Altered Serum Proteins Suggest Inflammation, Fibrogenesis and Angiogenesis in Adult Patients with a Fontan Circulation
Abstract
:1. Introduction
2. Results
2.1. Clinical Examination, Routine Laboratory Analytes, Imaging, Exercise Capacity Testing
2.2. Proteomics Analysis
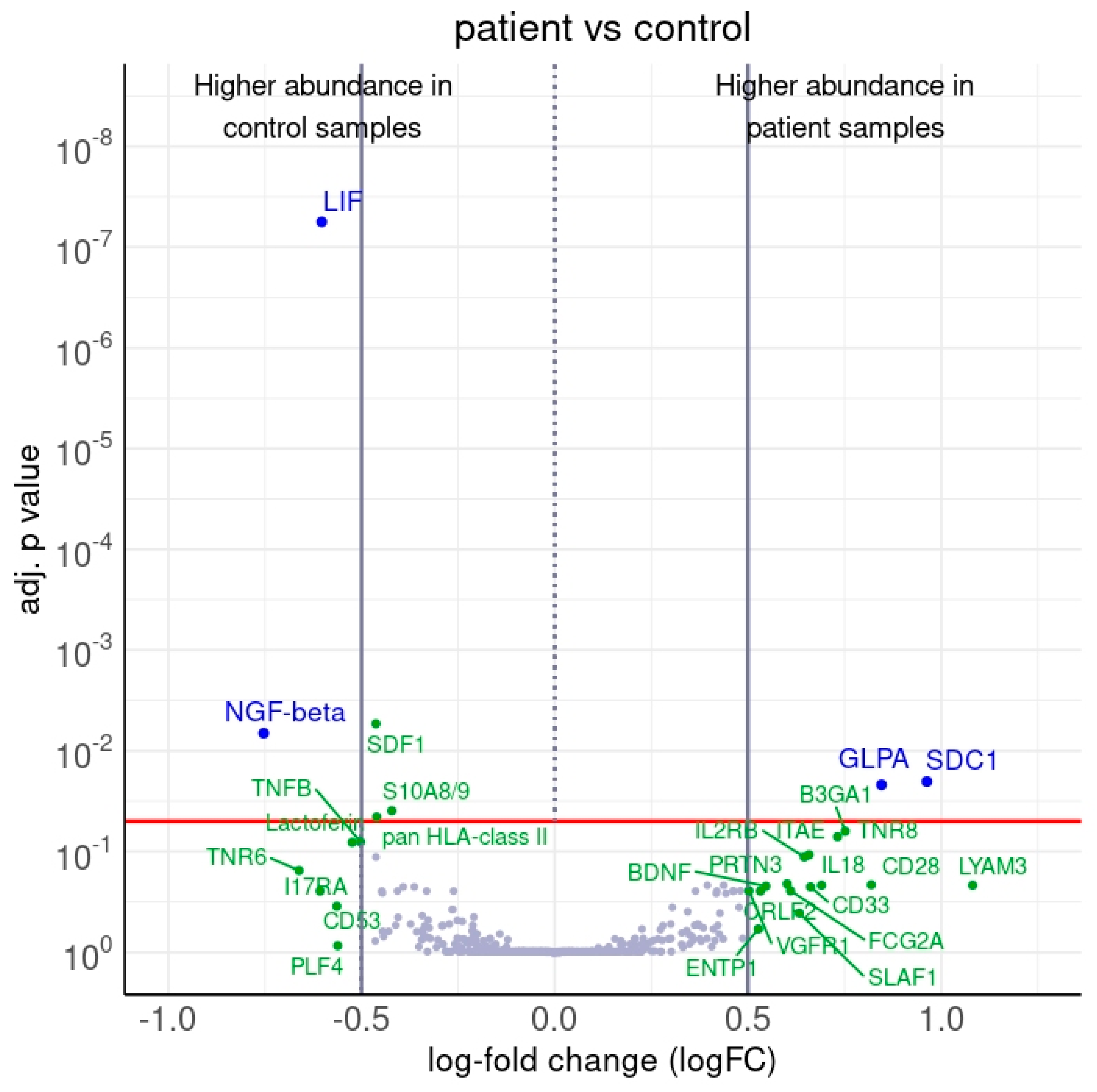
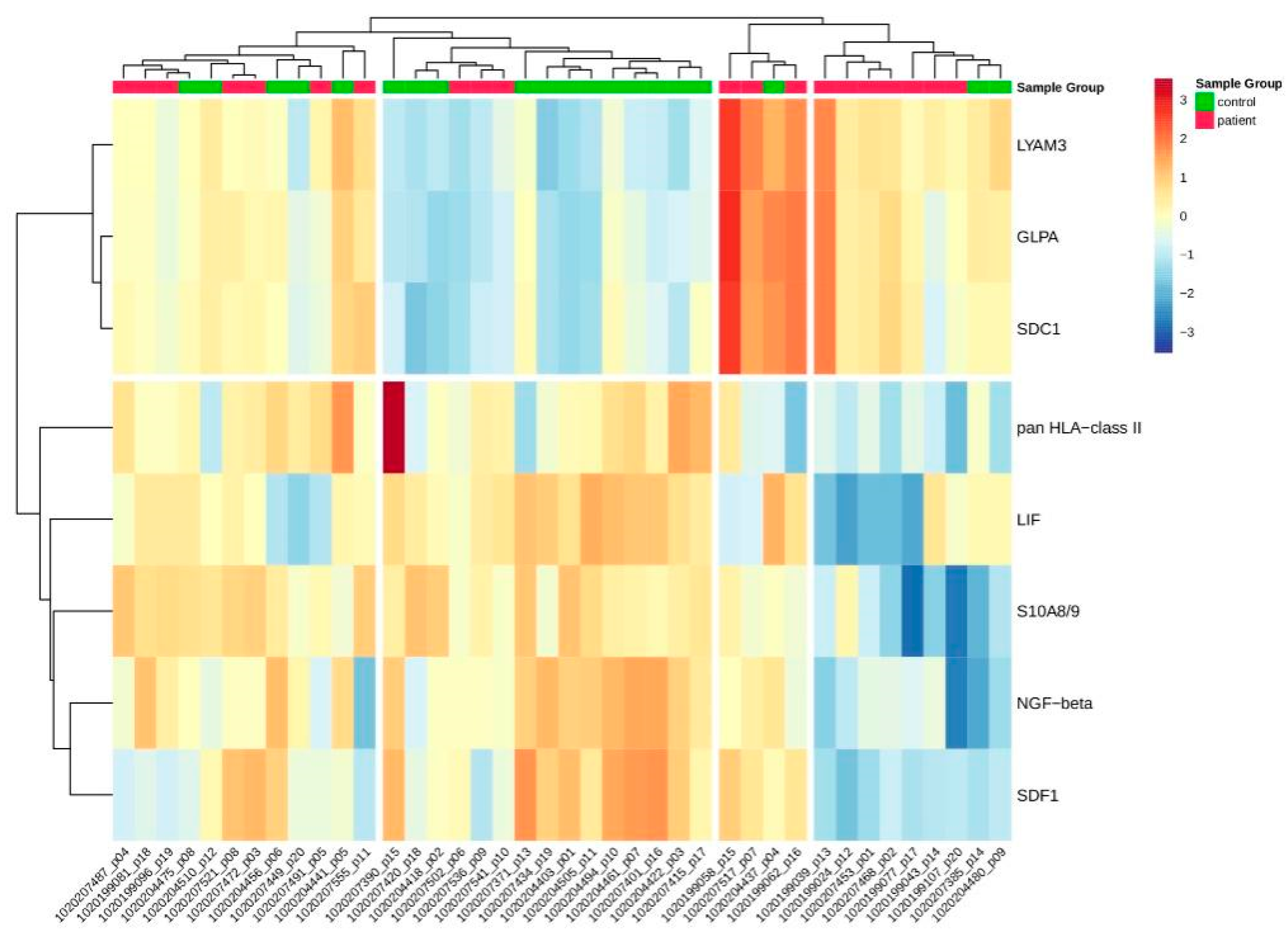
2.2.1. Differentially Abundant Proteins between Patients and Controls
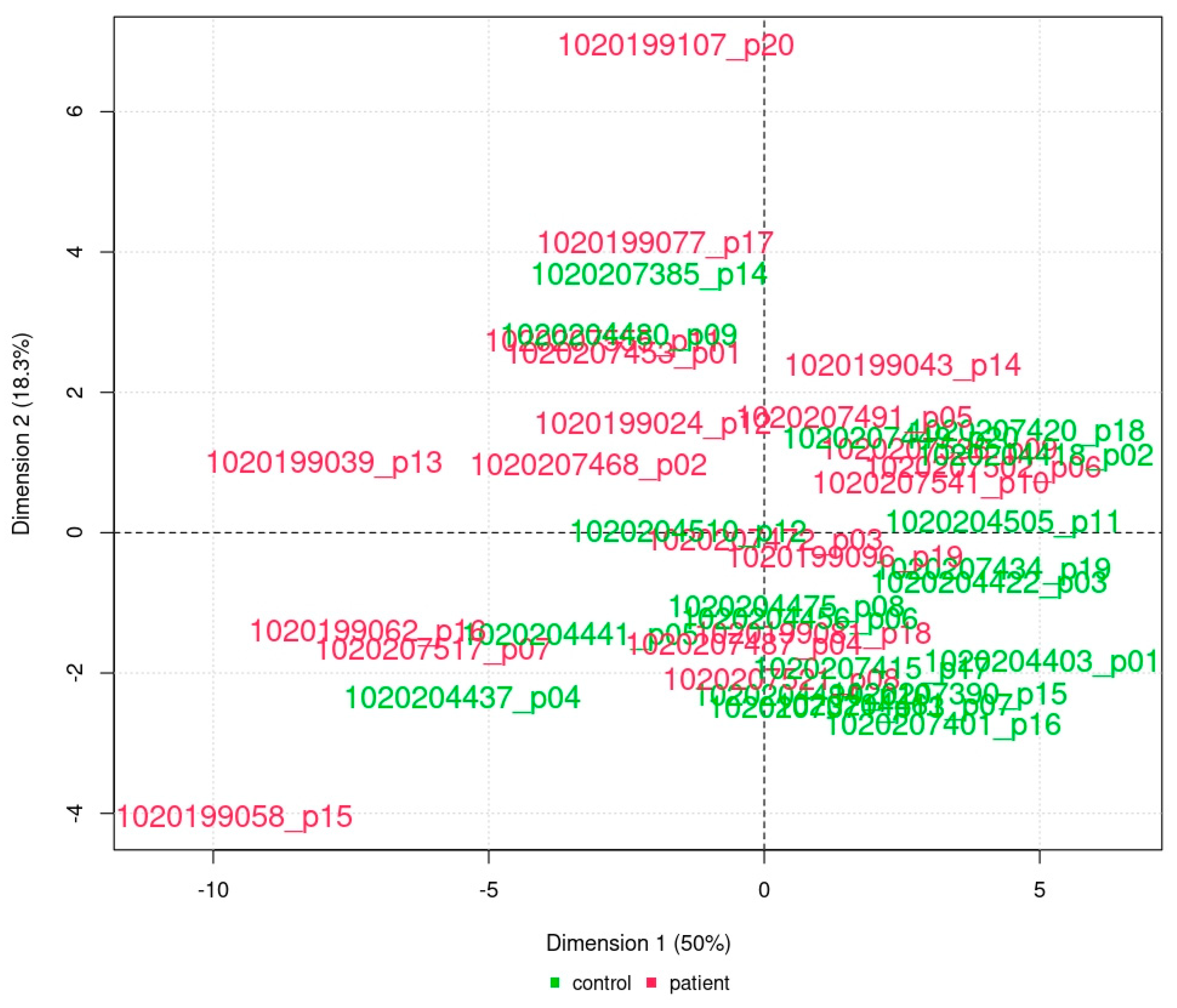
2.2.2. Principal Component Analysis
2.2.3. Receiver Operating Characteristic (ROC) Analysis
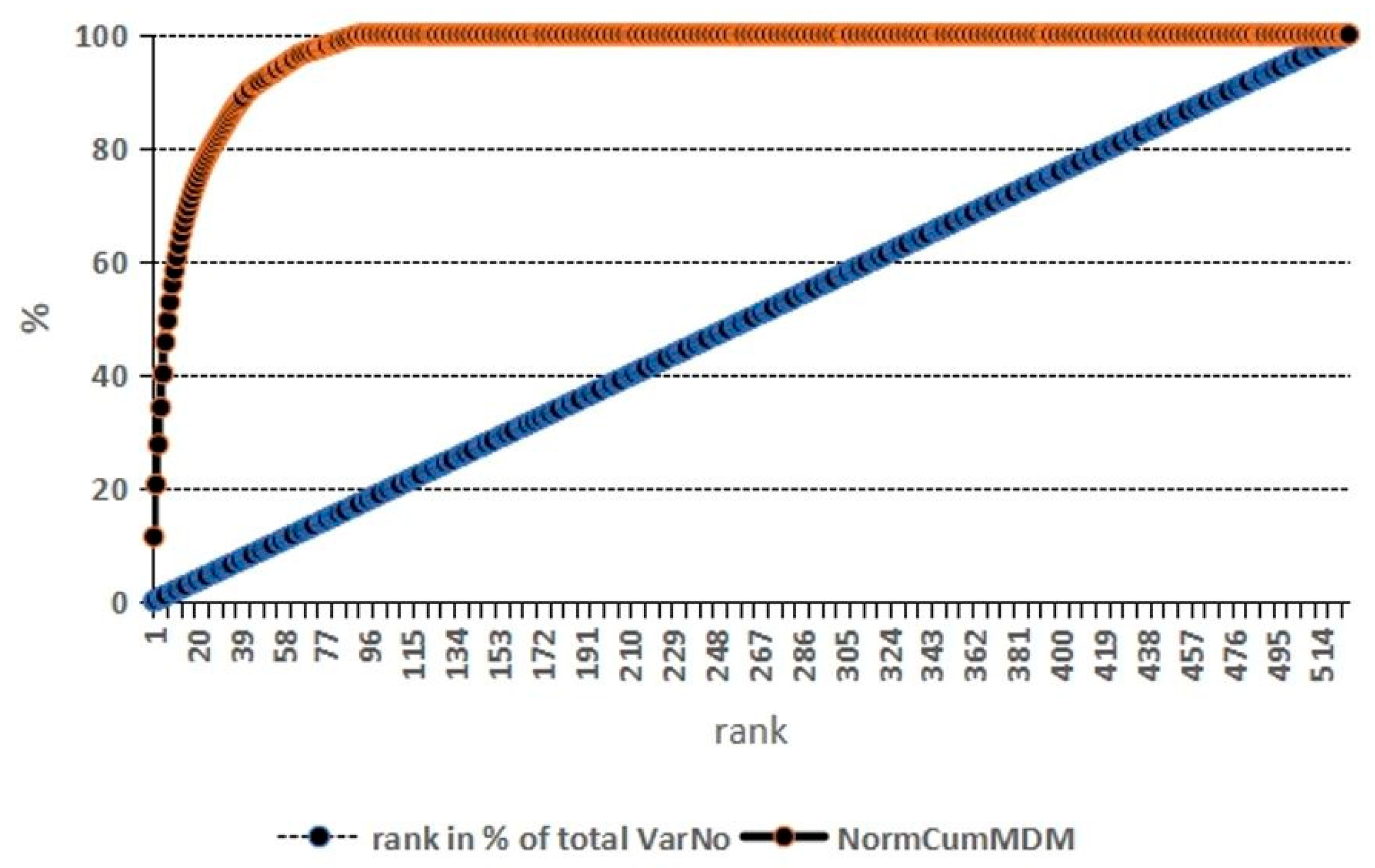
2.2.4. Random Forest Analysis of Proteomics Parameters with Respect to Group Assignment
3. Discussion
3.1. Fontan Is Never Normal—‘Good’ Fontan Patients Exhibit a Specific Proteomics Phenotype
3.2. Four Proteins as Inflammatory Biomarkers at Different Levels in Fontan Patients
3.2.1. Elevated SDC1—Inflammation, Cardiac and Hepatic Fibrosis, Angiogenesis
3.2.2. Elevated GLPA—Inflammation, Mutagenicity, Red Blood Cell Structure
3.2.3. Decreased LIF—While Pleiotropic, Expression of Cardioprotection
3.2.4. Decreased NGF-β—Alteration of the Autonomic Nervous System, from Cognitive Development to Cardiac Rhythm Abnormalities, and Metalloproteinase Inhibitor
3.3. Combining Four Proteomics-Based Proteins with Traditional Biomarkers for a Global Picture
3.3.1. Uric Acid—Antioxidative Scavenger
3.3.2. γ-Glutamyl-Transferase (γGT)—Liver Disease, and Antioxidative Glutathione Status
3.3.3. Cholesterol—Fontan Dyslipidemia Requiring Further Lipidology Research
3.4. Limitations, Confounders
4. Materials and Methods
4.1. Study Design and Inclusion and Exclusion Criteria
4.2. Routine Examination
4.3. Proteomics Analysis
4.3.1. Samples and Protein Extraction and Labelling
4.3.2. Sample Incubation
4.4. Data Acquisition and Analysis
4.5. Pathway Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rychik, J.; Atz, A.M.; Celermajer, D.S.; Deal, B.J.; Gatzoulis, M.A.; Gewillig, M.H.; Hsia, T.-Y.; Hsu, D.T.; Kovacs, A.H.; McCrindle, B.W.; et al. Evaluation and Management of the Child and Adult with Fontan Circulation: A Scientific Statement From the American Heart Association. Circulation 2019, 140, e234. [Google Scholar] [CrossRef] [PubMed]
- D’Udekem, Y.; Kisamori, E.; Yerebakan, C. We Were Wrong on the Benefits of the Extra-Cardiac Fontan. Should We Go Back to the Lateral Tunnel? Interdiscip. Cardiovasc. Thorac. Surg. 2023, 37, ivad187. [Google Scholar] [CrossRef] [PubMed]
- Choussat, A.; Fontan, F. Selection Criteria for the Fontan Procedure. In Pediatric Cardiology; Churchill Livingstone: Edinburgh, UK, 1977. [Google Scholar]
- Stern, H.J. Fontan “Ten Commandments” Revisited and Revised. Pediatr. Cardiol. 2010, 31, 1131–1134. [Google Scholar] [CrossRef] [PubMed]
- Gewillig, M.; Brown, S.C.; Eyskens, B.; Heying, R.; Ganame, J.; Budts, W.; Gerche, A.L.; Gorenflo, M. The Fontan Circulation: Who Controls Cardiac Output? Interact. Cardiovasc. Thorac. Surg. 2010, 10, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Gewillig, M.; Brown, S.C. The Fontan Circulation after 45 Years: Update in Physiology. Heart 2016, 102, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Rychik, J. Path Taken in a Fontan Circulation: Room for Optimism in the Face of Uncertainty. Heart 2021, 107, 521–522. [Google Scholar] [CrossRef] [PubMed]
- Alsoufi, B.; Gillespie, S.; Kim, D.; Shashidharan, S.; Kanter, K.; Maher, K.; Kogon, B. The Impact of Dominant Ventricle Morphology on Palliation Outcomes of Single Ventricle Anomalies. Ann. Thorac. Surg. 2016, 102, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, M.L.; Pelletier, G.J.; Pourmoghadam, K.K.; Mesia, C.I.; Madan, N.; Stern, H.; Schwartz, R.; Murphy, J.D. Protocols Associated with No Mortality in 100 Consecutive Fontan Procedures. Eur. J. Cardiothorac. Surg. 2008, 33, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Malec, E.; Schmidt, C.; Lehner, A.; Januszewska, K. Results of the Fontan Operation with No Early Mortality in 248 Consecutive Patients. Kardiol. Pol. 2017, 75, 255–260. [Google Scholar] [CrossRef]
- Atz, A.M.; Zak, V.; Mahony, L.; Uzark, K.; D’agincourt, N.; Goldberg, D.J.; Williams, R.V.; Breitbart, R.E.; Colan, S.D.; Burns, K.M.; et al. Longitudinal Outcomes of Patients with Single Ventricle after the Fontan Procedure. J. Am. Coll. Cardiol. 2017, 69, 2735–2744. [Google Scholar] [CrossRef]
- Michel, M.; Zlamy, M.; Entenmann, A.; Pichler, K.; Scholl-Bürgi, S.; Karall, D.; Geiger, R.; Salvador, C.; Niederwanger, C.; Ohuchi, H. Impact of the Fontan Operation on Organ Systems. Cardiovasc. Hematol. Disord. Drug Targets 2019, 19, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Davey, B.T.; Toro-Salazar, O.H.; Gauthier, N.; Valente, A.M.; Elder, R.W.; Wu, F.M.; Berman, N.; Pollack, P.; Lee, J.H.; Rathod, R.H. Surveillance and Screening Practices of New England Congenital Cardiologists for Patients after the Fontan Operation. Congenit. Heart Dis. 2019, 14, 1013–1023. [Google Scholar] [CrossRef] [PubMed]
- Smaś-Suska, M.; Dłużniewska, N.; Weryński, P.; Pająk, J.; Płazak, W.; Olszowska, M.; Podolec, P.; Tomkiewicz-Pająk, L. What Determines the Quality of Life of Adult Patients after Fontan Procedure? Cardiol. J. 2018, 25, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Takken, T.; Tacken, M.H.P.; Blank, A.C.; Hulzebos, E.H.; Strengers, J.L.M.; Helders, P.J.M. Exercise Limitation in Patients with Fontan Circulation: A Review. J. Cardiovasc. Med. 2007, 8, 775–781. [Google Scholar] [CrossRef]
- Ohuchi, H.; Miyamoto, Y.; Yamamoto, M.; Ishihara, H.; Takata, H.; Miyazaki, A.; Yamada, O.; Yagihara, T. High Prevalence of Abnormal Glucose Metabolism in Young Adult Patients with Complex Congenital Heart Disease. Am. Heart J. 2009, 158, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, W.; Tan, M.; Yu, S.; Rocchini, A. Low Total, Low-Density Lipoprotein, High-Density Lipoprotein, and Non–High-Density Lipoprotein Cholesterol Levels in Patients with Complex Congenital Heart Disease after Fontan Palliation. J. Pediatr. 2013, 162, 1199–1204. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, W.; Tan, M.; Ostlund, R.E.; Yu, S.; Ma, L.; Rocchini, A. Altered Cholesterol Metabolism and Hypocholesterolemia in Patients with Single Ventricle Following Fontan Palliation. J. Pediatr. 2016, 171, 73–77. [Google Scholar] [CrossRef]
- Zyblewski, S.C.; Argraves, W.S.; Graham, E.M.; Slate, E.H.; Atz, A.M.; Bradley, S.M.; McQuinn, T.C.; Wilkerson, B.A.; Wing, S.B.; Argraves, K.M. Reduction in Postoperative High-Density Lipoprotein Cholesterol Levels in Children Undergoing the Fontan Operation. Pediatr. Cardiol. 2012, 33, 1154–1159. [Google Scholar] [CrossRef]
- Renaud, D.; Scholl-Bürgi, S.; Karall, D.; Michel, M. Comparative Metabolomics in Single Ventricle Patients after Fontan Palliation: A Strong Case for a Targeted Metabolic Therapy. Metabolites 2023, 13, 932. [Google Scholar] [CrossRef]
- Michel, M.; Dubowy, K.-O.; Zlamy, M.; Karall, D.; Adam, M.G.; Entenmann, A.; Keller, M.A.; Koch, J.; Odri Komazec, I.; Geiger, R.; et al. Targeted Metabolomic Analysis of Serum Phospholipid and Acylcarnitine in the Adult Fontan Patient with a Dominant Left Ventricle. Ther. Adv. Chronic Dis. 2020, 11, 204062232091603. [Google Scholar] [CrossRef]
- Michel, M.; Dubowy, K.-O.; Entenmann, A.; Karall, D.; Adam, M.G.; Zlamy, M.; Odri Komazec, I.; Geiger, R.; Niederwanger, C.; Salvador, C.; et al. Targeted Metabolomic Analysis of Serum Amino Acids in the Adult Fontan Patient with a Dominant Left Ventricle. Sci. Rep. 2020, 10, 8930. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, S.W.; Shimada-Takaura, K.; Jong, C.J.; Ito, T.; Takahashi, K. Impaired Energy Metabolism of the Taurine-deficient Heart. Amino Acids 2016, 48, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.M.; Toni, L.S.; Miyano, C.A.; Sparagna, G.C.; Jonscher, R.; Phillips, E.K.; Karimpour-Fard, A.; Chapman, H.L.; Baybayon-Grandgeorge, A.N.; Pietra, A.E.; et al. Cardiac Transcriptome Remodeling and Impaired Bioenergetics in Single-Ventricle Congenital Heart Disease. JACC Basic Transl. Sci. 2023, 8, 258. [Google Scholar] [CrossRef] [PubMed]
- Thomson, L.M.; Mancuso, C.A.; Wolfe, K.R.; Khailova, L.; Niemiec, S.; Ali, E.; DiMaria, M.; Mitchell, M.; Twite, M.; Morgan, G.; et al. The Proteomic Fingerprint in Infants with Single Ventricle Heart Disease in the Interstage Period: Evidence of Chronic Inflammation and Widespread Activation of Biological Networks. Front. Pediatr. 2023, 11, 1308700. [Google Scholar] [CrossRef] [PubMed]
- Lopaschuk, G.D.; Persad, K.L. Failure to Launch: Impaired Cardiac Mitochondrial Metabolic Development in Newborns with Single Ventricle Heart Failure. JACC Basic Transl. Sci. 2023, 8, 280–282. [Google Scholar] [CrossRef] [PubMed]
- UniProt. Available online: https://www.uniprot.org/ (accessed on 31 January 2024).
- Michel, M.; Laser, K.T.; Dubowy, K.-O.; Scholl-Bürgi, S.; Michel, E. Metabolomics and Random Forests in Patients with Complex Congenital Heart Disease. Front. Cardiovasc. Med. 2022, 9, 994068. [Google Scholar] [CrossRef]
- Garcia, A.M.; Beatty, J.-T.; Nakano, S.J. Heart Failure in Single Right Ventricle Congenital Heart Disease: Physiological and Molecular Considerations. Am. J. Physiol. Heart Circ. Physiol. 2020, 318, H947–H965. [Google Scholar] [CrossRef] [PubMed]
- Wolff, D.; van Melle, J.P.; Willems, T.P.; Bartelds, B.; Ploegstra, M.-J.; Hillege, H.; Ebels, T.; Berger, R.M.F. N-Terminal pro-Brain Natriuretic Peptide Serum Levels Reflect Attrition of the Fontan Circulation. Cardiol. Young 2020, 30, 753–760. [Google Scholar] [CrossRef]
- Meyer, S.L.; Wolff, D.; Ridderbos, F.-J.S.; Eshuis, G.; Hillege, H.; Willems, T.P.; Ebels, T.; van Melle, J.P.; Berger, R.M.F. GDF-15 (Growth Differentiation Factor 15) Is Associated with Hospitalization and Mortality in Patients with a Fontan Circulation. J. Am. Heart Assoc. 2020, 9, e015521. [Google Scholar] [CrossRef]
- Buendía Fuentes, F.; Jover Pastor, P.; Arnau Vives, M.Á.; Lozano Edo, S.; Rodríguez Serrano, M.; Aguero, J.; Osa Sáez, A.; Conde Amiel, I.; Aguilera Sancho-Tello, V.; Martínez-Dolz, L.; et al. CA125: A New Biomarker in Patients with Fontan Circulation. Rev. Esp. Cardiol. Engl. Ed. 2023, 76, 112–120. [Google Scholar] [CrossRef]
- Renaud, D.; Michel, M. Biomarkers for Fontan Circulation and Comorbidities—A Scoping Review Protocol. 2024. Available online: https://doi.org/10.17605/OSF.IO/NVEMW (accessed on 2 May 2024).
- Gopal, S. Syndecans in Inflammation at a Glance. Front. Immunol. 2020, 11, 227. [Google Scholar] [CrossRef] [PubMed]
- Khoory, J.; Estanislau, J.; Elkhal, A.; Lazaar, A.; Melhorn, M.I.; Brodsky, A.; Illigens, B.; Hamachi, I.; Kurishita, Y.; Ivanov, A.R.; et al. Ligation of Glycophorin A Generates Reactive Oxygen Species Leading to Decreased Red Blood Cell Function. PLoS ONE 2016, 11, e0141206. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, M.M.; de la Puente, P. Leukemia Inhibitory Factor: An Important Cytokine in Pathologies and Cancer. Biomolecules 2022, 12, 217. [Google Scholar] [CrossRef] [PubMed]
- Minnone, G.; De Benedetti, F.; Bracci-Laudiero, L. NGF and Its Receptors in the Regulation of Inflammatory Response. Int. J. Mol. Sci. 2017, 18, 1028. [Google Scholar] [CrossRef] [PubMed]
- Ruparelia, N.; Chai, J.T.; Fisher, E.A.; Choudhury, R.P. Inflammatory Processes in Cardiovascular Disease: A Route to Targeted Therapies. Nat. Rev. Cardiol. 2017, 14, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Adamo, L.; Rocha-Resende, C.; Prabhu, S.D.; Mann, D.L. Reappraising the Role of Inflammation in Heart Failure. Nat. Rev. Cardiol. 2020, 17, 269–285. [Google Scholar] [CrossRef] [PubMed]
- Ashley, N.T.; Weil, Z.M.; Nelson, R.J. Inflammation: Mechanisms, Costs, and Natural Variation. Annu. Rev. Ecol. Evol. Syst. 2012, 43, 385–406. [Google Scholar] [CrossRef]
- Wienecke, L.M.; Cohen, S.; Bauersachs, J.; Mebazaa, A.; Chousterman, B.G. Immunity and Inflammation: The Neglected Key Players in Congenital Heart Disease? Heart Fail. Rev. 2021, 27, 1957. [Google Scholar] [CrossRef]
- Stosio, M.; Ruszkowski, J.; Mikosik-Roczyńska, A.; Haponiuk, I.; Witkowski, J.M. The Significance of Neonatal Thymectomy for Shaping the Immune System in Children with Congenital Heart Defects. Kardiochirurgia Torakochirurgia Pol. Pol. J. Cardio-Thorac. Surg. 2017, 14, 258–262. [Google Scholar] [CrossRef]
- Suriany, S.; Liu, H.; Cheng, A.L.; Wenby, R.; Patel, N.; Badran, S.; Meiselman, H.J.; Denton, C.; Coates, T.D.; Wood, J.C.; et al. Decreased Erythrocyte Aggregation in Glenn and Fontan: Univentricular Circulation as a Rheologic Disease Model. Pediatr. Res. 2024, 1, 1–11. [Google Scholar] [CrossRef]
- Ohuchi, H.; Hasegawa, S.; Yasuda, K.; Yamada, O.; Ono, Y.; Echigo, S. Severely Impaired Cardiac Autonomic Nervous Activity After the Fontan Operation. Circulation 2001, 104, 1513–1518. [Google Scholar] [CrossRef]
- Davos, C.H.; Francis, D.P.; Leenarts, M.F.E.; Yap, S.-C.; Li, W.; Davlouros, P.A.; Wensel, R.; Coats, A.J.S.; Piepoli, M.; Sreeram, N.; et al. Global Impairment of Cardiac Autonomic Nervous Activity Late After the Fontan Operation. Circulation 2003, 108, II-180. [Google Scholar] [CrossRef]
- Tkachenko, E.; Rhodes, J.M.; Simons, M. Syndecans. Circ. Res. 2005, 96, 488–500. [Google Scholar] [CrossRef]
- Miftode, R.-S.; Şerban, I.-L.; Timpau, A.-S.; Miftode, I.-L.; Ion, A.; Buburuz, A.-M.; Costache, A.-D.; Costache, I.-I. Syndecan-1: A Review on Its Role in Heart Failure and Chronic Liver Disease Patients’ Assessment. Cardiol. Res. Pract. 2019, 2019, 4750580. [Google Scholar] [CrossRef] [PubMed]
- Schellings, M.W.M.; Vanhoutte, D.; van Almen, G.C.; Swinnen, M.; Leenders, J.J.G.; Kubben, N.; van Leeuwen, R.E.W.; Hofstra, L.; Heymans, S.; Pinto, Y.M. Syndecan-1 Amplifies Angiotensin II-Induced Cardiac Fibrosis. Hypertension 2010, 55, 249–256. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Y.; Zheng, J.; Song, D.; Zheng, S.; Ren, L.; Wang, Y.; Yao, Y.; Wang, Y.; Liu, Y.; et al. Syndecan-1 as an Independent Risk Factor for the Incidence of Adverse Cardiovascular Events in Patients Having Stage C and D Heart Failure with Non-Ischemic Dilated Cardiomyopathy. Clin. Chim. Acta Int. J. Clin. Chem. 2019, 490, 63–68. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. Syndecan-1. Hypertension 2010, 55, 233–235. [Google Scholar] [CrossRef] [PubMed]
- Regős, E.; Abdelfattah, H.H.; Reszegi, A.; Szilák, L.; Werling, K.; Szabó, G.; Kiss, A.; Schaff, Z.; Kovalszky, I.; Baghy, K. Syndecan-1 Inhibits Early Stages of Liver Fibrogenesis by Interfering with TGFβ1 Action and Upregulating MMP. Matrix Biol. J. Int. Soc. Matrix Biol. 2018, 68, 474–489. [Google Scholar] [CrossRef] [PubMed]
- Reitsma, S.; Slaaf, D.W.; Vink, H.; van Zandvoort, M.A.M.J.; oude Egbrink, M.G.A. The Endothelial Glycocalyx: Composition, Functions, and Visualization. Pflug. Arch. 2007, 454, 345–359. [Google Scholar] [CrossRef]
- Kouwenberg, M.; Rops, A.; Bebber, M.B.; Diepeveen, L.; Götte, M.; Hilbrands, L.; van der Vlag, J. Role of Syndecan-1 in the Interaction between Dendritic Cells and T Cells. PLoS ONE 2020, 15, e0230835. [Google Scholar] [CrossRef]
- Angsana, J.; Chen, J.; Smith, S.; Xiao, J.; Wen, J.; Liu, L.; Haller, C.A.; Chaikof, E.L. Syndecan-1 Modulates the Motility and Resolution Responses of Macrophages. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Bruegger, D.; Brettner, F.; Rossberg, I.; Nussbaum, C.; Kowalski, C.; Januszewska, K.; Becker, B.F.; Chappell, D. Acute Degradation of the Endothelial Glycocalyx in Infants Undergoing Cardiac Surgical Procedures. Ann. Thorac. Surg. 2015, 99, 926–931. [Google Scholar] [CrossRef]
- Hahn, R.G.; Zdolsek, M.; Zdolsek, J. Plasma Concentrations of Syndecan-1 Are Dependent on Kidney Function. Acta Anaesthesiol. Scand. 2021, 65, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Poole, J. Red Cell Antigens on Band 3 and Glycophorin A. Blood Rev. 2000, 14, 31–43. [Google Scholar] [CrossRef]
- Hollox, E.J.; Louzada, S. Genetic Variation of Glycophorins and Infectious Disease. Immunogenetics 2023, 75, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Ouagari, K.E.; Teissié, J.; Benoist, H. Glycophorin A Protects K562 Cells from Natural Killer Cell Attack: Role of Oligosaccharides. J. Biol. Chem. 1995, 270, 26970–26975. [Google Scholar] [CrossRef]
- Jensen, R.H.; Reynolds, J.C.; Robbins, J.; Bigbee, W.L.; Grant, S.G.; Langlois, R.G.; Pineda, J.D.; Lee, T.; Barker, W.C. Glycophorin A as a Biological Dosimeter for Radiation Dose to the Bone Marrow from Iodine. Radiat. Res. 1997, 147, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Takaro, T.K.; Engel, L.S.; Keifer, M.; Bigbee, W.L.; Kavanagh, T.J.; Checkoway, H. Glycophorin A Is a Potential Biomarker for the Mutagenic Effects of Pesticides. Int. J. Occup. Environ. Health 2004, 10, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Tomkiewicz-Pajak, L.; Plazak, W.; Kolcz, J.; Pajak, J.; Kopec, G.; Dluzniewska, N.; Olszowska, M.; Moryl-Bujakowska, A.; Podolec, P. Iron Deficiency and Hematological Changes in Adult Patients after Fontan Operation. J. Cardiol. 2014, 64, 384–389. [Google Scholar] [CrossRef]
- Nicola, N.A.; Babon, J.J. Leukemia Inhibitory Factor (LIF). Cytokine Growth Factor Rev. 2015, 26, 533–544. [Google Scholar] [CrossRef]
- Rose-John, S. Interleukin-6 Family Cytokines. Cold Spring Harb. Perspect. Biol. 2018, 10, a028415. [Google Scholar] [CrossRef] [PubMed]
- Ulich, T.R.; Fann, M.J.; Patterson, P.H.; Williams, J.H.; Samal, B.; Del Castillo, J.; Yin, S.; Guo, K.; Remick, D.G. Intratracheal Injection of LPS and Cytokines. V. LPS Induces Expression of LIF and LIF Inhibits Acute Inflammation. Am. J. Physiol. Lung Cell. Mol. Physiol. 1994, 267, L442–L446. [Google Scholar] [CrossRef] [PubMed]
- Fischer, P.; Hilfiker-Kleiner, D. Survival Pathways in Hypertrophy and Heart Failure: The Gp130-STAT3 Axis. Basic Res. Cardiol. 2007, 102, 393–411. [Google Scholar] [CrossRef] [PubMed]
- Zouein, F.A.; Kurdi, M.; Booz, G.W. LIF and the Heart: Just Another Brick in the Wall? Eur. Cytokine Netw. 2013, 24, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Takano, H.; Mizukami, M.; Akazawa, H.; Qin, Y.; Toko, H.; Sakamoto, M.; Minamino, T.; Nagai, T.; Komuro, I. Leukemia Inhibitory Factor Enhances Survival of Cardiomyocytes and Induces Regeneration of Myocardium After Myocardial Infarction. Circulation 2003, 108, 748–753. [Google Scholar] [CrossRef] [PubMed]
- Florholmen, G.; Thoresen, G.H.; Rustan, A.C.; Jensen, J.; Christensen, G.; Aas, V. Leukaemia Inhibitory Factor Stimulates Glucose Transport in Isolated Cardiomyocytes and Induces Insulin Resistance after Chronic Exposure. Diabetologia 2006, 49, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Florholmen, G.; Aas, V.; Rustan, A.C.; Lunde, P.K.; Straumann, N.; Eid, H.; Ødegaard, A.; Dishington, H.; Andersson, K.B.; Christensen, G. Leukemia Inhibitory Factor Reduces Contractile Function and Induces Alterations in Energy Metabolism in Isolated Cardiomyocytes. J. Mol. Cell. Cardiol. 2004, 37, 1183–1193. [Google Scholar] [CrossRef] [PubMed]
- Saraf, A.; De Staercke, C.; Everitt, I.; Haouzi, A.; Ko, Y.-A.; Jennings, S.; Kim, J.H.; Rodriguez, F.H.; Kalogeropoulos, A.P.; Quyyumi, A.; et al. Biomarker Profile in Stable Fontan Patients. Int. J. Cardiol. 2020, 305, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Ohuchi, H.; Negishi, J.; Hayama, Y.; Miike, H.; Suzuki, D.; Nakajima, K.; Konagai, N.; Iwasa, T.; Sakaguchi, H.; Kurosaki, K.; et al. Abnormal Glucose Metabolism in Patients with Fontan Circulation: Unique Characteristics and Associations with Fontan Pathophysiology. Am. Heart J. 2019, 216, 125–135. [Google Scholar] [CrossRef]
- Clode, M.; Tran, D.; Majumdar, A.; Ayer, J.; Ferrie, S.; Cordina, R. Nutritional Considerations for People Living with a Fontan Circulation: A Narrative Review. Cardiol. Young 2024, 23, 1–12. [Google Scholar] [CrossRef]
- Tran, D.L.; Celermajer, D.S.; Ayer, J.; Grigg, L.; Clendenning, C.; Hornung, T.; Justo, R.; Davis, G.M.; d’Udekem, Y.; Cordina, R. The “Super-Fontan” Phenotype: Characterizing Factors Associated with High Physical Performance. Front. Cardiovasc. Med. 2021, 8, 764273. [Google Scholar] [CrossRef] [PubMed]
- Tuon, L.; Tramontin, N.S.; Custódio, I.; Comim, V.H.; Costa, B.; Tietbohl, L.T.W.; Muller, A.P. Serum Biomarkers to Mild Cognitive Deficits in Children and Adolescents. Mol. Neurobiol. 2023, 60, 7080–7087. [Google Scholar] [CrossRef] [PubMed]
- Syed, Z.; Dudbridge, F.; Kent, L. An Investigation of the Neurotrophic Factor Genes GDNF, NGF, and NT3 in Susceptibility to ADHD. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2007, 144B, 375–378. [Google Scholar] [CrossRef] [PubMed]
- Than, U.T.T.; Nguyen, L.T.; Nguyen, P.H.; Nguyen, X.-H.; Trinh, D.P.; Hoang, D.H.; Nguyen, P.A.T.; Dang, V.D. Inflammatory Mediators Drive Neuroinflammation in Autism Spectrum Disorder and Cerebral Palsy. Sci. Rep. 2023, 13, 22587. [Google Scholar] [CrossRef]
- Calderon, J.; Newburger, J.W.; Rollins, C.K. Neurodevelopmental and Mental Health Outcomes in Patients with Fontan Circulation: A State-of-the-Art Review. Front. Pediatr. 2022, 10, 826349. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhao, Y.; Dong, X.; Li, C.; Zhou, L.; Zou, C.; Li, X.; Zhou, N.; Liu, J.; Sun, Y.; et al. The Occurrence of Valvular Atrial Fibrillation: Involvement of NGF/TrKA Signaling Pathway. J. Investig. Surg. 2021, 34, 1379–1386. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Hong, S.Y.; Wi, J.; Lee, D.L.; Joung, B.; Lee, M.H.; Pak, H.-N. Catheter Ablation of Atrial Fibrillation Raises the Plasma Level of NGF-β Which Is Associated with Sympathetic Nerve Activity. Yonsei Med. J. 2015, 56, 1530–1537. [Google Scholar] [CrossRef] [PubMed]
- Becker, B.F.; Reinholz, N.; Leipert, B.; Ruschke, P.; Permanetter, B.; Gerlach, E. Role of Uric Acid as an Endogenous Radical Scavenger and Antioxidant. Chest 1991, 100, 176S–181S. [Google Scholar] [CrossRef]
- Ames, B.N.; Cathcart, R.; Schwiers, E.; Hochstein, P. Uric Acid Provides an Antioxidant Defense in Humans against Oxidant- and Radical-Caused Aging and Cancer: A Hypothesis. Proc. Natl. Acad. Sci. USA 1981, 78, 6858–6862. [Google Scholar] [CrossRef]
- Becker, B.F. Towards the Physiological Function of Uric Acid. Free Radic. Biol. Med. 1993, 14, 615–631. [Google Scholar] [CrossRef]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef] [PubMed]
- Ohuchi, H.; Negishi, J.; Hayama, Y.; Sasaki, O.; Taniguchi, Y.; Noritake, K.; Miyazaki, A.; Yamada, O. Hyperuricemia Reflects Global Fontan Pathophysiology and Associates with Morbidity and Mortality in Patients after the Fontan Operation. Int. J. Cardiol. 2015, 184, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Koenig, G.; Seneff, S. Gamma-Glutamyltransferase: A Predictive Biomarker of Cellular Antioxidant Inadequacy and Disease Risk. Dis. Markers 2015, 2015, 818570. [Google Scholar] [CrossRef] [PubMed]
- Kunutsor, S.K. Gamma-Glutamyltransferase—Friend or Foe Within? Liver Int. 2016, 36, 1723–1734. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]


| Variable | Unit | Patient | Control | p-Value |
|---|---|---|---|---|
| minimum SpO2 | % | 90 ± 3 | 98 ± 1 | <0.00001 |
| maximum SpO2 | % | 93 ± 3 | 99 ± 1 | <0.00001 |
| VO2AT | ml/kg/min | 24.5 ± 4.9 | 30.1 ± 3.6 | <0.00001 |
| VO2max | ml/kg/min | 28.8 ± 10.1 | 45.7 ± 6.4 | <0.00001 |
| heart rate at rest | 1/min | 83 ± 17 | 86 ± 19 | 0.08 |
| BP systolic at rest | mmHg | 123 ± 10 | 119 ± 12 | 0.04 |
| BP diastolic at rest | mmHg | 68 ± 8 | 71 ± 8 | 0.04 |
| hematocrit | % | 47 ± 5 | 39 ± 4 | <0.00001 |
| hemoglobin | g/dL | 16.4 ± 2.1 | 12.7 ± 1.4 | <0.00001 |
| platelet count | 1000/nL | 171 ± 73 | 279 ± 88 | 0.0002 |
| leucocytes | 1/nL | 6.7 ± 3.2 | 7.2 ± 2.6 | 0.23 |
| γGT | U/L | 86 ± 43 | 35 ± 19 | 0.00002 |
| alkaline phosphatase | U/l | 103 ± 53 | 99 ± 73 | 0.2 |
| ALT | U/L | 39 ± 11 | 31 ± 10 | 0.04 |
| AST | U/L | 35 ± 8 | 32 ± 8 | 0.12 |
| GLDH | U/L | 3.7 ± 1.9 | 3.5 ± 1.5 | 0.26 |
| total bilirubin | mg/dL | 1.22 ± 0.67 | 0.3 ± 0.29 | <0.00001 |
| CRP | mg/dL | 0.18 ± 0.2 | 0.16 ± 0.14 | 0.47 |
| fibrinogen | mg/dL | 239 ± 78 | 259 ± 57 | 0.17 |
| antithrombin III | % | 101 ± 11 | 105 ± 9 | 0.38 |
| INR | 2.1 ± 0.7 | 1 ± 0.04 | <0.00001 | |
| PTT | sec | 35 ± 6 | 27 ± 4 | 0.00004 |
| creatine kinase | U/L | 129 ± 66 | 95 ± 38 | 0.09 |
| uric acid | mg/dL | 5.9 ± 1.4 | 3.9 ± 1.3 | 0.0003 |
| creatinine | mg/dL | 0.8 ± 0.12 | 0.53 ± 0.18 | <0.00001 |
| urea | mg/dL | 32 ± 7 | 23 ± 9 | 0.0008 |
| triglycerides | mg/dL | 128 ± 86 | 47 ± 22 | 0.0003 |
| total cholesterol | mg/dL | 145 ± 27 | 149 ± 34 | 0.77 |
| HDL-cholesterol | mg/dL | 42 ± 15 | 51 ± 22 | 0.03 |
| non-HDL-cholesterol | mg/dL | 85 ± 25 | 73 ± 21 | 0.2 |
| total protein | g/dL | 7.2 ± 0.5 | 7.0 ± 0.7 | 0.31 |
| albumin | mg/dL | 4145 ± 492 | 4215 ± 218 | 0.64 |
| NT-proBNP * | pg/mL | 53 ± 69 | 41 ± 32 | 0.41 |
| Protein | Antibody ID | Uniprot-Entry-Name | Uniprot ID | logFC | AveExp | adj. p-Value | HGNC |
|---|---|---|---|---|---|---|---|
| SDC1 | ab1576 | SDC1_HUMAN | P18827 | 0.96 | 11.14 | 2.2 × 10−2 | SDC1 |
| GLPA | ab1491 | GLPA_HUMAN | P02724 | 0.85 | 11.09 | 2.18 × 10−2 | GYPA |
| LIF | ab1725 | LIF_HUMAN | P15018 | −0.6 | 15.2 | 5.61 × 10−8 | LIF |
| NGF-beta | ab1602 | NGF_HUMAN | P01138 | −0.75 | 12.44 | 6.69 × 10−3 | NGF |
| Protein | Antibody ID | Uniprot-Entry-Name | Uniprot ID | logFC | AveExp | adj. p-Value | HGNC |
|---|---|---|---|---|---|---|---|
| LYAM3 | ab1570 | LYAM3_HUMAN | P16109 | 1.08 | 11.45 | 2.17 × 10−1 | SELP |
| CD28 | ab1420 | CD28_HUMAN | P10747 | 0.82 | 12.14 | 2.14 × 10−1 | CD28 |
| B3GA1 | ab1568 | B3GA1_HUMAN | Q9P2W7 | 0.75 | 11.25 | 6.28 × 10−2 | B3GAT1 |
| TNR8 | ab1423 | TNR8_HUMAN | P28908 | 0.73 | 10.92 | 7.14 × 10−2 | TNFRSF8 |
| IL18 | ab1511 | IL18_HUMAN | Q14116 | 0.69 | 11.19 | 2.17 × 10−1 | IL18 |
| CD33 | ab1562 | CD33_HUMAN | P20138 | 0.66 | 11.31 | 2.24 × 10−1 | CD33 |
| ITAE | ab1573 | ITAE_HUMAN | P38570 | 0.66 | 10.88 | 1.08 × 10−1 | ITGAE |
| IL2RB | ab1575 | IL2RB_HUMAN | P14784 | 0.65 | 11.18 | 1.13 × 10−1 | IL2RB |
| SLAF1 | ab2132 | SLAF1_HUMAN | Q13291 | 0.63 | 10.35 | 4.10 × 10−1 | SLAMF1 |
| FCG2A | ab1561 | FCG2A_HUMAN | P12318 | 0.61 | 10.85 | 2.46 × 10−1 | FCGR2A |
| PRTN3 | ab1501 | PRTN3_HUMAN | P24158 | 0.60 | 11.04 | 2.10 × 10−1 | PRTN3 |
| BDNF | ab1502 | BDNF_HUMAN | P23560 | 0.55 | 10.96 | 2.21 × 10−1 | BDNF |
| CRLF2 | ab1498 | CRLF2_HUMAN | Q9HC73 | 0.53 | 11.73 | 2.47 × 10−1 | CRLF2 |
| ENTP1 | ab1111 | ENTP1_HUMAN | P49961 | 0.53 | 11.36 | 5.89 × 10−1 | ENTPD1 |
| VGFR1 | ab1618 | VGFR1_HUMAN | P17948 | 0.50 | 11.63 | 2.46 × 10−1 | FLT1 |
| S10A8/9 | ab1624 | −0.42 | 14.37 | 3.94 × 10−2 | |||
| pan HLA-class II | ab1496 | −0.46 | 9.86 | 4.53 × 10−2 | |||
| SDF1 | ab2491 | SDF1_HUMAN | P48061 | −0.46 | 11.20 | 5.39 × 10−3 | CXCL12 |
| TNFB | ab1921 | TNFB_HUMAN | P01374 | −0.50 | 12.00 | 8.04 × 10−2 | LTA |
| Lactoferin | ab1611 | TRFL_HUMAN | P02788 | −0.52 | 13.03 | 8.10 × 10−2 | LTF |
| PLF4 | ab1841 | PLF4_HUMAN | P02776 | −0.56 | 12.51 | 8.61 × 10−1 | PF4 |
| CD53 | ab1453 | CD53_HUMAN | P19397 | −0.56 | 13.55 | 3.50 × 10−1 | CD53 |
| I17RA | ab2407 | I17RA_HUMAN | Q96F46 | −0.61 | 12.62 | 2.47 × 10−1 | IL17RA |
| TNR6 | ab1478 | TNR6_HUMAN | P25445 | −0.66 | 14.34 | 1.55 × 10−1 | FAS |
| Random Forest | Classical Statistics | Protein | ||||
|---|---|---|---|---|---|---|
| Rank | Score | Rank (n = 64) | Fold Change | Adj. p | Rank (Only Adj. p < 0.05, n = 7) | |
| 1 | 0.0085 | 5 | −0.75 | 0.0067 | 3 | NGF-β |
| 2 | 0.0068 | 11 | −0.6 | 0.000000056 | 4 | LIF |
| 3 | 0.0052 | 28 | 0.36 | 0.25 | CCL14 | |
| 4 | 0.0048 | 21 | 0.44 | 0.22 | PD1L1 | |
| 5 | 0.0044 | 25 | −0.39 | 0.22 | CCL5 | |
| 6 | 0.0041 | 43 | −0.21 | 0.61 | CD38 | |
| 7 | 0.0028 | 19 | −0.46 | 0.0054 | 6 | SDF1 |
| 8 | 0.0024 | 25 | −0.2 | 0.76 | AMPN | |
| 9 | 0.0023 | 38 | −0.26 | 0.98 | LYAM1 | |
| 10 | 0.0018 | 23 | 0.42 | 0.25 | CADH2 | |
| 11 | 0.0017 | 28 | 0.36 | 0.51 | VCAM1 | |
| 12 | 0.0016 | 35 | 0.09 | 0.74 | CD27 | |
| 13 | 0.0015 | 1 | 1.08 | 0.98 | LYAM3 | |
| 14 | 0.0013 | 41 | −0.23 | 0.59 | DPP4 | |
| 15 | 0.0011 | 21 | 0.1 | 0.98 | CEAM1,3,5,6,8 | |
| 16 | 0.001 | 20 | −0.45 | 0.25 | BMP4 | |
| 17 | 0.001 | 30 | −0.34 | 0.55 | VGFR2 | |
| 18 | 0.001 | 20 | −0.45 | 0.62 | ITA2B (CD41a) | |
| 19 | 0.0009 | 28 | −0.36 | 0.22 | CD9 | |
| 20 | 0.0008 | 35 | −0.29 | 0.54 | IL34 | |
| 21 | 0.0008 | 9 | 0.63 | 0.41 | SLAF1 | |
| 22 | 0.0007 | 2 | 0.96 | 0.02 | 1 | SDC1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michel, M.; Renaud, D.; Schmidt, R.; Einkemmer, M.; Laser, L.V.; Michel, E.; Dubowy, K.O.; Karall, D.; Laser, K.T.; Scholl-Bürgi, S. Altered Serum Proteins Suggest Inflammation, Fibrogenesis and Angiogenesis in Adult Patients with a Fontan Circulation. Int. J. Mol. Sci. 2024, 25, 5416. https://doi.org/10.3390/ijms25105416
Michel M, Renaud D, Schmidt R, Einkemmer M, Laser LV, Michel E, Dubowy KO, Karall D, Laser KT, Scholl-Bürgi S. Altered Serum Proteins Suggest Inflammation, Fibrogenesis and Angiogenesis in Adult Patients with a Fontan Circulation. International Journal of Molecular Sciences. 2024; 25(10):5416. https://doi.org/10.3390/ijms25105416
Chicago/Turabian StyleMichel, Miriam, David Renaud, Ronny Schmidt, Matthias Einkemmer, Lea Valesca Laser, Erik Michel, Karl Otto Dubowy, Daniela Karall, Kai Thorsten Laser, and Sabine Scholl-Bürgi. 2024. "Altered Serum Proteins Suggest Inflammation, Fibrogenesis and Angiogenesis in Adult Patients with a Fontan Circulation" International Journal of Molecular Sciences 25, no. 10: 5416. https://doi.org/10.3390/ijms25105416






