Abstract
The Salento peninsula in southern Italy (Mediterranean Sea) is a strip of land between the Adriatic and the Ionian Seas, both characterized by local regimes of currents, different geological and physical backgrounds, and quite diversified fauna. In this area, specimens of the sea cucumber Holothuria tubulosa (Brünnich, 1768) (Echinodermata) were collected at four stations in the spring and autumn of 2020 to investigate a possible symbiotic association with the inquiline fish Carapus acus (Brünnich, 1768). Among the collected holothurians, five pearlfish specimens were found in the body cavity of four H. tubulosa collected at 10 m of depth, in autumn, at “Grotta Verde” in Marina di Andrano, Lecce (Ionian Sea). More than half of the sea cucumbers from the latter station hosted the symbiont, suggesting the presence of a shallow population of C. acus inhabiting this coastal area. Furthermore, morphometric analysis carried out on the collected fish helped to shed light on the population dynamics characterizing this neglected species. This is the first report of C. acus from Apulian waters, allowing us to unite previously disjoined areas and providing essential baseline knowledge for planning future in-depth analysis of this difficult-to-study fish in a geographical area that is strategic in terms of the conservation of Mediterranean biodiversity. Furthermore, the range of preferred host species is extended, as C. acus was previously known to prefer other sea cucumber species such as Parastichopus regalis (Cuvier, 1817) instead of H. tubulosa. Finally, the finding of C. acus in a single station and in only one season is not trivial and delivers baseline useful information for future conservation purposes.
1. Introduction
The Mediterranean Sea is the largest and deepest enclosed sea on Earth. Located between Africa, Europe, and Asia, it is a hotspot of terrestrial and marine biodiversity [1,2,3]. Currently, it is known to host more than 17,000 described marine species [3], and although it occupies only 0.82% of the ocean’s surface, it is home to about 10% of the world’s marine species [1]. Even though the Mediterranean basin is one of the most studied seas in the world, there are still important gaps in knowledge on taxonomy, distribution, abundance, and temporal trends of occurrence for many taxa [3,4]. Currently, increasing efforts are being made to investigate the distribution ranges of Mediterranean species, but to date, fine-scale knowledge of their biodiversity and geographical distribution is still lacking [5].
Holothuroidea, commonly known as sea cucumbers, is a class of Echinoderms widespread worldwide in both shallow and deep areas of the marine environment [6,7,8]. Holothurians are a key benthic group in marine ecosystems [9]. Being active sediment rearrangers [10,11], they alter bottom stability [12] by playing a key role in the recycling of organic matter [13,14]; act as a repository of biological materials [9]; and regulate water quality by influencing its carbonate content and pH [14]. They have recently also proved useful as indicators of microplastic pollution in marine sediments, allowing us to shed light on interactions between this widespread pollutant and marine detritivores [15,16]. Holothurians are an important component of the trophic chain; they are food for a wide range of predators [9,17,18] in both shallow and deep water [9,10,19]; in fact, they are preyed upon by starfish, sea otters (Enhydra lutris L. 1758), crustaceans (e.g., crabs), gastropods, fish, birds, turtles, and marine mammals. Due to their ecological role in the marine environment, holothurians fall under specific protection protocols that prohibit their collection (Italian Ministry of Agriculture and Forestry, Law No. 156; 27 February 2018). Holothurians contribute to ecosystem richness by hosting more than 200 species of commensal symbionts and parasites (both externally and within their celom or respiratory tree) [9,20]. Symbiosis is an intimate interaction between different organisms of different species [21]. It is common in marine environments among different species [22], and it takes place in various forms like commensalism, mutualism, and parasitism [23]. Indeed, numerous species live in association with larger invertebrates, such as echinoderms [24,25], with a variety of fish living in association with different invertebrates, including holothurians [26].
Carapus acus (Brünnich, 1768), commonly known as “pearlfish” [27], is a fish with an elongated, slender, scaleless, and eel-shaped body. It has long dorsal and anal fins that join at the end of the tail. Alive, the body is translucent and reddish with about 14–15 silver iridescent dots located on the flanks. The snout is round with a wide opening of the mouth [28] and—according to Parmentier and collaborators [29,30]—has conical teeth and several small internal ones. Reproduction occurs from July to September [31]. This species has an eastern Atlantic–Mediterranean distribution. In the Mediterranean Sea, the occurrence of C. acus is widespread, as it has been reported from Morocco, Tunisia, Spain, the Balearic Islands, France, Italy, Croatia, Malta, and Greece [16,26,32,33,34,35,36,37], but data about its distribution, biology, and ecology are very scarce [33]. In Italy, this species has been reported from Sicily, Calabria, Naples, and Genoa [16,32,38,39]. In the Mediterranean Sea, C. acus is among the species that infest sea cucumbers and has been reported in association with several species of Holothuriida (Holothuria helleri Marenzeller von, 1877; H. poli Delle Chiaje, 1824; H. sanctori Delle Chiaje, 1823; H. stellati and H. tubulosa (Brünnich, 1768)) [32,40,41,42,43]; however, it seems to prefer species of the order Synallactida (Parastichopus regalis (Cuvier, 1817)) [43,44,45,46]. The association between these two organisms is a commonly seen relationship between an invertebrate host and a vertebrate [47,48]. Carapus acus enters a respiratory tree, and afterward, it may quickly enter the celom of the holothurian, where it finds protection from predators, a source of food, and a place to spawn [47]. At night, the species leaves the host to feed on small benthic invertebrates and small fish [31,49,50]. As happens in certain clownfish, the protection system of carapids can be twofold: a non-dependent system, which is represented by the absence of discharge from the Cuvierian tubules when the fish enters the host, and the possible resistance of the gills to toxins. The latter still remains to be shown [51]. Although it is a very well-known species, reported almost everywhere in the Mediterranean Sea, knowledge of its distribution is very local and fragmented, as are its biology and ecology. In fact, apart from some specific work focused on a restricted geographical area and sporadic reports, to date, there remain many knowledge gaps regarding this very elusive species.
Taking all these issues into consideration, the main aim of the present work is to investigate the possible presence of neglected populations of C. acus in southern Italy along the Salento peninsula, right in the middle of the known distribution range of this species, as there are no reports so far, and to possibly fill some gaps in the knowledge of its biology and ecology.
2. Materials and Methods
2.1. Sampling Collection
In the framework of a project of the University of Salento aimed at investigating the possible presence of Carapus acus in the Salento peninsula, specimens of the sea cucumber Holothuria tubulosa (Echinodermata) were collected from the studied area, including both Ionian and Adriatic sites [15]. Two sampling campaigns were carried out in the spring and autumn of 2020, sampling at four different sites located in the Ionian Sea (Stations 1, 2, and 3) and in the Adriatic Sea (Station 4) (Figure 1).
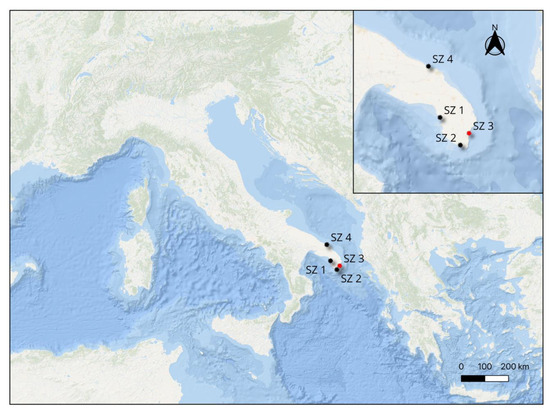
Figure 1.
Sampling stations along the Salento peninsula. SZ 1—“Chiapparo”, Santa Caterina, Lecce (40°08′28.2″ N 17°58′46.1″ E); SZ 2—“Torre Vado”, Morciano di Leuca, Lecce (39°49′46.3″ N 18°16′38.6″ E); SZ 3—“Grotta Verde”, Marina di Andrano, Lecce (39°57′48.1″ N 18°24′18.2″ E); SZ 4—“Torre Guaceto” M.P.A., Brindisi (40°42′48.2″ N 17°48′30.5″ E).
At least four adult specimens of H. tubulosa were collected per station and per each season through scuba diving at 10 m of depth, a bathymetric range poorly explored for this species. Considering the possible influence of the bathymetry in conditioning the presence/absence of the pearlfish [33], the sampling depth was kept constant to control this variable. Each collected individual was wrapped in aluminum foil directly underwater and placed in an aluminum thermos bottle with a wide opening to prevent the possible escape of the inquiline from the body of its host during transport. Once collected, the samples were cataloged with a tag indicating the station and the date and finally stored at −20 °C at the Department of Biological and Environmental Sciences and Technologies of the University of Salento (DiSTeBA) for anatomical dissection and further laboratory analysis. The collected samples were photographed, thawed at 37 °C, and then measured for both dimensions and weight. The dissection of the individuals was carried out by making a longitudinal incision on the dorsal side to keep the digestive system intact. Whenever dissection revealed the presence of one or more pearlfish specimens, they were individually photographed, measured, weighed, cataloged with a unique ID code, and preserved in EtOH 96%.
2.2. Morphometric Analyses
After the dissection of the sea cucumber and the isolation of the pearlfish specimens, the latter were weighed with a Napco Ja-210 precision electronic balance and measured with a ruler. Eight morphometric measurements were evaluated: total length (TL), body depth (BD), head length (HL), eye diameter (ED), pre-orbital length (PoL), pre-pectoral length (PpL), pre-dorsal length (PdL), and pre-anal length (PaL) [35] (Figure 2).

Figure 2.
Morphometric measurements acquired: total length (TL), body depth (BD), head length (HL), eye diameter (ED), pre-orbital length (PoL), pre-pectoral length (PpL), pre-dorsal length (PdL), and pre-anal length (PaL).
To assess the developmental stage of the recorded specimens, the relative ratios between body, head, and pectoral lengths [32] were calculated and considered together with all the measurements reported above. After morphometric analysis, all specimens were preserved in ethanol and stored at the Laboratory of Zoology and Marine Biology of the Department of Biological and Environmental Sciences and Technologies, of the University of Salento (Lecce, Italy).
3. Results
During the two sampling campaigns that occurred in the spring and autumn of 2020, 24 scuba dives were carried out at four sampling stations along the Salento peninsula. A total of 47 H. tubulosa specimens were collected at a 10 m of depth, but only within the four holothurian specimens collected in autumn from “Grotta Verde”, Marina di Andrano (LE) (SZ3 in Figure 1) did we find C. acus individuals (Table 1) (Figure 3). Additionally, three samples contained only one pearlfish, while one sea cucumber contained two individuals.

Table 1.
Localities, geographical coordinates, and seasons for the holothurian specimens collected during this study. Numbers above the seasons indicate the number of sea cucumbers collected. The asterisk (*) indicates the presence of the inquiline C. acus found in four out of the seven holothurians collected in autumn from Station 3 (SZ3).
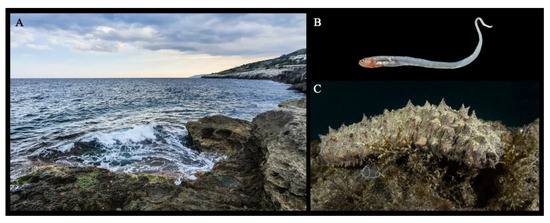
Figure 3.
(A) “Grotta Verde” in Marina di Andrano (LE) (SZ3); (B) Carapus acus individual from SZ3; (C) Holothuria tubulosa from the studied area.
3.1. Morphometric Analyses
The results of the fish morphometry (Table 2) showed a total length ranging from 5.9 to 17.9 cm, with an average of 9.73 ± 4.84 cm, while wet weight ranged from 0.08 to 0.34 g, with an average of 0.22 ± 0.10 g. Table 2 also shows the measurements and weights of the holothurians containing the pearlfish specimens.

Table 2.
Morphometric analyses of the four collected H. tubulosa specimens and their five respective C. acus symbionts. Legend: wet weight (Ww), total length (TL), body depth (BD), head length (HL), eye diameter (ED), pre-orbital length (PoL), pre-pectoral length (PpL), pre-dorsal length (PdL), and pre-anal length (PaL). The asterisk (*) highlights the holothurian specimen (ID: A13) that simultaneously hosted two pearlfish individuals (A13.1 and A13.2).
Based on the body, head, and pectoral lengths and the ratios between them, as reported in [33], the individuals in this study were identified as a tenuis larva (A10.1) and juveniles (A11.1, A12.1, A13.1, and A13.2) (Figure 4).
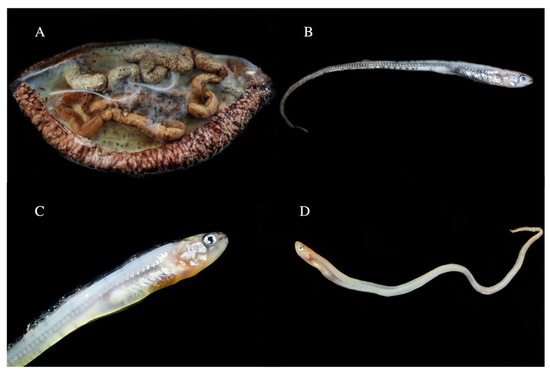
Figure 4.
(A) The tenuis larvae of Carapus acus found in the holothurian with code A10; (B–D) Juvenile specimens at different stages.
3.2. Comparison between Apulian C. acus and Populations from Other Localities
To investigate possible differences between Apulian and other populations, our results were compared with those observed by Enajjar and Bradai (2016) [35] on the Tunisian coast; El Aamri and Tamsouri (2018) [34] on the Moroccan coast; and Mezali and Khodja (2021) [28] on the western Algerian coast, and the results are reported in Table 3.

Table 3.
Ranges of the morphometric measurements of the five individuals of Carapus acus found in Salento (present study) and their comparison with those already published [28,34,35] and obtained from Tunisian, Moroccan, and Algerian specimens. Legend: wet weight (Ww), total length (TL), body depth (BD), head length (HL), eye diameter (ED), pre-orbital length (PoL), pre-pectoral length (PpL), pre-dorsal length (PdL), and pre-anal length (PaL).
4. Discussion
The present study reports, the first discover of the pearlfish Carapus acus at the Salento peninsula in southern Italy. Five individuals were found and collected from the body cavity of the Holothuroidea Holothuria tubulosa during an autumn sampling campaign at the “Grotta Verde” station in Marina di Andrano (SZ3) (Figure 3). Future and more specific samplings, considering both biotic and abiotic factors, will help to unveil specific environmental features positively influencing the presence of the pearlfish in this specific locality instead of in the other areas. The present finding, which is the first for this pearlfish in the Apulia region, allowed us to better investigate this neglected species shedding some light on the biology and ecology of this very cryptic fish. Interestingly, even if this Italian coast is considered a hot spot of biodiversity and constantly monitored—being a strategic area for early warnings of non-indigenous species—no records of C. acus have occurred until now. This case highlights the need to study Mediterranean biodiversity more in depth to better understand these species’ biology and their role in ecosystems, especially important for those taxa able to establish strong interspecific ecological relationships.
C. acus is a broadly well-known species; however, fragmented and, in some cases, discordant data have been reported so far. The pearlfish’s preference for one or another holothurian species is an example of this, as Trott [48] reported that, when pearlfish individuals have the choice to pick their host, they select only H. tubulosa, while, more recently, other authors [26] have indicated Parastichopus regalis as the preferred Holothuroidea. Our observations support the association with the former holothuroid, but additional comparisons should be performed to confirm this ecological aspect. In this context, it could be very interesting to test if bathymetry can alter this preference, shifting it from H. tubulosa to other holothurian species as depth increases. Another very interesting and confusing aspect concerns the exact location of the pearlfish in the holothurian body. In fact, some authors indicate the anus and the digestive system as the locus in which the C. acus lives, but others refer to the “celom” and the “celomic cavity” or the “respiratory tree” as the holothurian body cavity chosen by the fish. This aspect is not trivial and highlights the scanty information that is currently available on this interesting fish. Regarding this last point, it should be considered that C. acus shows different behaviors during its life cycle, as it seems to act as a parasite when the larva enters the holothurian, and it never moves from it as it becomes more commensal, or it simply becomes an inquiline in the juvenile and adult stages, when it seems to regularly come out from the sea cucumber at night to feed and then returns for protection during the day [46,48,52]. This complicated life cycle could have generated some confusion on the way the pearlfish can reach the celomic cavity from the cloaca during its movements to and from the outside, especially considering that the celoma is defined as an internal cavity that is not in communication with the outside. On the other hand, it seems impossible that the fish could live inside the digestive system, which, in holothurians, is full of compact hard detritus. Our observations confirm the presence of larvae and juveniles in the holothurian’s body cavity where the respiratory trees are located. Three of our sampled holothurians contained only one pearlfish specimen, while one contained two; this interesting situation is in line with what has already been noted by other authors [31,43], confirming that sexual pairs of pearlfish can be found in sea cucumbers, which are believed to also serve as breeding sites [26]. Finally, collecting holothurian specimens in two different seasons proved to be useful and showed the possible differential occurrence of the pearlfish in the same area throughout the year: in fact, all the C. acus individuals were found only in autumn from Station 3. Another interesting consideration should be made regarding the depth and habitat in which we found these Apulian pearlfish since they were collected in a shallower (10 m of depth) coastal area, contrary to what has already been reported for eastern Adriatic Sea [33,53,54,55,56]. Ecological observations and morphological data on interesting and poorly known species like the cryptic pearlfish C. acus are of fundamental importance because they allow us to fill some gaps in our knowledge of Mediterranean diversity and the delicate relationships occurring between different marine organisms. Knowledge of these biological and ecological aspects is indeed also the baseline step for planning future studies on this difficult-to-study fish in a strategic locality, like the Salento peninsula, and promoting effective conservation strategies aimed at preserving Mediterranean marine ecosystems and biodiversity.
Author Contributions
Conceptualization, G.F. and A.M.; methodology, G.F., A.M. and M.S.; software, A.M.; validation, G.F., A.M., M.S., E.T. and S.P.; formal analysis, A.M.; investigation, G.F. and M.S.; resources, G.F. and M.S; data curation, A.M., G.F., E.T. and M.S.; writing—original draft preparation, A.M. and G.F.; writing—review and editing, A.M., G.F., M.S., E.T. and S.P.; supervision, G.F. and S.P. All authors have read and agreed to the published version of the manuscript.
Funding
Project funded under the National Recovery and Resilience Plan (NRRP), Mission 4 Component 2 Investment 1.4—Call for tender No. 3138 of 16 December 2021, rectified by Decree n.3175 of 18 December 2021 of Italian Ministry of University and Research funded by the European Union-NextGenerationEU. EU Horizon Euro Research and innovation program ACTNOW (grant agreement No: 101060072). Awards Number: Project code CN_00000033, Concession Decree No. 1034 of 14 June 2022 adopted by the Italian Ministery of University and research, CUP D33C22000960007, Project title “ National Biodiversity Future Center- NBFC”.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bianchi, C.N.; Morri, C. Marine biodiversity of the Mediterranean Sea: Situation, problems and prospects for future research. Mar. Pollut. Bull. 2000, 40, 367–376. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Da Fonseca, G.A.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Coll, M.; Piroddi, C.; Steenbeek, J.; Kaschner, K.; Ben Rais Lasram, F.; Aguzzi, J.; Ballesteros, E.; Bianchi, C.N.; Corbera, J.; Dailianis, T.; et al. The Biodiversity of the Mediterranean Sea: Estimates, Patterns, and Threats. PLoS ONE 2010, 5, e11842. [Google Scholar] [CrossRef]
- Giangrande, A. Biodiversity, conservation, and the ‘taxonomic impediment’. Aquat. Conserv. Mar. Freshw. Ecosyst. 2003, 13, 451–459. [Google Scholar] [CrossRef]
- Kim, K.C.; Byrne, L.B. Biodiversity loss and the taxonomic bottleneck: Emerging biodiversity science. Ecol. Res. 2006, 21, 794–810. [Google Scholar] [CrossRef]
- Conand, C.; Mangion, P. Sea cucumbers on La Reunion Island fringing reefs: Diversity, distribution, abundance and structure of the populations. Beche Mer Inf. Bull. 2002, 17, 27–33. [Google Scholar]
- Du, H.; Bao, Z.; Hou, R.; Wang, S.; Su, H.; Yan, J.; Tian, M.; Li, Y.; Wei, W.; Lu, W. Transcriptome sequencing and characterization for the sea cucumber Apostichopus japonicus (Selenka, 1867). PLoS ONE 2012, 7, e33311. [Google Scholar] [CrossRef]
- Kiew, P.L.; Don, M.M. Jewel of the seabed: Sea cucumbers as nutritional and drug candidates. Int. J. Food Sci. Nutr. 2012, 63, 616–636. [Google Scholar] [CrossRef]
- Purcell, S.W.; Conand, C.; Uthicke, S.; Byrne, M. Ecological roles of exploited sea cucumbers. In Oceanography and Marine Biology, 1st ed.; CRC Press: Boca Raton, FL, USA, 2016; pp. 375–394. [Google Scholar]
- Bakus, G.J. The biology and ecology of tropical holothurians. In Biology and Geology of the Coral Reefs; Jones, O.A., Endean, R., Eds.; Academic Press: New York, NY, USA, 1973; Volume 2, pp. 325–367. [Google Scholar]
- Webb, K.L.; DuPaul, W.D.; D’Elia, C.F. Biomass and nutrient flux measurements on Holothuria atra populations on Holothuria atra populations on windward reef flats at Enewetak, Marshall Islands. In Proceedings of 3rd International Coral Reef Symposium; Taylor, D.L., Ed.; University of Florida: Miami, FL, USA, 1977; Volume 1, pp. 410–415. [Google Scholar]
- Massin, C. Food and feeding mechanisms: Holothuroidea. In Echinoderm Nutrition; Jangoux, M., Lawrence, J.M., Eds.; Balkema Publ Rotterdam: Rotterdam, The Netherlands, 1982; pp. 43–55. [Google Scholar]
- Francour, P. Dynamique de ‘Lécosystème à Posidonia oceanica Dans le Parc National de Port-Cros: Analyse des Compartiments Matte, Litière, Faune Vagile, Echinodermes et Poissons. Doctoral Dissertation, Université Pierre et Marie Curie (Paris 6), Paris, France, 1990; pp. 1–373. [Google Scholar]
- Massin, C. Effects of feeding on the environment: Holothuroidea. In Echinoderm Nutrition; Jangoux, M., Lawrence, J., Eds.; A.A. Balkema: Rotterdam, The Netherlands, 1982; pp. 494–497. [Google Scholar]
- Martines, A.; Furfaro, G.; Solca, M.; Muzzi, M.; Di Giulio, A.; Rossi, S. An Analysis of Microplastics Ingested by the Mediterranean Detritivore Holothuria tubulosa (Echinodermata: Holothuroidea) Sheds Light on Patterns of Contaminant Distribution in Different Marine Areas. Water 2023, 15, 1597. [Google Scholar] [CrossRef]
- Renzi, M.; Blašković, A.; Bernardi, G.; Russo, G.F. Plastic litter transfer from sediments towards marine trophic webs: A case study on holothurians. Mar. Pollut. Bull. 2018, 135, 376–385. [Google Scholar] [CrossRef]
- Francour, P. Predation on holothurians: A literature review. Invertebr. Biol. 1997, 116, 52–60. [Google Scholar] [CrossRef]
- Raghunathan, C.; Venkataraman, K. Status Survey of Holothurians (Sea cucumber) in the Territorial Waters of Andaman and Nicobar Islands; Zoological Survey of India Kolkata: Kolkata, India, 2014. [Google Scholar]
- Morton, B. The feeding strategy of the predatory Gyrineum natator (Gastropoda: Neotaenioglossa: Ranellidae) in the Cape d’Aguilar Marine Reserve, Hong Kong, with a review of sulphuric acid use in prey access by the Tonnoidea and experimentally derived estimates of consumption. J. Nat. Hist. 2015, 49, 483–507. [Google Scholar]
- Hamel, J.; Conand, C.; Pawson, D.L.; Mercier, A. The sea cucumber Holothuria scabra (Holothuroidae: Echinodermata): Its biology and exploitation as Beche-de-mer. Adv. Mar. Biol. 2001, 41, 131–223. [Google Scholar]
- Leung, T.L.; Poulin, R. Parasitism, commensalism, and mutualism: Exploring the many shades of symbioses. Vie Milieu/Life Environ. 2008, 58, 107–115. [Google Scholar]
- Ross, D.M. Symbiotic relations. In The Biology of the Crustacea; Bliss, D., Ed.; Academic Press: New York, NY, USA, 1983; Volume 7, pp. 163–212. [Google Scholar]
- Eeckhaut, I. Animal Life Encyclopedia; The Gale Group: Farmington Hills, MI, USA, 2003; pp. 31–34. [Google Scholar]
- Lyskin, S.; Britaev, T. Symbionts of holothurians from South Vietnam: Intra-and interspecific interactions. Proc. Dokl. Biol. Sci. 2005, 401, 116–119. [Google Scholar] [CrossRef]
- Martin, I.; Anker, A.; Britayev, T.A.; Palmer, A.R. Symbiosis between the alpheid shrimp, Athanas ornithorhynchus Banner and Banner, 1973 (Crustacea: Decapoda), and the brittle star, Macrophiothrix longipeda (Lamarck, 1816) (Echinodermata: Ophiuroidea). Zool. Stud. 2005, 44, 234. [Google Scholar]
- González-Wangüemert, M.; Maggi, C.; Valente, S.; Martínez-Garrido, J.; Vasco-Rodrigues, N. Parastichopus regalis-The main host of Carapus acus in temperate waters of the Mediterranean Sea and northeastern Atlantic Ocean. Beche Mer Inf. Bull. 2014, 34, 38–42. [Google Scholar]
- Markle, D.F.; Olney, J.E. Systematics of the pearlfishes (Pisces: Carapidae). Bull. Mar. Sci. 1990, 47, 269–410. [Google Scholar]
- Mezali, K.; Khodja, I. First record of pearlfish (Carapus acus) in the western Algerian coast. J. Black Sea Mediterr Mar Sci. 2021, 27, 365–371. [Google Scholar]
- Parmentier, E.; Chardon, M.; Poulicek, M.; Bussers, J.-C.; Vandewalle, P. Morphology of the buccal apparatus and related structures in four species of Carapidae. Aust. J. Zool. 1998, 46, 391–404. [Google Scholar] [CrossRef]
- Parmentier, E.; Castro-Aguirre, J.; Vandewalle, P. Morphological comparison of the buccal apparatus in two bivalve commensal Teleostei: Encheliophis dubius and Onuxodon fowleri (Carapidae, Ophidiiformes). Zoomorphology 2000, 120, 29–37. [Google Scholar] [CrossRef]
- Trott, L.B.; Olney, J.E. Carapidae. In Fish of the Northeastern Atlantic and the Mediterranean; Whitehead, P.J.P., Bauchot, M.-L., Hureau, J.-C., Nielsen, J., Tortonese, E., Eds.; UNESCO: Paris, France, 1986; Volume 3, pp. 1172–1176. [Google Scholar]
- Arnold, D.C. A systematic revision of the fishes of the teleost family Carapidae (Percomorphi, Blennioidea), with description of two new species. Bull. Br. Mus. (Nat. Hist.) 1956, 4, 247–307. [Google Scholar] [CrossRef]
- Despalatović, M.; Cvitković, I.; Žuljević, A.; Žunec, A.; Bogner, D.; Nejašmić, J.; Isajlović, I. Incidence of commensalism between the pearlfish Carapus acus (Brünnich, 1768) and the holothurian Parastichopus regalis (Cuvier, 1817) in the eastern Adriatic Sea. J. Appl. Ichthyol. 2020, 36, 834–836. [Google Scholar] [CrossRef]
- El Aamri, F.; Tamsouri, M. First finding of rare Pearlfish, Carapus acus (Brünnich, 1768) (Ophidiiformes: Carapidae) from Mediterranean coasts of Morocco. J. Mater. Environ. Sci. 2018, 9, 3134–3136. [Google Scholar]
- Enajjar, S.; Bradai, M.N. First Record of Carapus acus (Osteichthyes: Carapidae) in the Gulf of Gabès (southern Tunisia, central Mediterranean Sea). Mar. Biodivers. Rec. 2016, 9, 7. [Google Scholar] [CrossRef][Green Version]
- Tortonese, E. Osteichthyes (pesci ossei). Fauna D’italia 1975, 479–523. [Google Scholar]
- Borg, J.A.; Dandria, D.; Evans, J.; Knittweis, L.; Schembri, P.J. A Critical Checklist of the Marine Fishes of Malta and Surrounding Waters. Diversity 2023, 15, 225. [Google Scholar] [CrossRef]
- Relini Orsi, L.; Relini, G. La pesca a strascico mesobatiale in mar Ligure e la ricerca finalizzata. Nat. Sicil. 1982, 2, 375–387. [Google Scholar]
- Busalacchi, B.; Rinelli, P.; De Domenico, F.; Profeta, A.; Perdichizzi, F.; Bottari, T. Analysis of demersal fish assemblages off the Southern Tyrrhenian Sea (central Mediterranean). Hydrobiologia 2010, 654, 111–124. [Google Scholar] [CrossRef]
- Arnold, D.C. Further studies on the behaviour of the fish Carapus acus (Brünnich). Pubbl. Staz. Zool. Napoli 1957, 30, 263–268. [Google Scholar]
- Gustato, G. Osservazioni sulla biologica e sul comportamento di Carapus acus (Ophioidei, Percomorphi). Boll. Soc. Natur. Napoli. 1976, 85, 505–535. [Google Scholar]
- Kloss, K.; Pfeiffer, W. Zur biologie des «eingeweidefisches» C. acus (Brunnich, 1768) (Carapidae, Teleostei), mit hinweisen auf eine nich-parasitische ernähung. Rev. Suisse Zool. 2000, 107, 335–349. [Google Scholar] [CrossRef]
- Eeckhaut, I.; Parmentier, E.; Becker, P.; Gomez da Silva, S.; Jangoux, M. Parasites and biotic diseases in field and cultivated sea cucumbers. In Advances in Sea Cucumber Aquaculture and Management; FAO: Rome, Italy, 2004. [Google Scholar]
- Meyer-Rochow, V.B. Comparison between 15 Carapus mourlani in a Single Holothurian and 19 C. mourlani from Starfish. Copeia 1977, 1977, 582–584. [Google Scholar] [CrossRef]
- Mezali, K. Contribution à la systématique, la biologie, l’écologie et la dynamique de cinq espèces d’holothuries aspidochirotes [Holothuria (H.) tubulosa, H. (L.) polii, H. (H.) stellati, H. (P.) forskali et H (P.) sanctori] de l’herbier à Posidonia oceanica (L.) Delille de la Prèsqu’île de Sidi-Fredj. Thèse de Magister, Ecole National des Sciences de la Mer et de l’Aménagement du Littoral (Ex- ISMAL), Alger, Algérie, 1998; 192p. [Google Scholar]
- Parmentier, E.; Fine, M.; Vandewalle, P.; Ducamp, J.J.; Lagardère, J.P. Sound production in two carapids (Carapus acus and C. mourlani) and through the sea cucumber tegument. Acta Zool. 2006, 87, 113–119. [Google Scholar] [CrossRef]
- Trott, L.B. Contributions to the Biology of Carapid Fishes (Paracanthopterygii: Gadiformes). 1968. Available online: https://www.elibrary.ru/item.asp?id=6915511 (accessed on 12 September 2023).
- Trott, L.B. A general review of the pearlfishes (Pisces, Carapidae). Bull. Mar. Sci. 1981, 31, 623–629. [Google Scholar]
- Froese, R.; Pauly, D.; FishBase. World Wide Web Electronic Publication; Version (02/2018). Available online: www.fishbase.org (accessed on 9 May 2018).
- Golani, D.; Ozturk, B.; Basusta, N. Fishes of the eastern Mediterranean. Isr. J. Aquac. 2007, 59, 121. [Google Scholar] [CrossRef]
- Parmentier, E.; Vandewalle, P. Further insight on carapid-holothuroid relationships. Mar. Biol. 2005, 146, 455–465. [Google Scholar] [CrossRef]
- Parmentier, E.; Das, K. Commensal vs. parasitic relationship between Carapini fish and their hosts: Some fu12rther insight through δ13C and δ15N measurements. J. Exp. Mar. Biol. 2004, 310, 47–58. [Google Scholar] [CrossRef]
- Jukić, S. Koćarska područja u srednjem Jadranu. Acta Adriat. 1975, 17, 1–86. [Google Scholar]
- Županović, Š.; Jardas, I. Fauna i Flora Jadrana; Logos: Split, Croatia, 1989; Volume 2. [Google Scholar]
- Ungaro, N.; Marano, C.A.; Marsan, R.; Martino, M.; Marzano, M.C.; Strippoli, G.; Vlora, A. Analysis of demersal species as- semblages from trawl surveys in the South Adriatic Sea. Aquat. Living Resour. 1999, 12, 177–185. [Google Scholar] [CrossRef]
- Jardas, I. Jadranska Ihtiofauna; Školska knjiga: Zagreb, Croatia, 1996. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).