Genome-Wide Identification and Expression Pattern Analysis of TIFY Family Genes Reveal Their Potential Roles in Phalaenopsis aphrodite Flower Opening
Abstract
:1. Introduction
2. Results
2.1. Identification and Characterization of TIFY Genes in Phalaenopsis
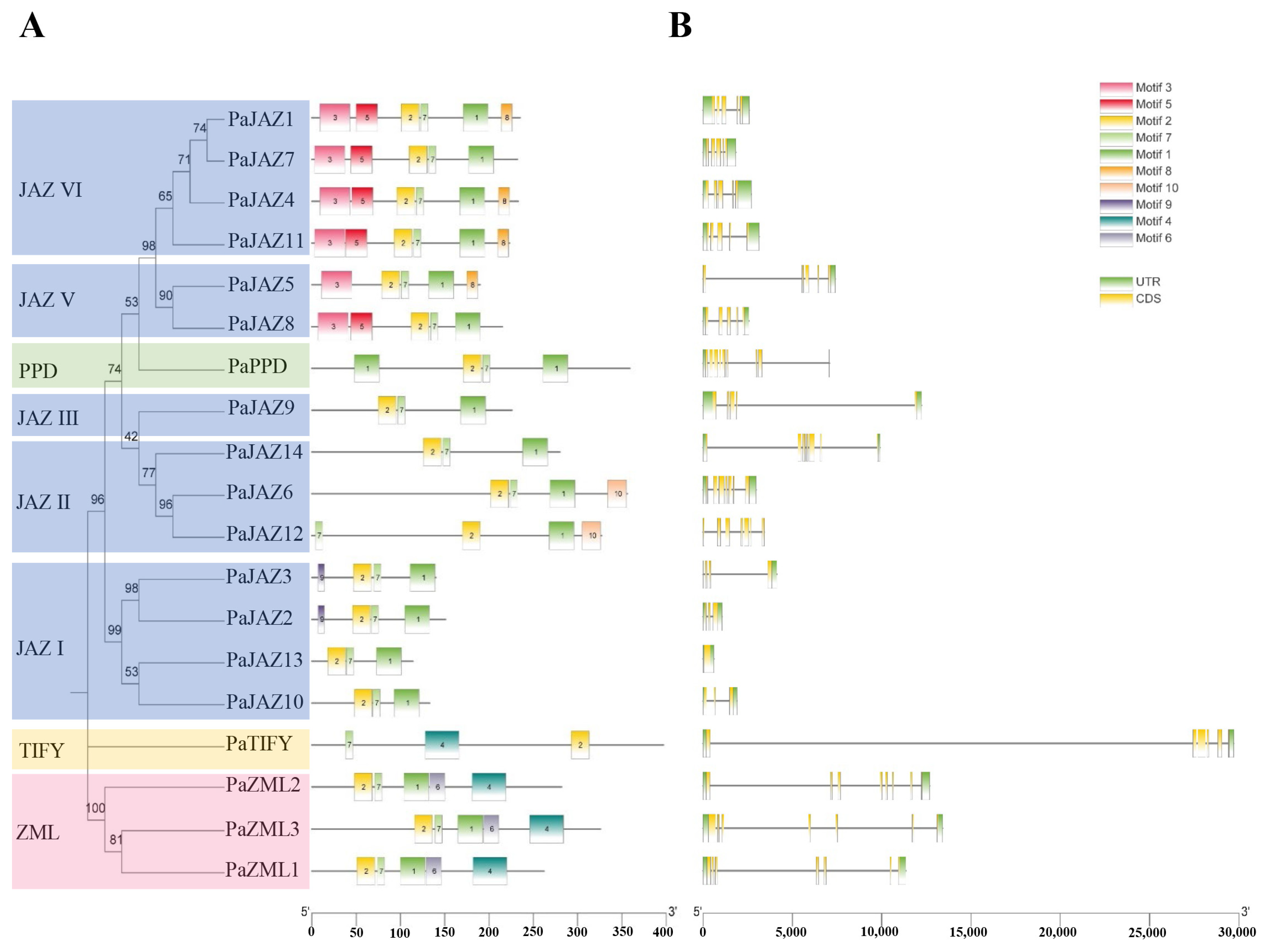
2.2. Phylogenetic Analyses and Classification of the Phalaenopsis TIFY Gene Family
2.3. Sequence Analysis of Phalaenopsis TIFY Family
2.4. Promoter cis-Elements Analysis of Phalaenopsis TIFY Genes
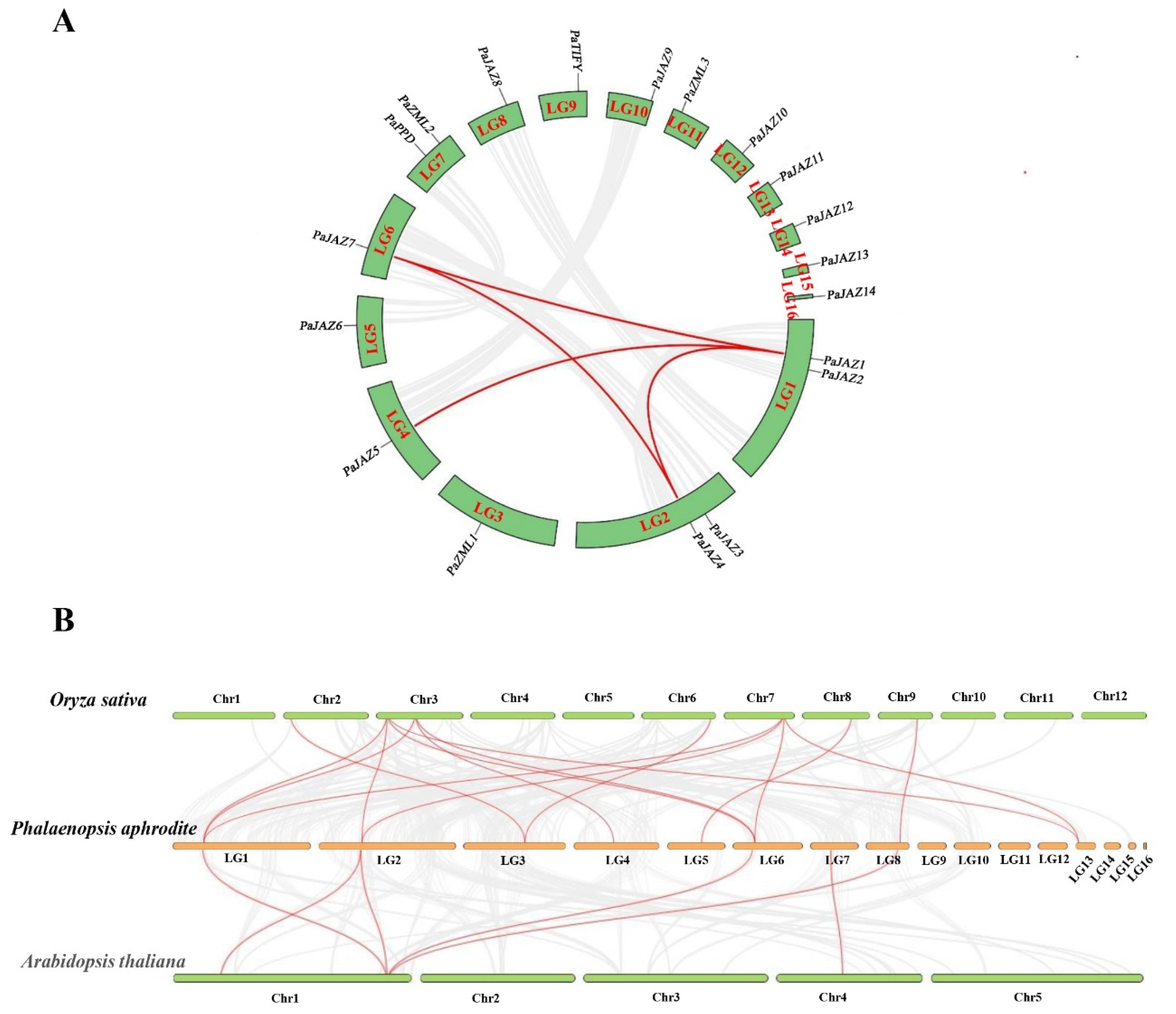
2.5. Synteny and Evolutionary Analyses
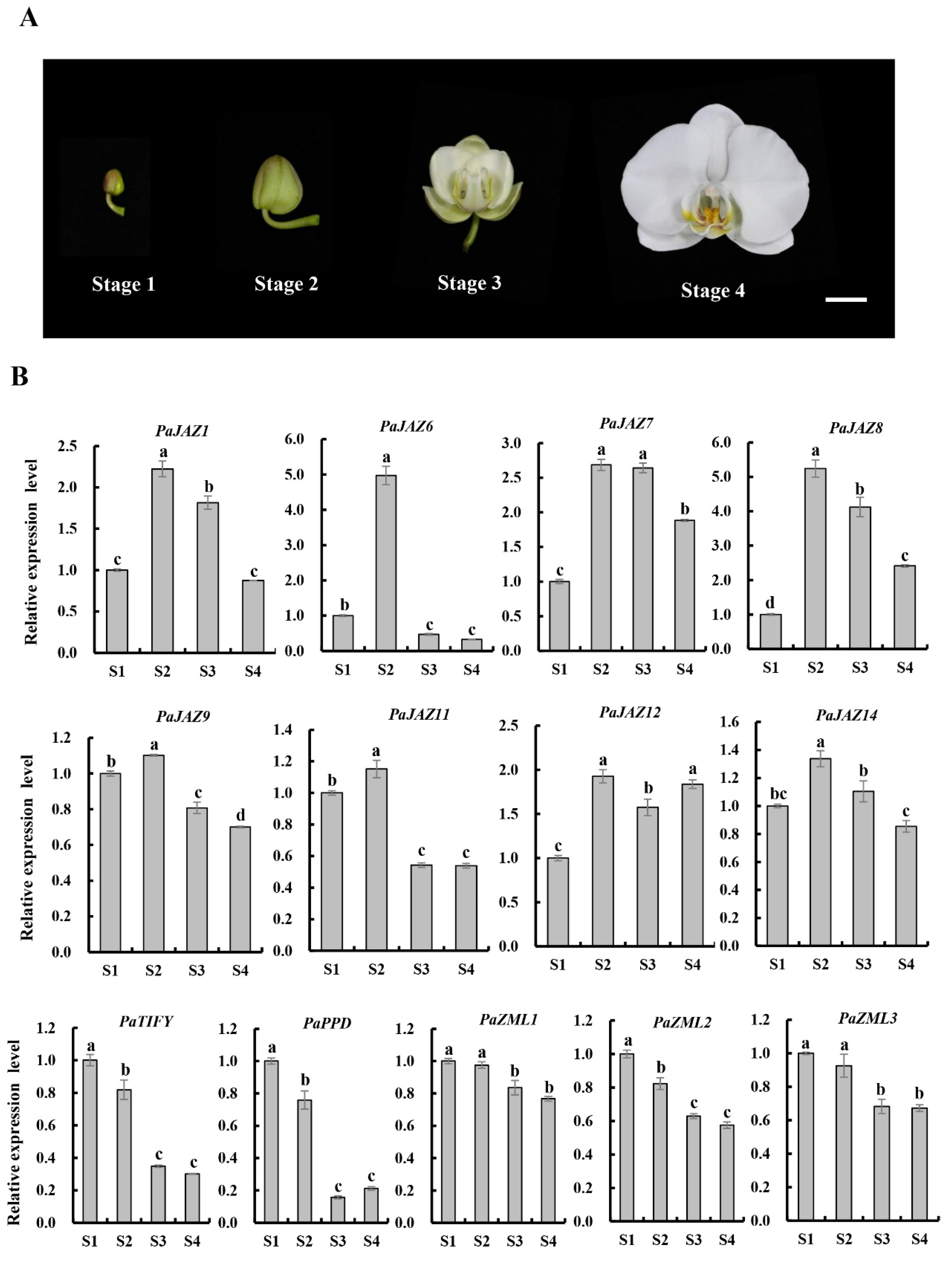
2.6. Expression Analysis of TIFY Genes in the Process of Phalaenopsis Flower Opening
2.7. Expression Analysis of Petal- or Lip-Associated TIFY Genes during Flower Opening in Phalaenopsis
3. Discussion
4. Materials and Methods
4.1. Identification and Physicochemical Properties Analysis of TIFY Family Genes
4.2. Phylogenetic Analysis
4.3. Gene Structure and Conservative Domain Analysis
4.4. Prediction of cis-Acting Elements
4.5. Gene Duplication and Synteny Analyses
4.6. Expression Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nishii, A.; Takemura, M.; Fujita, H.; Shikata, M.; Yokota, A.; Kohchi, T. Characterization of a novel gene encoding a putative single zinc-finger protein, ZIM, expressed during the reproductive phase in Arabidopsis thaliana. Biosci. Biotechnol. Biochem. 2000, 64, 1402–1409. [Google Scholar] [CrossRef] [PubMed]
- Vanholme, B.; Grunewald, W.; Bateman, A.; Kohchi, T.; Gheysen, G. The tify family previously known as ZIM. Trends Plant Sci. 2007, 12, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Meng, Y.; Huang, D.; Qi, Y.; Chen, M. Origin and evolutionary analysis of the plant-specific TIFY transcription factor family. Genomics 2011, 98, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; An, H.R.; Tong, C.; Jang, S. Flowering and flowering genes: From model plants to orchids. Hortic. Environ. Biotechnol. 2021, 62, 135–148. [Google Scholar] [CrossRef]
- Mondragon Palomino, M.; Theißen, G. Why are orchid flowers so diverse? Reduction of evolutionary constraints by paralogues of class B floral homeotic genes. Ann. Bot.-London 2009, 104, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Cheng, C.; Tsai, W.; Chung, M.; Chen, W.; Hu, J.; Chen, H. The duplicated B-class MADS-Box genes display dualistic characters in orchid floral organ identity and growth. Plant Cell Physiol. 2011, 52, 1515–1531. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.; Hsu, W.; Lee, Y.; Mao, W.; Yang, J.; Li, J.; Yang, C. Model for perianth formation in orchids. Nat. Plants 2015, 1, 15046. [Google Scholar] [CrossRef]
- Fernández-Calvo, P.; Chini, A.; Fernández-Barbero, G.; Chico, J.; Gimenez-Ibanez, S.; Geerinck, J.; Eeckhout, D.; Schweizer, F.; Godoy, M.; Franco-Zorrilla, J.M.; et al. The Arabidopsis bHLH transcription factors MYC3 and MYC4 are targets of JAZ repressors and act additively with MYC2 in the activation of jasmonate responses. Plant Cell 2011, 23, 701–715. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, Z.; An, F.; Hao, D.; Li, P.; Song, J.; Yi, C.; Guo, H. Jasmonate-activated MYC2 represses ETHYLENE INSENSITIVE3 activity to antagonize ethylene-promoted apical hook formation in Arabidopsis. Plant Cell 2014, 26, 1105–1117. [Google Scholar] [CrossRef]
- Qi, T.; Song, S.; Ren, Q.; Wu, D.; Huang, H.; Chen, Y.; Fan, M.; Peng, W.; Ren, C.; Xie, D. The Jasmonate-ZIM-domain proteins interact with the WD-Repeat/bHLH/MYB complexes to regulate Jasmonate-mediated anthocyanin accumulation and trichome initiation in Arabidopsis thaliana. Plant Cell 2011, 23, 1795–1814. [Google Scholar] [CrossRef]
- Jiang, Y.; Liang, G.; Yang, S.; Yu, D. Arabidopsis WRKY57 functions as a node of convergence for Jasmonic acid- and auxin-mediated signaling in Jasmonic acid-induced leaf senescence. Plant Cell 2014, 26, 230–245. [Google Scholar] [CrossRef] [PubMed]
- Begum, S.; Jing, S.; Yu, L.; Sun, X.; Wang, E.; Kawochar, M.; Qin, J.; Liu, J.; Song, B. Modulation of JA signalling reveals the influence of StJAZ1-like on tuber initiation and tuber bulking in potato. Plant J. 2022, 109, 952–964. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zhu, L.; Wassan, G.; Wang, Y.; Miao, Y.; Shaban, M.; Hu, H.; Sun, H.; Zhang, X. GhJAZ2 attenuates cotton resistance to biotic stresses via inhibiting the transcriptional activity of GhbHLH171. Mol. Plant Pathol. 2017, 19, 896–908. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, P.; Browse, J. Male-sterility in Arabidopsis induced by overexpression of a MYC5-SRDX chimeric repressor. Plant J. 2015, 81, 849–860. [Google Scholar] [CrossRef]
- Hori, Y.; Kurotani, K.; Toda, Y.; Hattori, T.; Takeda, S. Overexpression of the JAZ factors with mutated jas domains causes pleiotropic defects in rice spikelet development. Plant Signal. Behav. 2014, 9, e970414. [Google Scholar] [CrossRef]
- Yu, X.; Chen, G.; Tang, B.; Zhang, J.; Zhou, S.; Hu, Z. The Jasmonate ZIM-domain protein gene SlJAZ2 regulates plant morphology and accelerates flower initiation in Solanum lycopersicum plants. Plant Sci. 2018, 267, 65–73. [Google Scholar] [CrossRef]
- Guan, Y.; Ding, L.; Jiang, J.; Jia, D.; Li, S.; Jin, L.; Zhao, W.; Zhang, X.; Song, A.; Chen, S.; et al. The TIFY family protein CmJAZ1-like negatively regulates petal size via interaction with the bHLH transcription factor CmBPE2 in Chrysanthemum morifolium. Plant J. 2022, 112, 1489–1506. [Google Scholar] [CrossRef]
- Oh, Y.; Baldwin, I.T.; Galis, I.; Pandey, G.K. A jasmonate ZIM-domain protein NaJAZd regulates floral jasmonic acid levels and counteracts flower abscission in Nicotiana attenuata plants. PLoS ONE 2013, 8, e57868. [Google Scholar] [CrossRef]
- Chen, C.; Ma, Y.; Zuo, L.; Xiao, Y.; Jiang, Y.; Gao, J. The CALCINEURIN B-LIKE 4/CBL-INTERACTING PROTEIN 3 module degrades repressor JAZ5 during rose petal senescence. Plant Physiol. 2023, 193, 1605–1620. [Google Scholar] [CrossRef]
- Hu, R.; Guo, J.; Guo, X.; Chen, F.; Wang, W. Genome-wide identification and analysis of the TIFY gene family in Dendrobium officinale Kimura et Migo during protocorm development. J. Biol. 2021, 38, 53–58. [Google Scholar]
- Wilkins, M.R.; Gasteiger, E.; Bairoch, A.; Sanchez, J.C.; Williams, K.L.; Appel, R.D.; Hochstrasser, D.F. Protein identification and analysis tools on the ExPASy server. In The Proteomics Protocols Handbook; Walker, J.M., Ed.; Humana Press: Totowa, NJ, USA, 2005. [Google Scholar] [CrossRef]
- Ye, H.; Du, H.; Tang, N.; Li, X.; Xiong, L. Identification and expression profiling analysis of TIFY family genes involved in stress and phytohormone responses in rice. Plant Mol. Biol. 2009, 71, 291–305. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Jin, S.; Guo, H.; Zhong, X.; He, J.; Li, X.; Jiang, M.; Yu, X.; Long, H.; Ma, M.; et al. Genome-wide identification and characterization of TIFY family genes in Moso Bamboo (Phyllostachys edulis) and expression profiling analysis under dehydration and cold stresses. PeerJ 2016, 4, e2620. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; You, J.; Chan, Z. Identification and characterization of TIFY family genes in Brachypodium distachyon. J. Plant Res. 2015, 128, 995–1005. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gao, M.; Singer, S.D.; Fei, Z.; Wang, H.; Wang, X. Genome-wide identification and analysis of the TIFY gene family in grape. PLoS ONE 2012, 7, e44465. [Google Scholar] [CrossRef] [PubMed]
- White, D.W.R. PEAPOD regulates lamina size and curvature in Arabidopsis. Proc. Natl. Acad. Sci. USA 2006, 103, 13238–13243. [Google Scholar] [CrossRef] [PubMed]
- Chao, Y.T.; Chen, W.C.; Chen, C.Y.; Ho, H.Y.; Yeh, C.H.; Kuo, Y.T.; Su, C.L.; Yen, S.H.; Hsueh, H.Y.; Yeh, J.H.; et al. Chromosome-level assembly, genetic and physical mapping of Phalaenopsis aphrodite genome provides new insights into species adaptation and resources for orchid breeding. Plant Biotechnol. J. 2018, 16, 2027–2041. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Kang, Y.; Li, W.; Liu, W.; Xie, P.; Liao, L.; Huang, L.; Yao, M.; Qian, L.; Liu, Z.; et al. Genome-wide identification and functional analysis of the TIFY gene family in the response to multiple stresses in Brassica napus L. BMC Genom. 2020, 21, 736. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Jia, H.; Wu, M.; Zhong, W.; Jia, D.; Wang, Z.; Huang, C. Genome-wide identification and characterization of the TIFY gene family in kiwifruit. BMC Genom. 2022, 23, 179. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Wan, Q.; Wang, H.; Guo, C.; Niu, X.; Zhang, X.; Zhang, R.; Chen, Y.; Luo, K. Genome-wide identification and expression of TIFY family in cassava (Manihot esculenta Crantz). Front. Plant Sci. 2022, 13, 1017840. [Google Scholar] [CrossRef]
- Sheng, Y.; Yu, H.; Pan, H.; Qiu, K.; Xie, Q.; Chen, H.; Fu, S.; Zhang, J.; Zhou, H. Genome-wide analysis of the gene structure, expression and protein interactions of the peach (Prunus persica) TIFY gene family. Front. Plant Sci. 2022, 13, 792802. [Google Scholar] [CrossRef]
- Ahmadizadeh, M.; Heidari, P. Bioinformatics study of transcription factors involved in cold stress. Biharean Biol. 2014, 8, 83–86. [Google Scholar]
- Brioudes, F.; Joly, C.; Szécsi, J.; Varaud, E.; Leroux, J.; Bellvert, F.; Bertrand, C.; Bendahmane, M. Jasmonate controls late development stages of petal growth in Arabidopsis thaliana. Plant J. 2009, 60, 1070–1080. [Google Scholar] [CrossRef]
- Li, L.; Zhang, W.; Zhang, L.; Li, N.; Peng, J.; Wang, Y.; Zhong, C.; Yang, Y.; Sun, S.; Liang, S.; et al. Transcriptomic insights into antagonistic effects of gibberellin and abscisic acid on petal growth in Gerbera hybrida. Front. Plant Sci. 2015, 6, 168. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Li, L.; Huang, Y.; Wang, Y.; Zhang, W.; Zheng, R.; Zhong, C.; Wang, X. GhWIP2, a WIP zinc finger protein, suppresses cell expansion in Gerbera hybrida by mediating crosstalk between gibberellin, abscisic acid, and auxin. New Phytol. 2018, 219, 728–742. [Google Scholar] [CrossRef] [PubMed]
- Cannon, S.; Mitra, A.; Baumgarten, A.; Young, N.; May, G. The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis thaliana. BMC Plant Biol. 2004, 4, 10. [Google Scholar] [CrossRef]
- Krizek, B.A.; Anderson, J.T. Control of flower size. J. Exp. Bot. 2013, 64, 1427–1437. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- He, Z.; Zhang, H.; Gao, S.; Lercher, M.; Chen, W.; Hu, S. Evolview v2: An online visualization and management tool for customized and annotated phylogenetic trees. Nucleic Acids Res. 2016, 44, w370. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Jiang, Z.; Hsu, H.; Yang, C. Silencing of FOREVER YOUNG FLOWER-Like genes from Phalaenopsis orchids promotes flower senescence and abscission. Plant Cell Physiol. 2021, 62, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]




| Gene Name | Gene ID | ORF (bp) | AA (aa) | pI | Mw (KDa) | Instability Index | GRAVY | Subcellular Localization |
|---|---|---|---|---|---|---|---|---|
| PaJAZ1 | PAXXG009620 | 705 | 234 | 8.76 | 25.29 | 55.48 | −0.419 | nucleus |
| PaJAZ2 | PAXXG009920 | 453 | 150 | 8.24 | 16.3 | 96 | −0.597 | nucleus |
| PaJAZ3 | PAXXG013450 | 420 | 139 | 8.93 | 15.32 | 65.03 | −0.453 | nucleus |
| PaJAZ4 | PAXXG013820 | 699 | 232 | 8.49 | 25.38 | 56.71 | −0.526 | nucleus |
| PaJAZ5 | PAXXG029240 | 570 | 189 | 9.47 | 21.69 | 49.29 | −0.877 | nucleus |
| PaJAZ6 | PAXXG049350 | 1068 | 355 | 8.64 | 38.38 | 48.39 | −0.412 | nucleus |
| PaJAZ7 | PAXXG055930 | 696 | 231 | 8.69 | 24.82 | 47.29 | −0.428 | nucleus |
| PaJAZ8 | PAXXG098670 | 645 | 214 | 7.7 | 24.35 | 50.81 | −0.774 | nucleus |
| PaJAZ9 | PAXXG123530 | 678 | 225 | 6 | 23.85 | 67.31 | −0.249 | nucleus |
| PaJAZ10 | PAXXG152710 | 399 | 132 | 5.23 | 14.9 | 44.26 | −0.404 | nucleus |
| PaJAZ11 | PAXXG209060 | 669 | 222 | 7.74 | 24.35 | 35.93 | −0.491 | nucleus |
| PaJAZ12 | PAXXG229230 | 981 | 326 | 9.23 | 34.93 | 58.72 | −0.38 | nucleus |
| PaJAZ13 | PAXXG308040 | 342 | 113 | 9.51 | 12.74 | 75.96 | −0.658 | nucleus |
| PaJAZ14 | PAXXG348960 | 840 | 279 | 9.72 | 30.85 | 46.82 | −0.501 | nucleus |
| PaPPD | PAXXG088150 | 1077 | 358 | 9.36 | 38.87 | 68.71 | −0.638 | nucleus |
| PaZML1 | PAXXG018580 | 786 | 261 | 8.59 | 27.98 | 49.83 | −0.544 | nucleus |
| PaZML2 | PAXXG088460 | 846 | 281 | 5.61 | 30.41 | 53.62 | −0.594 | nucleus |
| PaZML3 | PAXXG144870 | 978 | 325 | 6.06 | 35.05 | 35.81 | −0.668 | nucleus |
| PaTIFY | PAXXG116340 | 1191 | 396 | 8.41 | 41.7 | 52.86 | −0.337 | nucleus |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, Y.; Zhang, Q.; Li, M.; Zhai, J.; Wu, S.; Ahmad, S.; Lan, S.; Peng, D.; Liu, Z.-J. Genome-Wide Identification and Expression Pattern Analysis of TIFY Family Genes Reveal Their Potential Roles in Phalaenopsis aphrodite Flower Opening. Int. J. Mol. Sci. 2024, 25, 5422. https://doi.org/10.3390/ijms25105422
Guan Y, Zhang Q, Li M, Zhai J, Wu S, Ahmad S, Lan S, Peng D, Liu Z-J. Genome-Wide Identification and Expression Pattern Analysis of TIFY Family Genes Reveal Their Potential Roles in Phalaenopsis aphrodite Flower Opening. International Journal of Molecular Sciences. 2024; 25(10):5422. https://doi.org/10.3390/ijms25105422
Chicago/Turabian StyleGuan, Yunxiao, Qiaoyu Zhang, Minghe Li, Junwen Zhai, Shasha Wu, Sagheer Ahmad, Siren Lan, Donghui Peng, and Zhong-Jian Liu. 2024. "Genome-Wide Identification and Expression Pattern Analysis of TIFY Family Genes Reveal Their Potential Roles in Phalaenopsis aphrodite Flower Opening" International Journal of Molecular Sciences 25, no. 10: 5422. https://doi.org/10.3390/ijms25105422






