Transcriptome Analysis of BAFF/BAFF-R System in Murine Nephrotoxic Serum Nephritis
Abstract
:1. Introduction
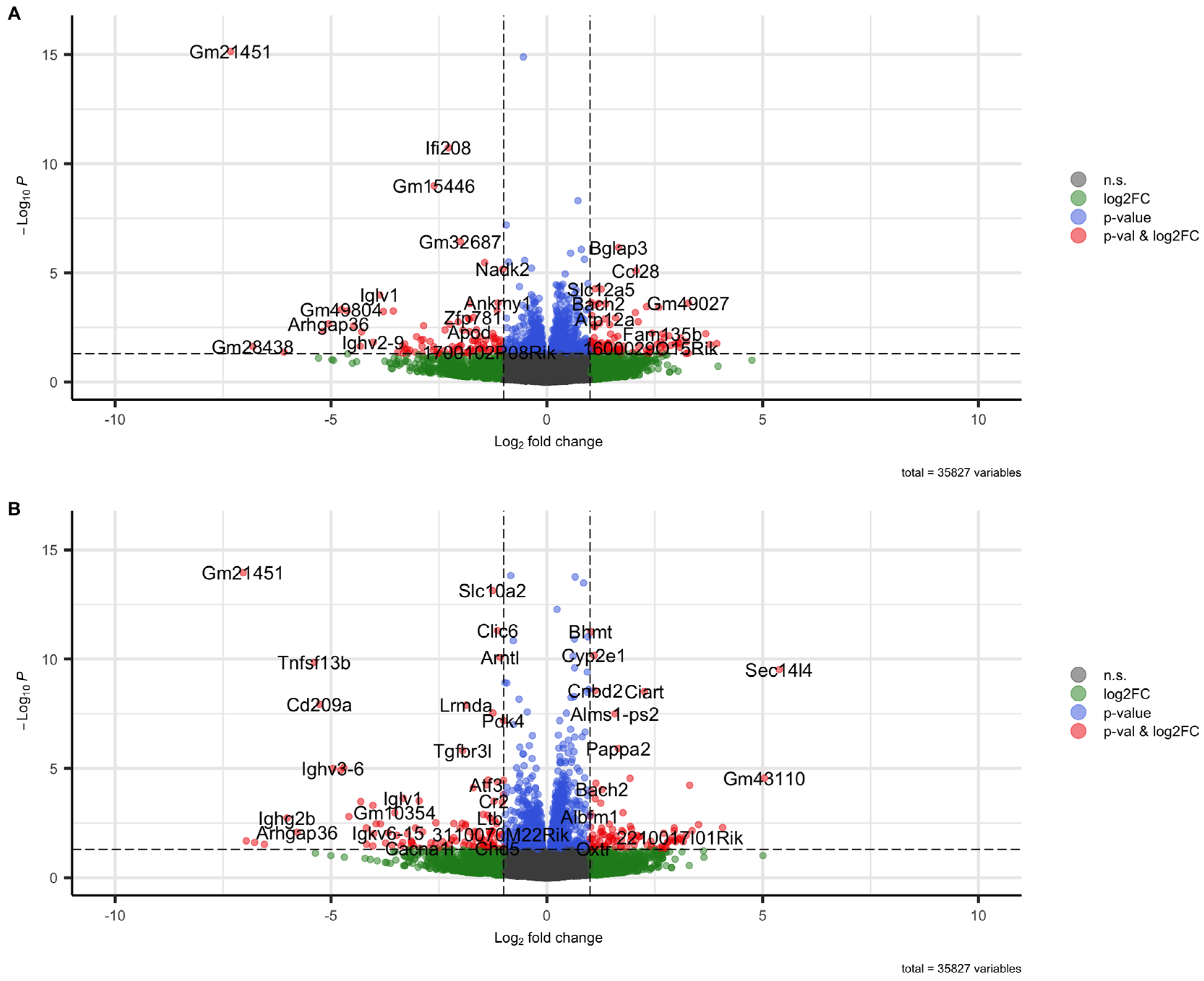
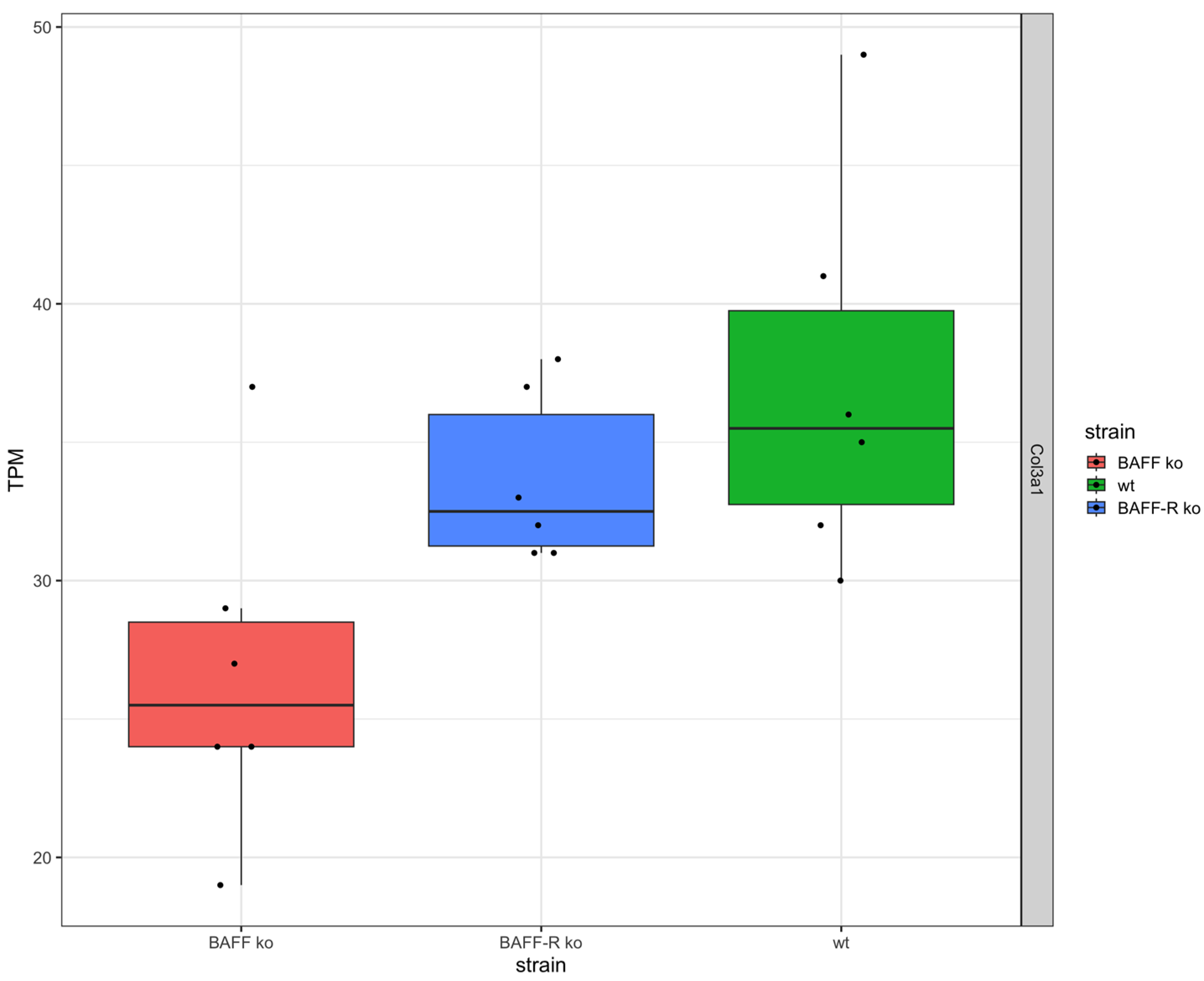
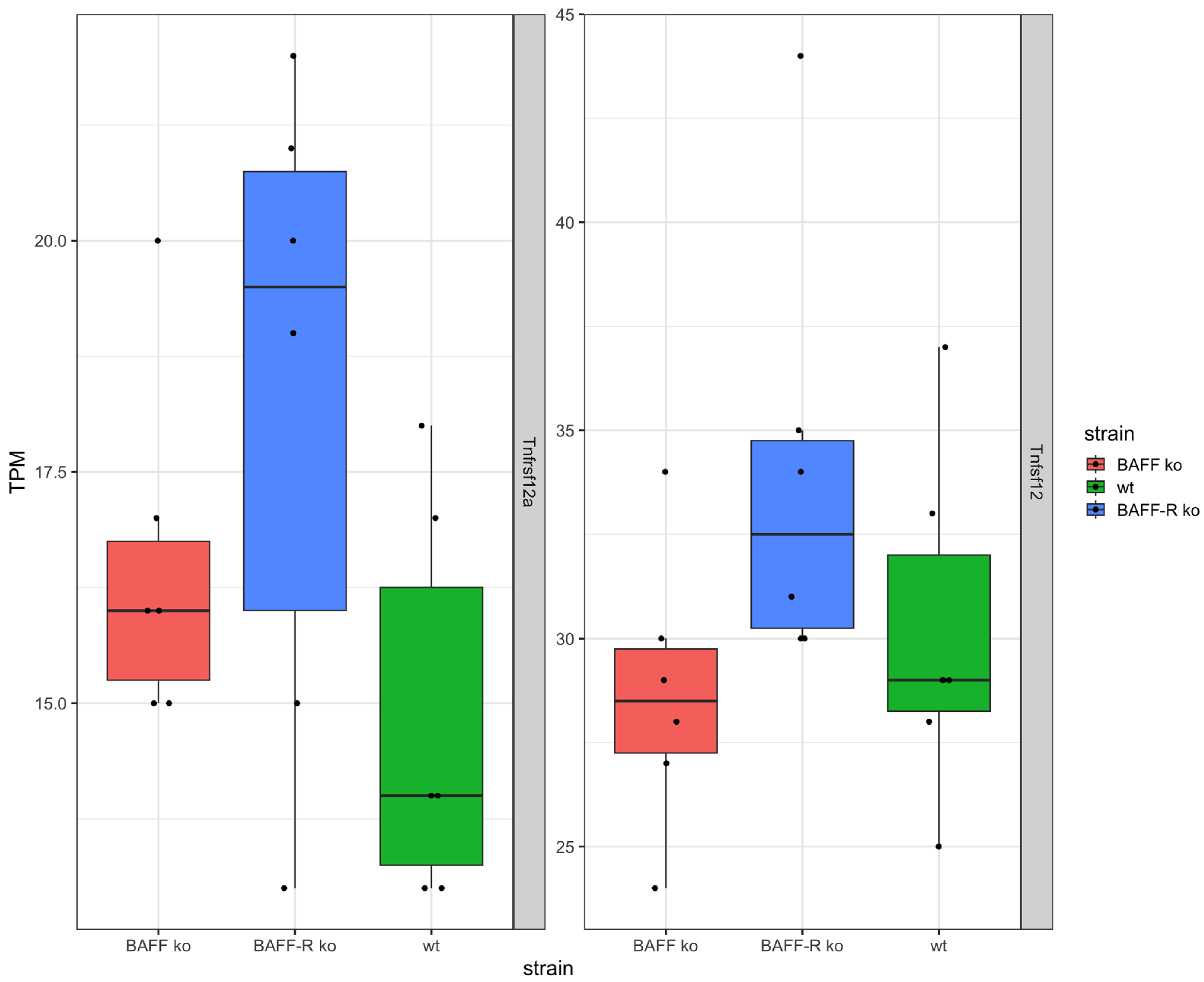
2. Results
3. Discussion
4. Materials and Methods
4.1. Ethical Statement
4.2. 3R Principle
4.3. Laboratory Animals
4.4. Experimental Study Design
4.5. Nephrotoxic Serum Nephritis (NTN)
4.6. RNA Isolation
4.7. RNA Sequencing
4.8. Bioinformatical and Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Imig, J.D.; Ryan, M.J. Immune and inflammatory role in renal disease. Compr. Physiol. 2013, 3, 957. [Google Scholar] [PubMed]
- Espi, M.; Koppe, L.; Fouque, D.; Thaunat, O. Chronic kidney disease-associated immune dysfunctions: Impact of protein-bound uremic retention solutes on immune cells. Toxins 2020, 12, 300. [Google Scholar] [CrossRef] [PubMed]
- Djudjaj, S.; Boor, P. Cellular and molecular mechanisms of kidney fibrosis. Mol. Asp. Med. 2019, 65, 16–36. [Google Scholar] [CrossRef] [PubMed]
- Kovesdy, C.P. Epidemiology of chronic kidney disease: An update 2022. Kidney Int. Suppl. 2022, 12, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.C.-T.; Zhang, Y.-Y.; Chan, M.K.-K.; Lam, W.W.-Y.; Chung, J.Y.-F.; Kang, W.; To, K.-F.; Lan, H.-Y.; Tang, P.M.-K. The emerging role of innate immunity in chronic kidney diseases. Int. J. Mol. Sci. 2020, 21, 4018. [Google Scholar] [CrossRef] [PubMed]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024, 105, S117–S314. [Google Scholar] [CrossRef] [PubMed]
- Webster, A.C.; Nagler, E.V.; Morton, R.L.; Masson, P. Chronic kidney disease. Lancet 2017, 389, 1238–1252. [Google Scholar] [CrossRef] [PubMed]
- Chadban, S.J.; Atkins, R.C. Glomerulonephritis. Lancet 2005, 365, 1797–1806. [Google Scholar] [CrossRef] [PubMed]
- Anders, H.-J.; Kitching, A.R.; Leung, N.; Romagnani, P. Glomerulonephritis: Immunopathogenesis and immunotherapy. Nat. Rev. Immunol. 2023, 23, 453–471. [Google Scholar] [CrossRef]
- Bikbov, B.; Purcell, C.A.; Levey, A.S.; Smith, M.; Abdoli, A.; Abebe, M.; Adebayo, O.M.; Afarideh, M.; Agarwal, S.K.; Agudelo-Botero, M. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef]
- Guo, Q.; Wu, S.; Xu, C.; Wang, J.; Chen, J. Global disease burden from acute glomerulonephritis 1990–2019. Kidney Int. Rep. 2021, 6, 2212–2217. [Google Scholar] [CrossRef]
- Burlingame, R.; Boey, M.; Starkebaum, G.; Rubin, R. The central role of chromatin in autoimmune responses to histones and DNA in systemic lupus erythematosus. J. Clin. Investig. 1994, 94, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Gewin, L.S. Renal fibrosis: Primacy of the proximal tubule. Matrix Biol. 2018, 68, 248–262. [Google Scholar] [CrossRef] [PubMed]
- Bonventre, J.V. Primary proximal tubule injury leads to epithelial cell cycle arrest, fibrosis, vascular rarefaction, and glomerulosclerosis. Kidney Int. Suppl. 2014, 4, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Bonventre, J.V. Maladaptive proximal tubule repair: Cell cycle arrest. Nephron Clin. Pract. 2014, 127, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Ferenbach, D.A.; Bonventre, J.V. Mechanisms of maladaptive repair after AKI leading to accelerated kidney ageing and CKD. Nat. Rev. Nephrol. 2015, 11, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Andrade, L.; Rodrigues, C.E.; Gomes, S.A.; Noronha, I.L. Acute kidney injury as a condition of renal senescence. Cell Transplant. 2018, 27, 739–753. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Humphreys, B.D.; Bonventre, J.V. Pathophysiology of acute kidney injury to chronic kidney disease: Maladaptive repair. Controv. Acute Kidney Inj. 2011, 174, 149–155. [Google Scholar]
- Qi, R.; Yang, C. Renal tubular epithelial cells: The neglected mediator of tubulointerstitial fibrosis after injury. Cell Death Dis. 2018, 9, 1126. [Google Scholar] [CrossRef]
- Takaori, K.; Nakamura, J.; Yamamoto, S.; Nakata, H.; Sato, Y.; Takase, M.; Nameta, M.; Yamamoto, T.; Economides, A.N.; Kohno, K. Severity and frequency of proximal tubule injury determines renal prognosis. J. Am. Soc. Nephrol. JASN 2016, 27, 2393. [Google Scholar] [CrossRef]
- O’sullivan, K.M.; Ford, S.L.; Longano, A.; Kitching, A.R.; Holdsworth, S.R. Intrarenal Toll-like receptor 4 and Toll-like receptor 2 expression correlates with injury in antineutrophil cytoplasmic antibody-associated vasculitis. Am. J. Physiol.-Ren. Physiol. 2018, 315, F1283–F1294. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Yiu, W.H.; Li, R.X.; Wu, H.J.; Wong, D.W.; Chan, L.Y.; Leung, J.C.; Lai, K.N.; Tang, S.C. The TLR4 antagonist CRX-526 protects against advanced diabetic nephropathy. Kidney Int. 2013, 83, 887–900. [Google Scholar] [CrossRef]
- Chen, J.-Q.; Szodoray, P.; Zeher, M. Toll-like receptor pathways in autoimmune diseases. Clin. Rev. Allergy Immunol. 2016, 50, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Zettel, K.; Korff, S.; Zamora, R.; Morelli, A.E.; Darwiche, S.; Loughran, P.A.; Elson, G.; Shang, L.; Salgado-Pires, S.; Scott, M.J. Toll-like receptor 4 on both myeloid cells and dendritic cells is required for systemic inflammation and organ damage after hemorrhagic shock with tissue trauma in mice. Front. Immunol. 2017, 8, 1672. [Google Scholar] [CrossRef] [PubMed]
- Hou, B.; Reizis, B.; DeFranco, A.L. Toll-like receptors activate innate and adaptive immunity by using dendritic cell-intrinsic and-extrinsic mechanisms. Immunity 2008, 29, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Lan, H.Y.; Chung, A.C.-K. TGF-β/Smad signaling in kidney disease. Semin. Nephrol. 2012, 32, 236–243. [Google Scholar] [CrossRef]
- Grech, A.P.; Amesbury, M.; Chan, T.; Gardam, S.; Basten, A.; Brink, R. TRAF2 differentially regulates the canonical and noncanonical pathways of NF-κB activation in mature B cells. Immunity 2004, 21, 629–642. [Google Scholar] [CrossRef] [PubMed]
- Li, P.-P.; Liu, D.-D.; Liu, Y.-J.; Song, S.-S.; Wang, Q.-T.; Chang, Y.; Wu, Y.-J.; Chen, J.-Y.; Zhao, W.-D.; Zhang, L.-L. BAFF/BAFF-R involved in antibodies production of rats with collagen-induced arthritis via PI3K-Akt-mTOR signaling and the regulation of paeoniflorin. J. Ethnopharmacol. 2012, 141, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Khan, W.N. B cell receptor and BAFF receptor signaling regulation of B cell homeostasis. J. Immunol. 2009, 183, 3561–3567. [Google Scholar] [CrossRef]
- Moore, P.A.; Belvedere, O.; Orr, A.; Pieri, K.; LaFleur, D.W.; Feng, P.; Soppet, D.; Charters, M.; Gentz, R.; Parmelee, D.; et al. BLyS: Member of the tumor necrosis factor family and B lymphocyte stimulator. Science 1999, 285, 260–263. [Google Scholar] [CrossRef]
- Schneider, P.; MacKay, F.; Steiner, V.; Hofmann, K.; Bodmer, J.-L.; Holler, N.; Ambrose, C.; Lawton, P.; Bixler, S.; Acha-Orbea, H.; et al. BAFF, a Novel Ligand of the Tumor Necrosis Factor Family, Stimulates B Cell Growth. J. Exp. Med. 1999, 189, 1747–1756. [Google Scholar] [CrossRef] [PubMed]
- Vincent, F.B.; Morand, E.F.; Mackay, F. BAFF and innate immunity: New therapeutic targets for systemic lupus erythematosus. Immunol. Cell Biol. 2012, 90, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, T.; Sato, S. The role of BAFF in autoimmune diseases. Jpn. J. Clin. Immunol. 2005, 28, 333–342. [Google Scholar] [CrossRef]
- Itotagawa, E.; Tomofuji, Y.; Kato, Y.; Konaka, H.; Tsujimoto, K.; Park, J.; Nagira, D.; Hirayama, T.; Jo, T.; Hirano, T. SLE stratification based on BAFF and IFN-I bioactivity for biologics and implications of BAFF produced by glomeruli in lupus nephritis. Rheumatology 2023, 62, 1988–1997. [Google Scholar] [CrossRef] [PubMed]
- Friebus-Kardash, J.; Branco, L.; Ribi, C.; Chizzolini, C.; Huynh-Do, U.; Dubler, D.; Roux-Lombard, P.; Dolff, S.; Kribben, A.; Eisenberger, U. Immune complexes containing serum B-cell activating factor and immunoglobulin G correlate with disease activity in systemic lupus erythematosus. Nephrol. Dial. Transplant. 2018, 33, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Fedoriw, Y.; Brenneman, E.K.; Truong, Y.K.; Kikly, K.; Vilen, B.J. BAFF induces tertiary lymphoid structures and positions T cells within the glomeruli during lupus nephritis. J. Immunol. 2017, 198, 2602–2611. [Google Scholar] [CrossRef] [PubMed]
- Nawata, A.; Nakayamada, S.; Hisano, S.; Miyazaki, Y.; Miyamoto, T.; Shiba, E.; Hisaoka, M.; Tanaka, Y. Differential expression of IFN-α, IL-12 and BAFF on renal immune cells and its relevance to disease activity and treatment responsiveness in patients with proliferative lupus nephritis. Lupus Sci. Med. 2023, 10, e000962. [Google Scholar] [CrossRef] [PubMed]
- Suso, J.; Posso-Osorio, I.; Jiménez, C.; Naranjo-Escobar, J.; Ospina, F.; Sánchez, A.; Cañas, C.; Tobón, G. Profile of BAFF and its receptors’ expression in lupus nephritis is associated with pathological classes. Lupus 2018, 27, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Lu, G.; Chen, X.; Chen, X.; Guo, N.; Li, W. BAFF is involved in the pathogenesis of IgA nephropathy by activating the TRAF6/NF-κB signaling pathway in glomerular mesangial cells. Mol. Med. Rep. 2020, 21, 795–805. [Google Scholar] [CrossRef]
- McCarthy, D.D.; Kujawa, J.; Wilson, C.; Papandile, A.; Poreci, U.; Porfilio, E.A.; Ward, L.; Lawson, M.A.; Macpherson, A.J.; McCoy, K.D. Mice overexpressing BAFF develop a commensal flora–dependent, IgA-associated nephropathy. J. Clin. Investig. 2011, 121, 3991–4002. [Google Scholar] [CrossRef]
- Ye, M.; Peng, Y.; Liu, C.; Yan, W.; Peng, X.; He, L.; Liu, H.; Liu, F. Vibration induces BAFF overexpression and aberrant O-Glycosylation of IgA1 in cultured human tonsillar mononuclear cells in IgA nephropathy. BioMed Res. Int. 2016, 2016, 9125960. [Google Scholar] [CrossRef] [PubMed]
- Zheng, N.; Fan, J.; Wang, B.; Wang, D.; Feng, P.; Yang, Q.; Yu, X. Expression profile of BAFF in peripheral blood from patients of IgA nephropathy: Correlation with clinical features and Streptococcus pyogenes infection. Mol. Med. Rep. 2017, 15, 1925–1935. [Google Scholar] [CrossRef]
- Zheng, N.; Wang, D.; Ming, H.; Zhang, H.; Yu, X. BAFF promotes proliferation of human mesangial cells through interaction with BAFF-R. BMC Nephrol. 2015, 16, 72. [Google Scholar] [CrossRef]
- Neusser, M.A.; Lindenmeyer, M.T.; Edenhofer, I.; Gaiser, S.; Kretzler, M.; Regele, H.; Segerer, S.; Cohen, C.D. Intrarenal production of B-cell survival factors in human lupus nephritis. Mod. Pathol. 2011, 24, 98–107. [Google Scholar] [CrossRef]
- Schwarting, A.; Relle, M.; Meineck, M.; Fohr, B.; Triantafyllias, K.; Weinmann, A.; Roth, W.; Weinmann-Menke, J. Renal tubular epithelial cell-derived BAFF expression mediates kidney damage and correlates with activity of proliferative lupus nephritis in mouse and men. Lupus 2018, 27, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Quach, T.D.; Dascalu, C.; Liu, Z.; Leung, T.; Byrne-Steele, M.; Pan, W.; Yang, Q.; Han, J.; Lesser, M. Belimumab promotes negative selection of activated autoreactive B cells in systemic lupus erythematosus patients. JCI Insight 2018, 3, e122525. [Google Scholar] [CrossRef]
- Stohl, W.; Kwok, A. Belimumab for the treatment of pediatric patients with lupus nephritis. Expert Opin. Biol. Ther. 2023, 23, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Bae, S.-C.; Bass, D.; Chu, M.; Egginton, S.; Gordon, D.; Roth, D.A.; Zheng, J.; Tanaka, Y. A pivotal phase III, randomised, placebo-controlled study of belimumab in patients with systemic lupus erythematosus located in China, Japan and South Korea. Ann. Rheum. Dis. 2018, 77, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Eddy, A.A.; López-Guisa, J.M.; Okamura, D.M.; Yamaguchi, I. Investigating mechanisms of chronic kidney disease in mouse models. Pediatr. Nephrol. 2012, 27, 1233–1247. [Google Scholar] [CrossRef]
- Kaneko, Y.; Nimmerjahn, F.; Madaio, M.P.; Ravetch, J.V. Pathology and protection in nephrotoxic nephritis is determined by selective engagement of specific Fc receptors. J. Exp. Med. 2006, 203, 789–797. [Google Scholar] [CrossRef]
- Xie, C.; Sharma, R.; Wang, H.; Zhou, X.J.; Mohan, C. Strain distribution pattern of susceptibility to immune-mediated nephritis. J. Immunol. 2004, 172, 5047–5055. [Google Scholar] [CrossRef] [PubMed]
- Nagai, H.; Yamada, H.; Nishigaki, T.; Nakazawa, M.; Koda, A. The susceptibility of experimental glomerulonephritis in six different strains of mice. J. Pharmacobiodyn. 1985, 8, 586–589. [Google Scholar] [CrossRef] [PubMed]
- Ougaard, M.K.E.; Kvist, P.H.; Jensen, H.E.; Hess, C.; Rune, I.; Søndergaard, H. Murine nephrotoxic nephritis as a model of chronic kidney disease. Int. J. Nephrol. 2018, 2018, 8424502. [Google Scholar] [CrossRef] [PubMed]
- Möckel, T.; Boegel, S.; Schwarting, A. Transcriptome analysis of renal ischemia/reperfusion (I/R) injury in BAFF and BAFF-R deficient mice. PLoS ONE 2023, 18, e0291619. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, T.; Fujimoto, M.; Hasegawa, M.; Matsushita, Y.; Komura, K.; Ogawa, F.; Watanabe, R.; Takehara, K.; Sato, S. BAFF antagonist attenuates the development of skin fibrosis in tight-skin mice. J. Investig. Dermatol. 2007, 127, 2772–2780. [Google Scholar] [CrossRef] [PubMed]
- François, A.; Chatelus, E.; Wachsmann, D.; Sibilia, J.; Bahram, S.; Alsaleh, G.; Gottenberg, J.-E. B lymphocytes and B-cell activating factor promote collagen and profibrotic markers expression by dermal fibroblasts in systemic sclerosis. Arthritis Res. Ther. 2013, 15, R168. [Google Scholar] [CrossRef] [PubMed]
- Thapa, M.; Tedesco, D.; Gumber, S.; Elrod, E.J.; Han, J.-H.; Kitchens, W.H.; Magliocca, J.F.; Adams, A.B.; Grakoui, A. Blockade of BAFF reshapes the hepatic B cell receptor repertoire and attenuates autoantibody production in cholestatic liver disease. J. Immunol. 2020, 204, 3117–3128. [Google Scholar] [CrossRef] [PubMed]
- Mo, F.; Luo, Y.; Yan, Y.; Li, J.; Lai, S.; Wu, W. Are activated B cells involved in the process of myocardial fibrosis after acute myocardial infarction? An in vivo experiment. BMC Cardiovasc. Disord. 2021, 21, 5. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Li, R.; Hou, Y.; Song, S.; Han, W.; Chen, N.; Du, Y.; Ren, Y.; Shi, Y. Thioredoxin-interacting protein deficiency ameliorates kidney inflammation and fibrosis in mice with unilateral ureteral obstruction. Lab. Investig. 2018, 98, 1211–1224. [Google Scholar] [CrossRef]
- Park, S.-J.; Kim, Y.; Li, C.; Suh, J.; Sivapackiam, J.; Goncalves, T.M.; Jarad, G.; Zhao, G.; Urano, F.; Sharma, V. Blocking CHOP-dependent TXNIP shuttling to mitochondria attenuates albuminuria and mitigates kidney injury in nephrotic syndrome. Proc. Natl. Acad. Sci. USA 2022, 119, e2116505119. [Google Scholar] [CrossRef]
- He, Q.; Li, Y.; Zhang, W.; Chen, J.; Deng, W.; Liu, Q.; Liu, Y.; Liu, D. Role and mechanism of TXNIP in ageing-related renal fibrosis. Mech. Ageing Dev. 2021, 196, 111475. [Google Scholar] [CrossRef] [PubMed]
- Avissar, N.; Ornt, D.B.; Yagil, Y.; Horowitz, S.; Watkins, R.H.; Kerl, E.A.; Takahashi, K.; Palmer, I.S.; Cohen, H.J. Human kidney proximal tubules are the main source of plasma glutathione peroxidase. Am. J. Physiol.-Cell Physiol. 1994, 266, C367–C375. [Google Scholar] [CrossRef] [PubMed]
- Whitin, J.C.; Bhamre, S.; Tham, D.M.; Cohen, H.J. Extracellular glutathione peroxidase is secreted basolaterally by human renal proximal tubule cells. Am. J. Physiol.-Ren. Physiol. 2002, 283, F20–F28. [Google Scholar] [CrossRef] [PubMed]
- Olson, G.E.; Whitin, J.C.; Hill, K.E.; Winfrey, V.P.; Motley, A.K.; Austin, L.M.; Deal, J.; Cohen, H.J.; Burk, R.F. Extracellular glutathione peroxidase (Gpx3) binds specifically to basement membranes of mouse renal cortex tubule cells. Am. J. Physiol.-Ren. Physiol. 2010, 298, F1244–F1253. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Takekoshi, Y.; Itami, N.; Honjo, T.; Kojima, H.; Yano, S.; Takahashi, H.; Saito, I.; Takahashi, K. Enzyme-linked immunosorbent assay for extracellular glutathione peroxidase in serum of normal individuals and patients with renal failure on hemodialysis. Clin. Chim. Acta 1995, 236, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Tian, X.; Yu, C.; Luo, J.; Zhang, J.; Hua, Y.; Wei, G. GPX3 and GSTT1 as biomarkers related to oxidative stress during renal ischemia reperfusion injuries and their relationship with immune infiltration. Front. Immunol. 2023, 14, 1136146. [Google Scholar] [CrossRef] [PubMed]
- Kashani, K.; Al-Khafaji, A.; Ardiles, T.; Artigas, A.; Bagshaw, S.M.; Bell, M.; Bihorac, A.; Birkhahn, R.; Cely, C.M.; Chawla, L.S. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit. Care 2013, 17, R25. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, A.; Faubel, S.; Askenazi, D.J.; Cerda, J.; Fissell, W.H.; Heung, M.; Humphreys, B.D.; Koyner, J.L.; Liu, K.D.; Mour, G. Clinical use of the urine biomarker [TIMP-2]×[IGFBP7] for acute kidney injury risk assessment. Am. J. Kidney Dis. 2016, 68, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Ngov, C.; Henley, N.; Boufaied, N.; Gerarduzzi, C. Characterization of matricellular protein expression signatures in mechanistically diverse mouse models of kidney injury. Sci. Rep. 2019, 9, 16736. [Google Scholar] [CrossRef]
- Rayego-Mateos, S.; Morgado-Pascual, J.L.; Rodrigues-Diez, R.R.; Rodrigues-Diez, R.; Falke, L.L.; Mezzano, S.; Ortiz, A.; Egido, J.; Goldschmeding, R.; Ruiz-Ortega, M. Connective tissue growth factor induces renal fibrosis via epidermal growth factor receptor activation. J. Pathol. 2018, 244, 227–241. [Google Scholar] [CrossRef]
- Rayego-Mateos, S.; Campillo, S.; Rodrigues-Diez, R.R.; Tejera-Muñoz, A.; Marquez-Exposito, L.; Goldschmeding, R.; Rodríguez-Puyol, D.; Calleros, L.; Ruiz-Ortega, M. Interplay between extracellular matrix components and cellular and molecular mechanisms in kidney fibrosis. Clin. Sci. 2021, 135, 1999–2029. [Google Scholar] [CrossRef] [PubMed]
- Phanish, M.K.; Winn, S.; Dockrell, M. Connective tissue growth factor-(CTGF, CCN2)–a marker, mediator and therapeutic target for renal fibrosis. Nephron Exp. Nephrol. 2010, 114, e83–e92. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-López, E.; Rodríguez Díez, R.; Rodríguez Vita, J.; Rayego Mateos, S.; Rodríguez Díez, R.; Rodríguez García, E.; Lavoz Barria, C.; Mezzano, S.; Selgas, R.; Egido, J. Connective tissue growth factor (CTGF): A key factor in the onset and progression of kidney damage. Nefrología 2009, 29, 382–391. [Google Scholar] [PubMed]
- Rayego-Mateos, S.; Morgado-Pascual, J.L.; Lavoz, C.; Rodrigues-Díez, R.R.; Márquez-Expósito, L.; Tejera-Muñoz, A.; Tejedor-Santamaría, L.; Rubio-Soto, I.; Marchant, V.; Ruiz-Ortega, M. CCN2 Binds to Tubular Epithelial Cells in the Kidney. Biomolecules 2022, 12, 252. [Google Scholar] [CrossRef] [PubMed]
- Furie, R.; Rovin, B.H.; Houssiau, F.; Malvar, A.; Teng, Y.K.O.; Contreras, G.; Amoura, Z.; Yu, X.; Mok, C.C.; Santiago, M.B.; et al. Two-Year, Randomized, Controlled Trial of Belimumab in Lupus Nephritis. N. Engl. J. Med. 2020, 383, 1117–1128. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Campbell, S.R.; Broder, A.; Herlitz, L.; Abadi, M.; Wu, P.; Michaelson, J.S.; Burkly, L.C.; Putterman, C. Inhibition of the TWEAK/Fn14 pathway attenuates renal disease in nephrotoxic serum nephritis. Clin. Immunol. 2012, 145, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.; Burkly, L.C.; Gao, H.-X.; Berman, J.W.; Su, L.; Browning, B.; Zheng, T.; Schiffer, L.; Michaelson, J.S.; Putterman, C. Proinflammatory effects of TWEAK/Fn14 interactions in glomerular mesangial cells. J. Immunol. 2006, 176, 1889–1898. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.-X.; Campbell, S.R.; Burkly, L.C.; Jakubowski, A.; Jarchum, I.; Banas, B.; Saleem, M.A.; Mathieson, P.W.; Berman, J.W.; Michaelson, J.S. TNF-like weak inducer of apoptosis (TWEAK) induces inflammatory and proliferative effects in human kidney cells. Cytokine 2009, 46, 24–35. [Google Scholar] [CrossRef]
- Lynch, C.N.; Wang, Y.C.; Lund, J.K.; Chen, Y.-W.; Leal, J.A.; Wiley, S.R. TWEAK induces angiogenesis and proliferation of endothelial cells. J. Biol. Chem. 1999, 274, 8455–8459. [Google Scholar] [CrossRef]
- Justo, P.; Sanz, A.; Sanchez-Nino, M.; Winkles, J.; Lorz, C.; Egido, J.; Ortiz, A. Cytokine cooperation in renal tubular cell injury: The role of TWEAK. Kidney Int. 2006, 70, 1750–1758. [Google Scholar] [CrossRef]
- Sanz, A.B.; Sanchez-Niño, M.D.; Izquierdo, M.C.; Jakubowski, A.; Justo, P.; Blanco-Colio, L.M.; Ruiz-Ortega, M.; Egido, J.; Ortiz, A. Tweak induces proliferation in renal tubular epithelium: A role in uninephrectomy induced renal hyperplasia. J. Cell. Mol. Med. 2009, 13, 3329–3342. [Google Scholar] [CrossRef] [PubMed]
- Sanz, A.B.; Sanchez-Niño, M.D.; Ortiz, A. TWEAK, a multifunctional cytokine in kidney injury. Kidney Int. 2011, 80, 708–718. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, N.; Su, L.; Burkly, L.C.; Mackay, M.; Aranow, C.; Kollaros, M.; Michaelson, J.S.; Rovin, B.; Putterman, C. Urinary TWEAK and the activity of lupus nephritis. J. Autoimmun. 2006, 27, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, N.; Rubinstein, T.; Burkly, L.C.; Collins, C.E.; Blanco, I.; Su, L.; Hojaili, B.; Mackay, M.; Aranow, C.; Stohl, W. Urinary TWEAK as a biomarker of lupus nephritis: A multicenter cohort study. Arthritis Res. Ther. 2009, 11, R143. [Google Scholar] [CrossRef]
- Lu, J.; Kwan, B.C.H.; Lai, F.M.M.; Choi, P.C.L.; Tam, L.S.; Li, E.K.M.; Chow, K.M.; Wang, G.; Li, P.K.T.; Szeto, C.C. Gene expression of TWEAK/Fn14 and IP-10/CXCR3 in glomerulus and tubulointerstitium of patients with lupus nephritis. Nephrology 2011, 16, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Baisantry, A.; Bhayana, S.; Rong, S.; Ermeling, E.; Wrede, C.; Hegermann, J.; Pennekamp, P.; Sörensen-Zender, I.; Haller, H.; Melk, A. Autophagy induces prosenescent changes in proximal tubular S3 segments. J. Am. Soc. Nephrol. JASN 2016, 27, 1609. [Google Scholar] [CrossRef]
- Meseguer, A.; Catterall, J.F. Mouse kidney androgen-regulated protein messenger ribonucleic acid is expressed in the proximal convoluted tubules. Mol. Endocrinol. 1987, 1, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Tornavaca, O.; Pascual, G.; Barreiro, M.; Grande, M.; Carretero, A.; Riera, M.; Garcia-Arumi, E.; Bardaji, B.; González-Núñez, M.; Montero, M. Kidney androgen-regulated protein transgenic mice show hypertension and renal alterations mediated by oxidative stress. Circulation 2009, 119, 1908–1917. [Google Scholar] [CrossRef] [PubMed]
- de Quixano, B.B.; Villena, J.A.; Aranda, M.; Brils, G.; Cuevas, A.; Hespel, T.; Lekuona, H.; Súarez, C.; Tornavaca, O.; Meseguer, A. Kidney androgen-regulated protein (KAP) transgenic mice are protected against high-fat diet induced metabolic syndrome. Sci. Rep. 2017, 7, 16102. [Google Scholar] [CrossRef] [PubMed]
- Tornavaca, O.; Sarro, E.; Pascual, G.; Bardaji, B.; Montero, M.A.; Salcedo, M.T.; Plana, M.; Lopez-Hellin, J.; Itarte, E.; Meseguer, A. KAP degradation by calpain is associated with CK2 phosphorylation and provides a novel mechanism for cyclosporine A-induced proximal tubule injury. PLoS ONE 2011, 6, e25746. [Google Scholar] [CrossRef]
- Melchinger, H.; Calderon-Gutierrez, F.; Obeid, W.; Xu, L.; Shaw, M.M.; Luciano, R.L.; Kuperman, M.; Moeckel, G.W.; Kashgarian, M.; Wilson, F.P. Urine uromodulin as a biomarker of kidney tubulointerstitial fibrosis. Clin. J. Am. Soc. Nephrol. 2022, 17, 1284–1292. [Google Scholar] [CrossRef] [PubMed]
- Rampoldi, L.; Scolari, F.; Amoroso, A.; Ghiggeri, G.; Devuyst, O. The rediscovery of uromodulin (Tamm–Horsfall protein): From tubulointerstitial nephropathy to chronic kidney disease. Kidney Int. 2011, 80, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Köttgen, A.; Glazer, N.L.; Dehghan, A.; Hwang, S.-J.; Katz, R.; Li, M.; Yang, Q.; Gudnason, V.; Launer, L.J.; Harris, T.B. Multiple loci associated with indices of renal function and chronic kidney disease. Nat. Genet. 2009, 41, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Köttgen, A.; Pattaro, C.; Böger, C.A.; Fuchsberger, C.; Olden, M.; Glazer, N.L.; Parsa, A.; Gao, X.; Yang, Q.; Smith, A.V. New loci associated with kidney function and chronic kidney disease. Nat. Genet. 2010, 42, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Jennings, P.; Aydin, S.; Kotanko, P.; Lechner, J.; Lhotta, K.; Williams, S.; Thakker, R.V.; Pfaller, W. Membrane targeting and secretion of mutant uromodulin in familial juvenile hyperuricemic nephropathy. J. Am. Soc. Nephrol. 2007, 18, 264–273. [Google Scholar] [CrossRef] [PubMed]
- El-Achkar, T.M.; Wu, X.-R.; Rauchman, M.; McCracken, R.; Kiefer, S.; Dagher, P.C. Tamm-Horsfall protein protects the kidney from ischemic injury by decreasing inflammation and altering TLR4 expression. Am. J. Physiol.-Ren. Physiol. 2008, 295, F534–F544. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, N.R.; Masi, S.; Taddei, S. The renin-angiotensin-aldosterone system: A crossroad from arterial hypertension to heart failure. Heart Fail. Rev. 2020, 25, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Sparks, M.A.; Crowley, S.D.; Gurley, S.B.; Mirotsou, M.; Coffman, T.M. Classical renin-angiotensin system in kidney physiology. Compr. Physiol. 2014, 4, 1201. [Google Scholar] [PubMed]
- Živná, M.; Hůlková, H.; Matignon, M.; Hodaňová, K.; Vylet’al, P.; Kalbáčová, M.; Barešová, V.; Sikora, J.; Blažková, H.; Živný, J. Dominant renin gene mutations associated with early-onset hyperuricemia, anemia, and chronic kidney failure. Am. J. Hum. Genet. 2009, 85, 204–213. [Google Scholar] [CrossRef]
- Gorelik, L.; Gilbride, K.; Dobles, M.; Kalled, S.L.; Zandman, D.; Scott, M.L. Normal B cell homeostasis requires B cell activation factor production by radiation-resistant cells. J. Exp. Med. 2003, 198, 937–945. [Google Scholar] [CrossRef]
- Schiemann, B.; Gommerman, J.L.; Vora, K.; Cachero, T.G.; Shulga-Morskaya, S.; Dobles, M.; Frew, E.; Scott, M.L. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science 2001, 293, 2111–2114. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.; Casola, S.; Kutok, J.L.; Rajewsky, K.; Schmidt-Supprian, M. TNF family member B cell-activating factor (BAFF) receptor-dependent and-independent roles for BAFF in B cell physiology. J. Immunol. 2004, 173, 2245–2252. [Google Scholar] [CrossRef] [PubMed]
- Song, H.K.; Hwang, D.Y. Use of C57BL/6N mice on the variety of immunological researches. Lab. Anim. Res. 2017, 33, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Salant, D.J.; Cybulsky, A.V. [38] Experimental glomerulonephritis glomerulonephritis. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1988; Volume 162, pp. 421–461. [Google Scholar]
- Dülsner, A.; Hack, R.; Krüger, C.; Pils, M.; Scherer, K.; Schmelting, B.; Schmidt, M.; Weinert, H.; Jourdan, T. Empfehlung zur Substanzapplikation bei Versuchstieren. Fachinf. Aus Dem Aussch. Für Tierschutzbeauftragte Und Dem Arbeitskreis 2017, 4, 1–8. [Google Scholar]
- Bray, N.L.; Pimentel, H.; Melsted, P.; Pachter, L. Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol. 2016, 34, 525–527. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Blighe, K.; Rana, S.; Lewis, M. EnhancedVolcano: Publication-Ready Volcano Plots with Enhanced Colouring and Labeling. Available online: https://github.com/kevinblighe/EnhancedVolcano (accessed on 4 November 2022).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]

| Gene | Expression | Current Status | References |
|---|---|---|---|
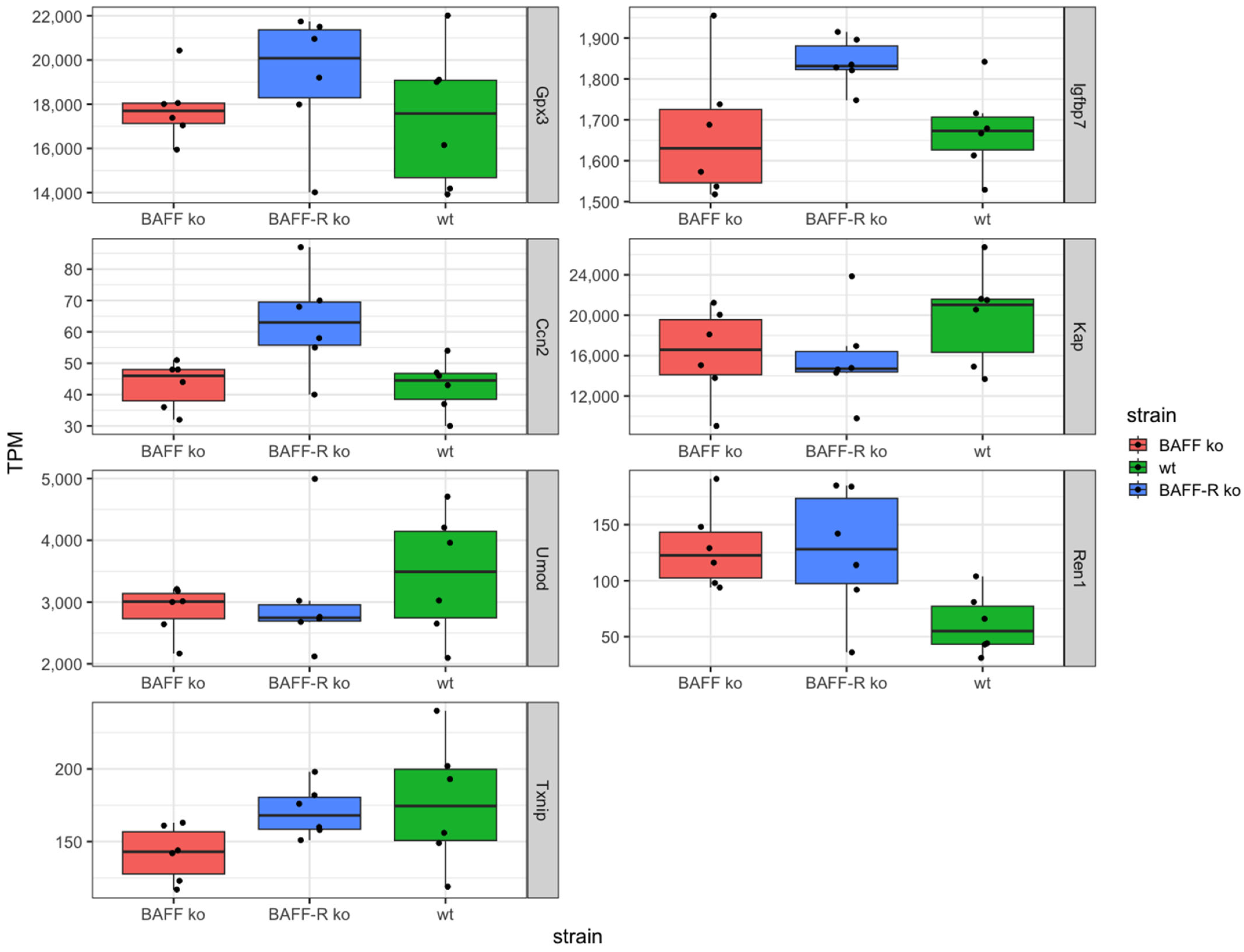
| Txnip | lower in BAFF ko BAFF-R ko and wt similar | associated with kidney disease | [59,60,61] |
| Gpx3 | higher in BAFF-R ko BAFF ko and wt similar | associated with CKD | [62,63,64,65,66] |
| Igfbp7 | higher in BAFF-R ko BAFF ko and wt similar | approved urinary biomarker | [67,68] |
| Ccn2 | higher in BAFF-R ko BAFF ko and wt similar | related to renal fibrosis, discussed as fibrotic marker | [69,70,71,72,73,74] |
| Kap | higher in wt BAFF and BAFF-R ko similar | unknown function, expressed in proximal section of TECs | [87,88,89,90] |
| Umod | higher in wt BAFF and BAFF-R ko similar | associated with kidney disease | [91,92,93,94,95,96] |
| Ren1 | lower in wt BAFF and BAFF-R ko similar | associated with kidney disease | [97,98,99] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Möckel, T.; Boegel, S.; Schwarting, A. Transcriptome Analysis of BAFF/BAFF-R System in Murine Nephrotoxic Serum Nephritis. Int. J. Mol. Sci. 2024, 25, 5415. https://doi.org/10.3390/ijms25105415
Möckel T, Boegel S, Schwarting A. Transcriptome Analysis of BAFF/BAFF-R System in Murine Nephrotoxic Serum Nephritis. International Journal of Molecular Sciences. 2024; 25(10):5415. https://doi.org/10.3390/ijms25105415
Chicago/Turabian StyleMöckel, Tamara, Sebastian Boegel, and Andreas Schwarting. 2024. "Transcriptome Analysis of BAFF/BAFF-R System in Murine Nephrotoxic Serum Nephritis" International Journal of Molecular Sciences 25, no. 10: 5415. https://doi.org/10.3390/ijms25105415





