | Low-carbon steel | Low-pressure cold spraying | [135] |
| 10 | Ni | SiO2 | Aluminum alloy | Electrodeposition | [8] |
| 11 | Fe | WC | Low-carbon steel | Laser cladding | [136] |
| 12 | Al | SiC | ZE41 magnesium alloys | Laser cladding | [9] |
| 13 | Al | CNTs | Stainless steel | Cold-spray deposition | [137] |
| 14 | Al | Al2O3 | AA 2024-T3 alloy | Low-pressure cold gas spray | [138] |
| 15 | Al | Al2O3 | Al-Si Al-Si-Cu | Internal oxidation | [125] |
| 16 | Cu | Al2O3 | Steel | Electrodeposition | [81] |
| 17 | Leaded brass | Al2O3 | - | Stir-casting technique | [139] |
| No. | Matrix | Reinforcement Material | Substrate | Obtaining Methods | Reference |
|---|---|---|---|---|---|
| 1 | Polytetrafluoroethylene | Carbon Nanotube | Stainless Steel | Doctor blade method | [147] |
| 2 | Polystyrene | Graphene | Steel | In situ miniemulsion polymerization + automatic coating | [32] |
| 3 | Epoxy | Functionalized multiwall carbon nanotubes | Carbon steel | Film applicator | [94] |
| 4 | Epoxy | Nanocellulose | Mild steel | Brushing | [148] |
| 5 | Epoxy | Graphene nanoplateles and montmorillonite | Mild steel | Doctor blade film applicator | [31] |
| 6 | Polyurethane | Multiwalled carbon nanotube (MWCNTs) | Stainless Steel | Airless spraying + curing at 70 °C | [141] |
| 7 | Chitosan | Zn | Mild Steel | Electrodeposition | [22] |
| 8 | Chitosan | Hydroxyapatite | Stainless steel | Electrophoretic Deposition | [149] |
| 9 | Chitosan | Silica + 2-mercapto-benzothiazole | Copper | Self-assembly | [150] |
| 10 | Chitosan | Montmorillonite | Mild steel | Direct melt intercalation | [151] |
| 11 | Alkyd resin | PANI -Fe2O3 | Mild steel | Dip coating | [152] |
| 12 | Styrene acrylic | MWCNTs, Zn particles, graphene nanoplatelets (first layer) hexagonal boron nitrides (second layer) | Steel | Spraying | [153] |
| 13 | PANI | Fe3O4 + clay | Carbon steel | Doctor blade application | [154] |
| No. | Matrix | Reinforcement Material | Obtaining Method | Advantages | Reference |
|---|---|---|---|---|---|
| 1 | Treated high-strength aluminium alloy AA7075 | α-Al2O3 and m-ZrO2 nanoparticles | Plasma electrolytic oxidation | Enhanced corrosion resistance | [56] |
| 2 | Zirconia toughed alumina (ZTA) | Nanosized oxide powders | High-velocity suspension flame spraying | Ease in safe nanoparticle handling | [61] |
| 3 | Nanotubular TiO2 layer on Ti | Hydroxiapatite (HA) | Electrodeposition | Improved stability of implant coatings | [162] |
| 4 | Al2O3/Al | SiO2 | Plasma electrolytic oxidation of Al in dilute alkaline electrolytes with sodium silicate | Decrease of the cathodic process rate for Al corrosion reaction | [57] |
| 5 | Al2O3/Ti | Ti | Sol–gel dip coating | Improved wear resistance | [59] |
| 6 | Ceramic/metal | Ceramic particles | Electroplating | High-density and porosity-free coatings | [163] |
| 7 | [Cr+(Cr,Al)2O3] eutectic | Al-Cr2O3-Al2O3 powders | Reactive plasma spraying | Increased hardness and toughness | [164] |
| 8 | Mg, Mg3(PO4)2/AZ31 magnesium alloy | ZnO nanoparticles | Plasma electrolytic oxidation | Improved corrosion resistance | [55] |
| 9 | Trimethoxy(propyl)silane/steel | TiO2 nanoparticles | One-step electrophoretic deposition | Superhydrophobicity, decreased corrosion rate | [60] |
| 10 | Sintered oxides/Al | Al2O3, SiO2, Al2(SiO3)3 | Plasma electrolytic oxidation | Better corrosion resistance | [57] |
| 11 | Porous Ti scaffold | HA/TiO2 | Sponge replication process and micro-arc oxidation | Improved osteoblastic activity of the porous Ti | [156] |
| 12 | Si3N4 | TiN | Spark plasma sintering | High strength and high toughness; good erosion and corrosion resistance | [52] |
| 13 | HA/stainless steel | rGO-HA Ti-rGO-HA | Sol–gel technique | Bioactivity | [165] |
| 14 | Yttria-stabilized zirconia (YSZ) and aluminium oxide (Al2O3) | MWCNT | Vacuum hot-pressing technique | High stability, increased porosity | [166] |
| 15 | Al2O3 | Graphene platelets | Metallurgical route | Resistance at elevated temperatures under critical load | [167] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muresan, L.M. Nanocomposite Coatings for Anti-Corrosion Properties of Metallic Substrates. Materials 2023, 16, 5092. https://doi.org/10.3390/ma16145092
Muresan LM. Nanocomposite Coatings for Anti-Corrosion Properties of Metallic Substrates. Materials. 2023; 16(14):5092. https://doi.org/10.3390/ma16145092
Chicago/Turabian StyleMuresan, Liana Maria. 2023. "Nanocomposite Coatings for Anti-Corrosion Properties of Metallic Substrates" Materials 16, no. 14: 5092. https://doi.org/10.3390/ma16145092
APA StyleMuresan, L. M. (2023). Nanocomposite Coatings for Anti-Corrosion Properties of Metallic Substrates. Materials, 16(14), 5092. https://doi.org/10.3390/ma16145092





