The Influence of a High-Cholesterol Diet and Forced Training on Lipid Metabolism and Intestinal Microbiota in Male Wistar Rats
Abstract
:1. Introduction
2. Results
2.1. Body Weight and Food Intake
2.2. Body Composition
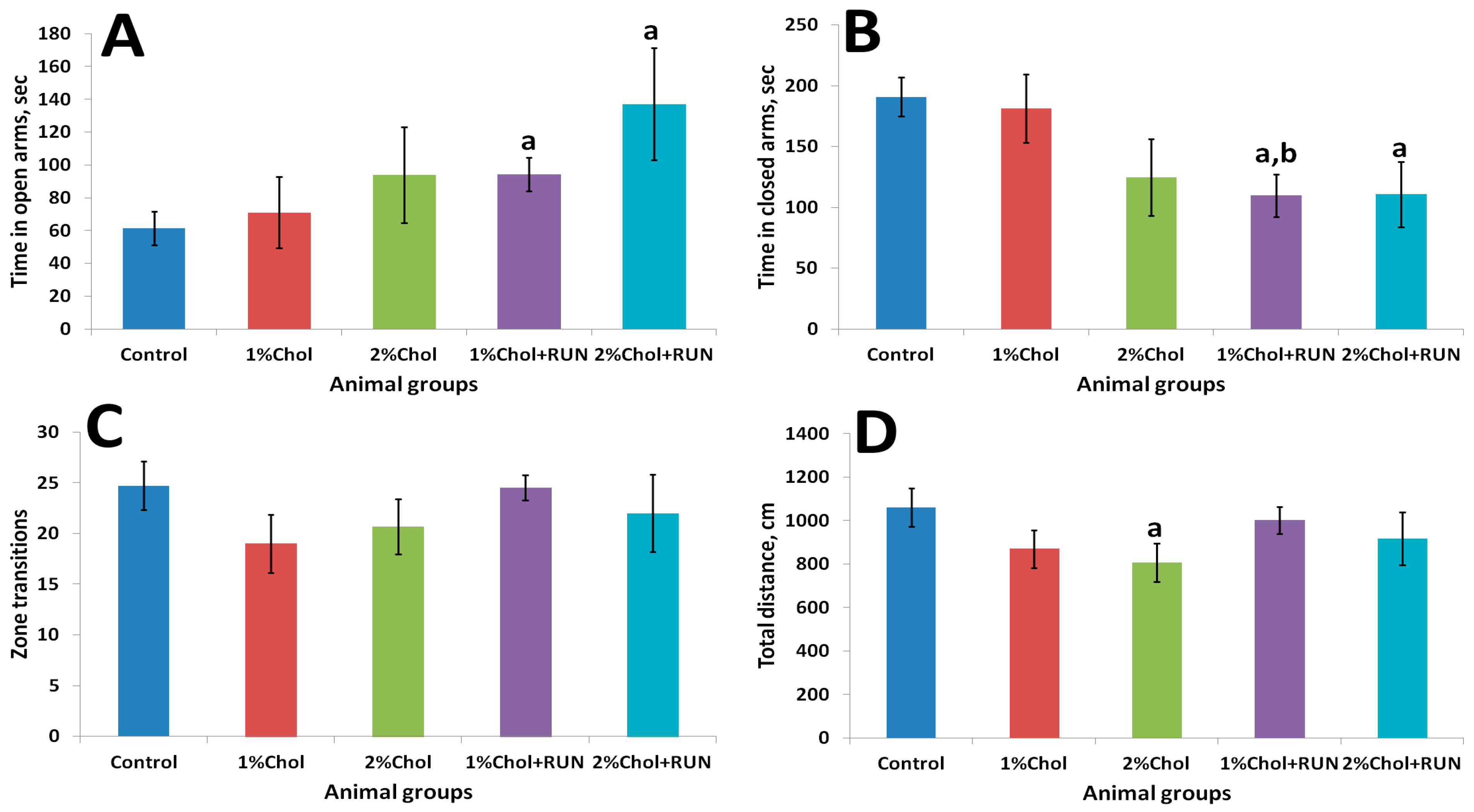
2.3. Elevated Plus Maze
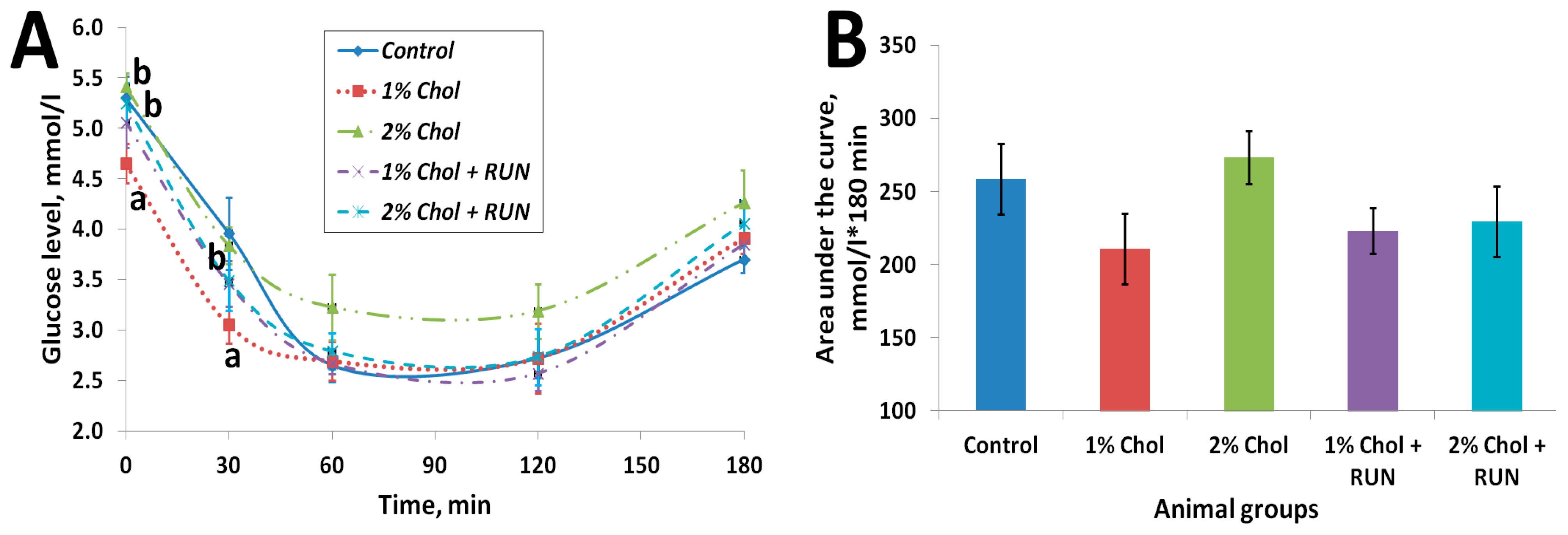
2.4. Insulin Resistance Test
2.5. Blood and Liver Biochemical Analysis
2.6. Blood Hematological Analysis
2.7. Study of the Microbiota
3. Discussion
4. Materials and Methods
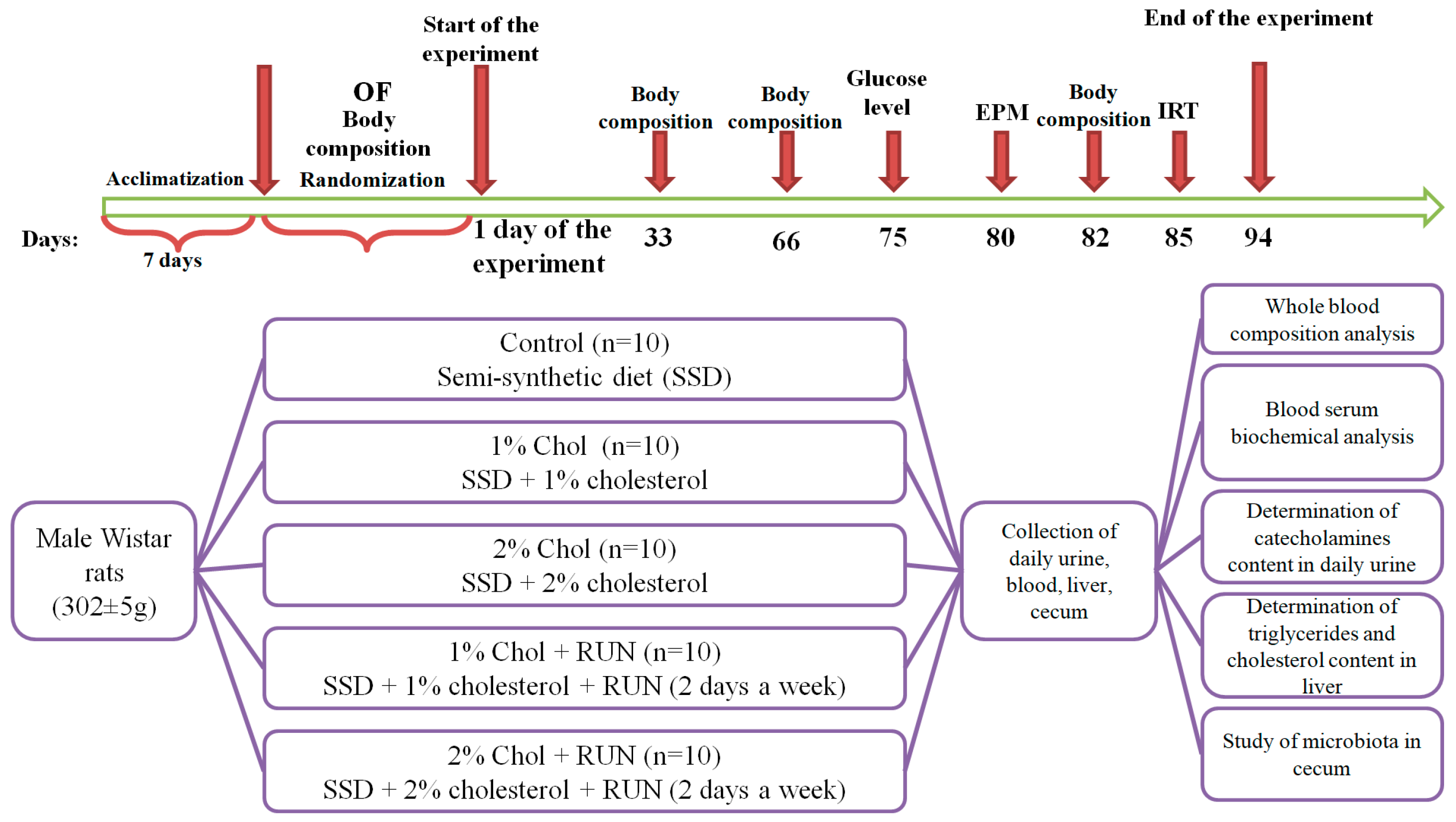
4.1. Experimental Animals
4.2. Experimental Design
4.2.1. Preliminary Division of the Animals
4.2.2. Animal Treatment
4.2.3. Animal Body Composition
4.2.4. Elevated Plus Maze
4.2.5. Insulin Resistance Test
4.2.6. Sample Collection
4.2.7. ELISA Kits
4.2.8. Serum and Liver Biochemical Analysis
4.2.9. Blood Hematological Analysis
4.2.10. Study of Microbiota
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Piché, M.-E.; Tchernof, A.; Després, J.-P. Obesity Phenotypes. Diabetes. and Cardiovascular Diseases. Circ. Res. 2020, 126, 1477–1500. [Google Scholar] [CrossRef]
- Babenko, A.Y.; Golikova, T.I. Obesity as a predictor of metabolic disorders and a target for personalized interventions. Ross. Zhurnal Pers. Meditsiny 2021, 1, 59–94. [Google Scholar]
- Chai, Z.; Yan, Y.; Zan, S.; Meng, X.; Zhang, F. Probiotic-fermented blueberry pomace alleviates obesity and hyperlipidemia in high-fat diet C57BL/6J mice. Food Res. Int. 2022, 157, 111396. [Google Scholar] [CrossRef]
- Perdomo, C.M.; Cohen, R.V.; Sumithran, P.; Clément, K.; Frühbeck, G. Contemporary medical. device. and surgical therapies for obesity in adults. Lancet 2023, 401, 1116–1130. [Google Scholar] [CrossRef]
- Karr, S. Epidemiology and management of hyperlipidemia. Am. J. Manag. Care 2017, 23 (Suppl. S9), S139–S148. [Google Scholar]
- Santos-Baez, L.S.; Ginsberg, H.N. Hypertriglyceridemia—Causes. Significance. and Approaches to Therapy. Front. Endocrinol. (Lausanne) 2020, 11, 574865. [Google Scholar] [CrossRef]
- Gao, M.; Jebb, S.A.; Aveyard, P.; Ambrosini, G.L.; Perez-Cornago, A.; Carter, J.; Sun, X.; Piernas, C. Associations between dietary patterns and the incidence of total and fatal cardiovascular disease and all-cause mortality in 116.806 individuals from the UK Biobank: A prospective cohort study. BMC Med. 2021, 19, 83. [Google Scholar] [CrossRef]
- AlFaris, N.A.; Alshammari, G.M.; AlTamimi, J.Z.; AlMousa, L.A.; AlKehayez, N.M.; Aljabryn, D.H.; Alagal, R.I.; Yahya, M.A. The protective effect of shrimp cooked in different methods on high-cholesterol-induced fatty liver in rats. Saudi J. Biol. Sci. 2021, 28, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Tirosh, O. Hypoxic Signaling and Cholesterol Lipotoxicity in Fatty Liver Disease Progression. Oxid. Med. Cell. Longev. 2018, 2018, 2548154. [Google Scholar] [CrossRef]
- Tripathi, A.; Debelius, J.; Brenner, D.A.; Karin, M.; Loomba, R.; Schnabl, B.; Knight, R. The gut-liver axis and the intersection with the microbiome. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 397–411. [Google Scholar] [CrossRef]
- Brown, E.M.; Clardy, J.; Xavier, R.J. Gut microbiome lipid metabolism and its impact on host physiology. Cell Host Microbe 2023, 31, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Schoeler, M.; Caesar, R. Dietary lipids. gut microbiota and lipid metabolism. Rev. Endocr. Metab. Disord. 2019, 20, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Lau, K.; Srivatsav, V.; Rizwan, A.; Nashed, A.; Liu, R.; Shen, R.; Akhtar, M. Bridging the Gap between Gut Microbial Dysbiosis and Cardiovascular Diseases. Nutrients 2017, 9, 859. [Google Scholar] [CrossRef] [PubMed]
- Matey-Hernandez, M.L.; Williams, F.M.K.; Potter, T.; Valdes, A.M.; Spector, T.D.; Menni, C. Genetic and microbiome influence on lipid metabolism and dyslipidemia. Physiol. Genom. 2018, 50, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Vourakis, M.; Mayer, G.; Rousseau, G. The Role of Gut Microbiota on Cholesterol Metabolism in Atherosclerosis. Int. J. Mol. Sci. 2021, 22, 8074. [Google Scholar] [CrossRef] [PubMed]
- Oynotkinova, O.S.; Nikonov, E.L.; Demidova, T.Y.; Baranov, A.P.; Kryukov, E.V.; Dedov, E.I.; Karavashkina, E.A. Changes in the intestinal microbiota as a risk factor for dyslipidemia. atherosclerosis and the role of probiotics in their prevention. Ter. Arkh. 2020, 92, 94–101. [Google Scholar] [CrossRef]
- Albillos, A.; de Gottardi, A.; Rescigno, M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J. Hepatol. 2020, 72, 558–577. [Google Scholar] [CrossRef]
- Wu, L.; Li, J.; Feng, J.; Ji, J.; Yu, Q.; Li, Y.; Zheng, Y.; Dai, W.; Wu, J.; Guo, C. Crosstalk between PPARs and gut microbiota in NAFLD. Biomed. Pharmacother. 2021, 136, 111255. [Google Scholar] [CrossRef]
- Cunha, L.F.; Ongaratto, M.A.; Endres, M.; Barschak, A.G. Modelling hypercholesterolaemia in rats using high cholesterol diet. Int. J. Exp. Pathol. 2021, 102, 74–79. [Google Scholar] [CrossRef]
- Saigo, Y.; Sasase, T.; Tohma, M.; Uno, K.; Shinozaki, Y.; Maekawa, T.; Sano, R.; Miyajima, K.; Ohta, T. High-Cholesterol Diet in Combination With Hydroxypropyl-β Cyclodextrin Induces NASH-Like Disorders in the Liver of Rats. Physiol. Res. 2023, 72, 371. [Google Scholar] [CrossRef]
- Jang, S.; Lee, M.-S.; Kang, S.-A.; Kim, C.-T.; Kim, Y. Portulaca oleracea L. Extract Regulates Hepatic Cholesterol Metabolism via the AMPK/MicroRNA-33/34a Pathway in Rats Fed a High-Cholesterol Diet. Nutrients 2022, 14, 3330. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, M.; Jiang, F.; Luo, D.; Liu, S.; Su, Y.; Guan, Q.; Yu, C. High cholesterol diet–induced testicular dysfunction in rats. Hormones 2023, 22, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Jiang, F.; Zhang, M.; Luo, D.; Shao, S.; Zhao, J.; Gao, L.; Zuo, C.; Guan, Q. HC diet inhibited testosterone synthesis by activating endoplasmic reticulum stress in testicular Leydig cells. J. Cell. Mol. Med. 2019, 23, 3140–3150. [Google Scholar] [CrossRef] [PubMed]
- Zafar, H.; Ain, N.u.; Alshammari, A.; Alghamdi, S.; Raja, H.; Ali, A.; Siddique, A.; Tahir, S.D.; Akbar, S.; Arif, M.; et al. Lacticaseibacillus rhamnosus FM9 and Limosilactobacillus fermentum Y57 Are as Effective as Statins at Improving Blood Lipid Profile in High Cholesterol. High-Fat Diet Model in Male Wistar Rats. Nutrients 2022, 14, 1654. [Google Scholar] [CrossRef] [PubMed]
- Biryulina, N.A.; Sidorova, Y.S.; Petrov, N.A.; Guseva, G.V.; Mazo, V.K. The effect of semi-synthetic and high-fat high-carbohydrate diets containing cholesterol on lipid and cholesterol metabolism of male Wistar rats. Bull. Exp. Biol. Med. 2023, 175, 451–456. [Google Scholar] [CrossRef]
- Kovacs, P.; Hajnal, A. Short-term high-fat diet consumption increases body weight and body adiposity and alters brain stem taste information processing in rats. Chem. Senses 2022, 47, bjac020. [Google Scholar] [CrossRef]
- Moreno-Fernández, S.; Garcés-Rimón, M.; Vera, G.; Astier, J.; Landrier, J.F.; Miguel, M. High Fat/High Glucose Diet Induces Metabolic Syndrome in an Experimental Rat Model. Nutrients 2018, 10, 1502. [Google Scholar] [CrossRef] [PubMed]
- Pathak, R.; Kumar, A.; Palfrey, H.A.; Stone, K.P.; Raju, N.R.; Gettys, T.W.; Murthy, S.N. Prolonged effects of DPP-4 inhibitors on steato-hepatitic changes in Sprague–Dawley rats fed a high-cholesterol diet. Inflamm. Res. 2022, 71, 711–722. [Google Scholar] [CrossRef]
- Oda, E. A decrease in total bilirubin predicted hyper-LDL cholesterolemia in a health screening population. Atherosclerosis 2014, 235, 334–338. [Google Scholar] [CrossRef]
- Su, Q.; Chen, H.; Du, S.; Dai, Y.; Chen, C.; He, T.; Feng, R.; Tao, T.; Hu, Z.; Zhao, H.; et al. Association Between Serum Bilirubin. Lipid Levels. and Prevalence of Femoral and Carotid Atherosclerosis: A Population-Based Cross-Sectional Study. Arterioscler. Thromb. Vasc. Biol. 2023, 43, 136–145. [Google Scholar] [CrossRef]
- Li, X.-H.; Lin, H.-Y.; Guan, L.-Y.; Peng, H.; Wen, M.-M.; Cao, Y.-Q.; Jiang, X.-Y.; Wang, Y.-B. Direct Bilirubin Levels and Risk of Metabolic Syndrome in Healthy Chinese Men. Biomed. Res. Int. 2017, 2017, 9621615. [Google Scholar] [CrossRef] [PubMed]
- Wu, I.-T.; Yeh, W.-J.; Huang, W.-C.; Yang, H.-Y. Very low-carbohydrate diet with higher protein ratio improves lipid metabolism and inflammation in rats with diet-induced nonalcoholic fatty liver disease. J. Nutr. Biochem. 2024, 126, 109583. [Google Scholar] [CrossRef] [PubMed]
- Pouwels, S.; Sakran, N.; Graham, Y.; Leal, A.; Pintar, T.; Yang, W.; Kassir, R.; Singhal, R.; Mahawar, K.; Ramnarain, D. Non-alcoholic fatty liver disease (NAFLD): A review of pathophysiology. clinical management and effects of weight loss. BMC Endocr. Disord. 2022, 22, 63. [Google Scholar] [CrossRef] [PubMed]
- Spassiani, N.A.; Kuk, J.L. Exercise and the fatty liver. Appl. Physiol. Nutr. Metab. 2008, 33, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Charatcharoenwitthaya, P.; Lindor, K.D.; Angulo, P. The spontaneous course of liver enzymes and its correlation in nonalcoholic fatty liver disease. Dig. Dis. Sci. 2012, 57, 1925–1931. [Google Scholar] [CrossRef] [PubMed]
- Van Herck, M.A.; Vonghia, L.; Francque, S.M. Animal Models of Nonalcoholic Fatty Liver Disease-A Starter’s Guide. Nutrients 2017, 9, 1072. [Google Scholar] [CrossRef] [PubMed]
- Lau, J.K.; Zhang, X.; Yu, J. Animal models of non-alcoholic fatty liver disease: Current perspectives and recent advances. J. Pathol. 2017, 241, 36–44. [Google Scholar] [CrossRef]
- Gu, X.; Ma, X.; Mo, L.; Wang, Q. The Role of Exercise Intensity on Fatty Liver in Rats. Chin. J. Physiol. 2022, 65, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Song, Y.; Xu, J.; Gan, H. Melatonin ameliorates nonalcoholic fatty liver induced by high-fat diet in rats. J. Pineal Res. 2006, 41, 79–84. [Google Scholar] [CrossRef]
- Qi, J.; Kim, J.-W.; Zhou, Z.; Lim, C.-W.; Kim, B. Ferroptosis Affects the Progression of Nonalcoholic Steatohepatitis via the Modulation of Lipid Peroxidation–Mediated Cell Death in Mice. Am. J. Pathol. 2020, 190, 68–81. [Google Scholar] [CrossRef]
- Münzberg, H.; Morrison, C.D. Structure. production and signaling of leptin. Metabolism 2015, 64, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Martelli, D.; Brooks, V.L. Leptin Increases: Physiological Roles in the Control of Sympathetic Nerve Activity. Energy Balance. and the Hypothalamic–Pituitary–Thyroid Axis. Int. J. Mol. Sci. 2023, 24, 2684. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Halder, C.V.; de Sousa Faria, A.V.; Andrade, S.S. Action and function of Faecalibacterium prausnitzii in health and disease. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Leylabadlo, H.E.; Ghotaslou, R.; Feizabadi, M.M.; Farajnia, S.; Moaddab, S.Y.; Ganbarov, K.; Khodadadi, E.; Tanomand, A.; Sheykhsaran, E.; Yousefi, B.; et al. The critical role of Faecalibacterium prausnitzii in human health: An overview. Microb. Pathog. 2020, 149, 104344. [Google Scholar] [CrossRef] [PubMed]
- Sitkin, S.I.; Vakhitov, T.Y.; Tkachenko, E.I.; Oreshko, L.S.; Zhigalova, T.N.; Radchenko, V.G.; Seliverstov, P.V.; Avalueva, E.B.; Suvorova, M.A.; Komlichenko, E.V. Gut microbiota in ulcerative colitis and celiac disease. Exp. Clin. Gastroenterol. 2017, 137, 8–30. [Google Scholar]
- Mohammad, S.; Thiemermann, C. Role of Metabolic Endotoxemia in Systemic Inflammation and Potential Interventions. Front. Immunol. 2021, 11, 594150. [Google Scholar] [CrossRef]
- Khare, A.; Gaur, S. Cholesterol-Lowering Effects of Lactobacillus Species. Curr. Microbiol. 2020, 77, 638–644. [Google Scholar] [CrossRef] [PubMed]
- O’Flaherty, S.; Briner Crawley, A.; Theriot, C.M.; Barrangou, R. The Lactobacillus Bile Salt Hydrolase Repertoire Reveals Niche-Specific Adaptation. mSphere 2018, 3. [Google Scholar] [CrossRef]
- Frappier, M.; Auclair, J.; Bouasker, S.; Gunaratnam, S.; Diarra, C.; Millette, M. Screening and Characterization of Some Lactobacillaceae for Detection of Cholesterol-Lowering Activities. Probiot. Antimicrob. Proteins 2022, 14, 873–883. [Google Scholar] [CrossRef]
- de Araújo Henriques Ferreira, G.; Magnani, M.; Cabral, L.; Brandão, L.R.; Noronha, M.F.; de Campos Cruz, J.; de Souza, E.L.; de Brito Alves, J.L. Potentially Probiotic Limosilactobacillus fermentum Fruit-Derived Strains Alleviate Cardiometabolic Disorders and Gut Microbiota Impairment in Male Rats Fed a High-Fat Diet. Probiot. Antimicrob. Proteins 2022, 14, 349–359. [Google Scholar] [CrossRef]
- Li, X.; Xiao, Y.; Huang, Y.; Song, L.; Li, M.; Ren, Z. Lactobacillus gasseri RW2014 Ameliorates Hyperlipidemia by Modulating Bile Acid Metabolism and Gut Microbiota Composition in Rats. Nutrients 2022, 14, 4945. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Nagpal, R.; Kumar, R.; Hemalatha, R.; Verma, V.; Kumar, A.; Chakraborty, C.; Singh, B.; Marotta, F.; Jain, S.; et al. Cholesterol-Lowering Probiotics as Potential Biotherapeutics for Metabolic Diseases. Exp. Diabetes Res. 2012, 2012, 902917. [Google Scholar] [CrossRef]
- DiRienzo, D.B. Effect of probiotics on biomarkers of cardiovascular disease: Implications for heart-healthy diets. Nutr. Rev. 2014, 72, 18–29. [Google Scholar] [CrossRef]
- Tonucci, L.B.; Olbrich dos Santos, K.M.; Licursi de Oliveira, L.; Rocha Ribeiro, S.M.; Duarte Martino, H.S. Clinical application of probiotics in type 2 diabetes mellitus: A randomized. double-blind. placebo-controlled study. Clin. Nutr. 2017, 36, 85–92. [Google Scholar] [CrossRef]
- Choi, B.S.-Y.; Brunelle, L.; Pilon, G.; Cautela, B.G.; Tompkins, T.A.; Drapeau, V.; Marette, A.; Tremblay, A. Lacticaseibacillus rhamnosus HA-114 improves eating behaviors and mood-related factors in adults with overweight during weight loss: A randomized controlled trial. Nutr. Neurosci. 2023, 26, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Wang, G.; Xia, Y.; Xiong, Z.; Ai, L. Bile salt hydrolase-overexpressing Lactobacillus strains can improve hepatic lipid accumulation in vitro in an NAFLD cell model. Food Nutr. Res. 2020, 64, 3751. [Google Scholar] [CrossRef] [PubMed]
- GOST 33647-2015; Principles of Good Laboratory Practice. Standartinform: Moscow, Russia, 2019; 16p.
- GOST 33216-2014; Guide to the Care and Maintenance of Laboratory Animals. Rules for Keeping and Caring for Laboratory Rodents and Rabbits. Standartinform: Moscow, Russia, 2019; 10p.
- Reeves, P.G. Components of the AIN-93 Diets as Improvements in the AIN-76A Diet. J. Nutr. 1997, 127, 838S–841S. [Google Scholar] [CrossRef]
- Omar, A.M.; Zhang, Q. Evaluation of Lipid Extraction Protocols for Untargeted Analysis of Mouse Tissue Lipidome. Metabolites 2023, 13, 1002. [Google Scholar] [CrossRef]


| Parameter | Animal Group | ||||
|---|---|---|---|---|---|
| Control | 1% Chol | 2% Chol | 1% Chol + RUN | 2% Chol + RUN | |
| Test 1 (before the start of the experiment) | |||||
| Fat content, % | 9.2 ± 0.5 | 8.3 ± 0.5 | 10.0 ± 1.2 | 8.2 ± 0.7 | 9.8 ± 0.7 |
| Lean weight, % | 85.5 ± 0.5 | 86.4 ± 0.9 | 84.8 ± 1.2 | 86.8 ± 0.7 | 84.7 ± 0.6 |
| Free water, % | 0.25 ± 0.03 | 0.26 ± 0.02 | 0.32 ± 0.05 | 0.22 ± 0.01 | 0.22 ± 0.02 |
| Total water, % | 74.1 ± 0.5 | 74.7 ± 0.8 | 73.4 ± 1.0 | 74.9 ± 0.7 | 73.9 ± 0.6 |
| Test 2 (33rd day) | |||||
| Fat content, % | 9.5 ± 1.1 | 5.3 ± 0.8 a,* | 10.4 ± 2.02 | 5.1 ± 1.0 a,c,* | 6,5 ± 1,3 * |
| Lean weight, % | 85.6 ± 1.1 | 90.2 ± 0.8 a,* | 85.0 ± 2.1 b | 90.6 ± 1.0 a,c,* | 88.6 ± 1.2 * |
| Free water, % | 0.24 ± 0.02 | 0.27 ± 0.03 | 0.30 ± 0.03 a | 0.29 ± 0.02 a | 0.30 ± 0.02 a |
| Total water, % | 72.3 ± 1.0 | 76.1 ± 0.6 a | 70.7 ± 1.7 b | 76.5 ± 0.9 a,c | 75.0 ± 1.1 |
| Test 3 (66th day) | |||||
| Fat content, % | 10.3 ± 1.2 | 5.4 ± 0.7 a,* | 12.7 ± 2.6 b | 5.5 ± 1.2 a,c,* | 6.3 ± 1.4 a,c,* |
| Lean weight, % | 83.5 ± 1.2 | 88.9 ± 0.7 a,* | 81.3 ± 2.7 b | 89.1 ± 1.1 a,c | 88.0 ± 1.4 a,c,* |
| Free water, % | 0.18 ± 0.01 | 0.26 ± 0.05 | 0.20 ± 0.02 | 0.24 ± 0.05 | 0.19 ± 0.03 |
| Total water, % | 70.2 ± 0.9 * | 74.7 ± 0.6 a | 68.4 ± 2.2 b | 75.1 ± 1.0 a,c | 74.1 ± 1.2 a,c |
| Test 4 (82nd day) | |||||
| Fat content, % | 10.5 ± 1.0 | 5.4 ± 0.7 a,c,* | 15.4 ± 2.2 a | 5.8 ± 1.3 a,c | 6.7 ± 1.5 a,c,* |
| Lean weight, % | 83.2 ± 1.0 * | 88.6 ± 0.7 a,c | 78.2 ± 2.3 a | 88.2 ± 1.2 a,c | 87.1 ± 1.6 a,c |
| Free water, % | 0.27 ± 0.02 | 0.31 ± 0.03 | 0.27 ± 0.03 | 0.24 ± 0.02 | 0.24 ± 0.03 |
| Total water, % | 69.2 ± 1.0 * | 74.5 ± 0.6 a,c | 67.1 ± 2.2 a,* | 74.5 ± 1.1 a,c | 73.3 ± 1.3 a,c |
| Parameter | Animal Groups | ||||
|---|---|---|---|---|---|
| Control | 1% Chol | 2% Chol | 1% Chol + RUN | 2% Chol + RUN | |
| Total protein, g/L | 75.0 [69.7–77.8] | 77.3 [74.2–82.6] | 82.1 [78.2–88.7] a | 76.4 [73.8–82.1] | 81.9 [77.4–83.9] a |
| Albumin, g/L | 35.8 [35.5–36.0] | 35.5 [35.1–36.8] | 37.2 [35.9–38.1] | 36.1 [35.6–36.9] | 36.7 [36.0–37.4] |
| Globulins, g/L | 38.2 ± 1.5 | 42.6 ± 1.6 | 45.7 ± 2.5 a | 41.7 ± 1.8 | 44.6 ± 1.5 a |
| Cholesterol, mmol/L | 1.5 [1.4–1.6] | 1.8 [1.3–1.9] | 2.8 [2.2–2.9] a,b | 1.8 [1.7–1.9] c | 2.2 [1.8–2.8] a,b |
| Triglycerides, mmol/L | 1.0 [0.8–2.3] | 0.8 [0.7–1.1] | 0.9 [0.7–1.6] | 0.8 [0.6–0.9] | 1.0 [0.6–1.1] |
| HDL, mmol/L | 1.2 [1.1–1.4] | 0.9 [0.8–1.0] a | 1.1 [1.1–1.2] b | 0.9 [0.8–0.9] a,c | 1.1 [1.0–1.2] b,d |
| LDL, mmol/L | 0.07 [0.05–0.08] | 0.13 [0.09–0.27] a | 0.20 [0.11–0.24] a | 0.12 [0.10–0.14] a | 0.12 [0.08–0.21] a |
| Atherogenic coefficient | 0.23 [0.14–0.27] | 0.85 [0.49–1.15] a | 1.20 [0.99–1.63] a | 1.03 [0.89–1.13] a | 1.07 [0.45–1.88] a |
| Total bilirubin, μmol/L | 4.27 ± 0.22 | 3.77 ± 0.14 a | 3.48 ± 0.11 a | 3.57 ± 0.07 a | 3.24 ± 0.22 a,b |
| Creatinine, μmol/L | 53.1 [51.3–56.1] | 52.4 [51.7–53.1] | 57.5 [53.1–59.0] b | 53.3 [53.0–56.1] | 57.0 [55.3–58.6] a,b |
| Alkaline phosphatase, U/L | 101 [88–121] | 115 [98–124] | 123 [112–155] | 117 [97–147] | 162 [129–183] a |
| ALT, U/L | 56.6 [55.4–65.4] | 56.4 [49.0–64.3] | 80.7 [75.3–109.5] a,b | 59.7 [50.2–65.5] c | 91.7 [72.2–157.0] a,b,d |
| AST, U/L | 39.4 ± 3.9 | 53.5 ± 5.1 | 60.1 ± 4.6 a | 60.0 ± 7.2 a | 42.3 ± 4.9 c |
| AST/ALT | 0.70 ± 0.10 | 0.92 ± 0.14 | 0.67 ± 0.10 | 0.98 ± 0.09 | 0.45 ± 0.11 b,d |
| Urea, mmol/L | 5.0 ± 0.2 | 4.7 ± 0.1 | 5.1 ± 0.3 | 4.8 ± 0.2 | 5.0 ± 0.1 |
| Uric acid, μmol/L | 86.6 ± 3.4 | 91.1 ± 4.6 | 88.2 ± 5.5 | 83.1 ± 2.6 | 87.1 ± 3.4 |
| Phosphor, mmol/L | 2.27 ± 0.04 | 2.37 ± 0.06 | 2.28 ± 0.05 | 2.39 ± 0.09 | 2.45 ± 0.07 |
| Magnesium, mmol/L | 0.89 [0.87–0.96] | 0.83 [0.76–0.86] | 0.91 [0.86–0.96] | 0.91 [0.83–0.99] | 0.90 [0.90–0.92] b |
| Calcium, mmol/L | 2.95 [2.94–3.00] | 3.01 [2.97–3.03] | 3.05 [2.99–3.08] a | 3.02 [2.97–3.08] | 3.16 [3.06–3.21] a,b,d |
| Glucose, mmol/L | 5.7 ± 0.3 | 5.7 ± 0.1 | 6.2 ± 0.3 | 5.7 ± 0.2 | 6.3 ± 0.2 |
| Parameter | Animal Groups | ||||
|---|---|---|---|---|---|
| Control | 1% Chol | 2% Chol | 1% Chol + RUN | 2% Chol + RUN | |
| Liver weight, g | 11.4 [10.0–11.7] | 14.3 [13.9–17.2] a | 14.2 [13.8–17.7] a | 15.1 [13.8–16.6] a | 14.5 [14.0–18.4] a |
| Relative liver weight, % | 2.3 [2.2–2.4] | 3.0 [2.6–3.2] a | 3.2 [2.9–3.4] a | 2.9 [2.9–3.1] a | 3.0 [2.9–3.7] a |
| Fat, mg/g of liver | 66.4 [63.6–70.2] | 138.7 [118.2–163.5] a | 154.5 [128.3–223.2] a | 150.4 [129.0–159.5] a | 158.2 [108.9–210.4] a |
| Triglycerides, mg/g | 6.6 [4.4–13.4] | 31.2 [16.3–37.3] a | 31.4 [23.2–36.2] a | 18.8 [6.6–30.9] | 20.4 [9.8–30.2] a |
| Cholesterol, mg/g | 3.0 [2.5–3.4] | 20.6 [16.4–22.4] a | 23.6 [17.5–28.2] a | 18.6 [15.6–22.8] a | 17.5 [11.9–22.1] a |
| Parameter | Animal Groups | ||||
|---|---|---|---|---|---|
| Control | 1% Chol | 2% Chol | 1% Chol + RUN | 2% Chol + RUN | |
| Leukocytes, 109/L | 5.5 [4.6–6.4] | 6.5 [5.4–7.4] | 4.3 [3.8–5.5] b | 7.8 [5.2–9.2] c | 5.9 [4.9–6.7] c |
| Lymphocytes, 109/L | 3.6 [3.1–4.5] | 4.0 [3.3–4.9] | 2.8 [1.9–3.5] b | 4.8 [3.3–6.6] c | 4.0 [2.9–4.4] |
| Lymphocytes, % | 66.2 ± 2.1 | 64.1 ± 2.8 | 61.5 ± 3.3 | 66.1 ± 2.3 | 61.6 ± 2.1 |
| Monocytes, 109/L | 0.24 ± 0.03 | 0.36 ± 0.05 | 0.20 ± 0.05 b | 0.26 ± 0.03 | 0.31 ± 0.05 |
| Monocytes, % | 4.3 [3.8–7.3] | 7.2 [5.1–7.7] | 4.5 [4.0–7.6] | 3.8 [3.3–4.9] | 7.3 [3.7–8.1] |
| Granulocytes, 109/L | 1.6 [1.2–1.8] | 2.1 [1.3–2.3] | 1.1 [0.9–1.5] b | 2.0 [1.7–2.3] c | 1.7 [1.5–2.0] |
| Granulocytes, % | 29.7 [23.6–34.7] | 28.9 [27.3–34.5] | 28.4 [22.4–43.4] | 29.9 [24.5–33.8] | 30.6 [28.5–35.6] |
| Hemoglobin, g/L | 153 [151–157] | 146 [146–150] | 150 [147–152] | 150 [144–155] | 155 [154–156] b |
| Average hemoglobin content in an erythrocyte, pg | 18.7 ± 0.2 | 18.4 ± 0.3 | 17.9 ± 0.2 a | 18.6 ± 0.1 c | 18.7 ± 0.2 c |
| Average concentration of cellular hemoglobin in erythrocytes, g/L | 365.7 ± 2.4 | 374.8 ± 1.6 | 371.2 ± 1.5 | 370.1 ± 1.4 | 367.4 ± 1.0 |
| Erythrocytes, 1012/L | 8.3 [8.0–8.5] | 8.1 [7.9–8.2] | 8.4 [8.2–8.5] | 7.9 [7.8–8.3] | 8.3 [8.2–8.3] |
| Average erythrocyte volume, fl | 50.6 [50.0–51.9] | 48.9 [47.4–50.9] | 48.0 [47.4–48.7] a,b | 50.6 [49.6–51.1] c | 51.2 [50.0–51.7] b,c |
| Hematocrit, % | 42.2 ± 0.6 | 39.7 ± 0.7 a | 40.3 ± 0.3 a | 40.3 ± 0.6 a | 42.1 ± 0.5 b,c,d |
| Width of distribution of red blood cells by volume, % | 13.0 [12.8–13.5] | 13.2 [13.1–13.7] | 13.7 [13.2–14.0] | 13.3 [13.1–13.5] | 13.1 [12.7–13.6] |
| Width of distribution of red blood cells by volume, fl | 31.2 ± 0.4 | 29.9 ± 0.4 | 29.9 ± 0.4 | 30.7 ± 0.2 | 31.3 ± 0.4 |
| Platelets, 109/L | 705.3 ± 71.2 | 738.7 ± 73.8 | 726.0 ± 63.5 | 761.6 ± 54.9 | 705.6 ± 45.7 |
| Platelets average volume, fl | 6.39 ± 0.14 | 6.20 ± 0.10 | 6.10 ± 0.10 | 6.21 ± 0.09 | 6.33 ± 0.07 |
| Populations and Groups of Microorganisms | Control | 1% Chol | 2% Chol | 1% Chol + RUN | 2% Chol + RUN | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Me (Q1–Q3) | % Detected | Me (Q1–Q3) | % Detected | Me (Q1–Q3) | % Detected | Me (Q1–Q3) | % Detected | Me (Q1–Q3) | % Detected | |
| Total bacterial mass | 12.9 [12.8–13.0] | 100 | 13.0 [13.0–13.2] | 100 | 12.8 [12.8–13.0] | 83 | 12.9 [12.8–13.0] | 100 | 12.9 [12.7–13.0] | 100 |
| Lactobacillus spp. | 7.8 [7.6–8.5] | 100 | 8.7 [8.4–9.3] | 100 | 9.0 a [8.8–9.4] | 100 | 9.2 a [8.8–9.4] | 100 | 9.0 a [8.8–9.2] | 100 |
| Bifidobacterium spp. | 10.5 [8.9–11.5] | 100 | 8.7 [8.5–8.8] | 100 | 8.5 [8.1–8.8] | 100 | 8.3 a [8.3–8.7] | 100 | 8.3 a [8.1–8.4] | 100 |
| Escherichia coli | 6.8 [6.6–7.6] | 100 | 8.5 a [8.2–8.9] | 100 | 8.2 a [8.0–8.3] | 100 | 8.5 a [7.3–8.7] | 100 | 8.0 [7.9–8.2] | 100 |
| Bacteroides spp. | 11.7 [11.4–11.9] | 100 | 12.3 [12.0–12.3] | 100 | 11.9 [11.6–11.9] | 100 | 11.8 [11.7–12.0] | 100 | 11.8 [11.6–12.0] | 100 |
| Faecalibacterium prausnitzii | 9.0 [8.8–9.2] | 100 | 8.0 a [8.0–8.2] | 100 | 8.2 a [7.9–8.5] | 100 | 8.7 b [8.4–8.8] | 100 | 8.2 a [7.8–8.5] | 100 |
| Enterococcus spp. | 5.7 [5.6–5.7] | 33 | 7.3 [7.3–7.3] | 17 | – | 0 | 7.2 [6.0–8.5] | 33 | – | 0 |
| Staphylococcus aureus | – | 0 | 7.5 [6.7–8.2] | 75 | 6.5 [6.5–6.5] | 33 | – | 0 | 6.5 [6.5–6.5] | 33 |
| Proteus vulgaris/mirabilis | – | 0 | – | 0 | – | 0 | 5.8 [5.8–5.8] | 33 | – | 0 |
| Blautia spp. | 10.4 [10.3–10.8] | 100 | 11.5 a [11.4–11.8] | 100 | 11.5 [11.1–11.5] | 100 | 11.3 [11.1–11.4] | 100 | 11.1 [10.9–11.5] | 100 |
| Acinetobacter spp. | 8.5 [8.3–8.6] | 100 | 8.6 [8.5–8.8] | 100 | 8.2 a,b [8.0–8.3] | 100 | 8.3 b [8.1–8.3] | 100 | 8.0 a,b [8.0–8.0] | 100 |
| Streptococcus spp. | 7.5 [7.2–7.8] | 67 | 7.7 [7.7–7.8] | 100 | 7.6 [7.3–7.7] | 100 | 7.3 [7.1–7.7] | 100 | 7.4 b [7.3–7.6] | 100 |
| Roseburia inulinivorans | 8.5 [8.5–8.7] | 100 | 8.7 [8.6–8.8] | 100 | 8.7 [8.5–8.8] | 100 | 8.6 [8.5–8.8] | 100 | 8.7 [8.6–8.9] | 100 |
| Prevotella spp. | 8.0 [7.1–8.5] | 67 | 7.5 [7.2–7.7] | 50 | 6.8 [6.6–6.9] | 100 | 6.3 [5.7–7.8] | 83 | 6.7 [6.4–6.9] | 100 |
| Ruminococcus spp. | 7.7 [7.7–7.7] | 17 | 9.8 a [9.4–11.5] | 83 | 8.7 [8.6–8.7] | 33 | 10.5 [10.2–10.9] | 33 | 8.5 [8.2–10.2] | 50 |
| Bacteroides spp. and F. prausnitzii (Bfr/Fprau) ratio | 338 [133.3–1361.1] | – | 12,571 a [4285–20,000] | – | 5476 a [2821–8416] | – | 2500 b [1160–3125] | – | 4166 a [1833–8750] | – |
| Parameter | Animal Group | ||||
|---|---|---|---|---|---|
| Control | 1% Chol | 2% Chol | 1% Chol + RUN | 2% Chol + RUN | |
| Body weight, g | 302.2 ± 4.0 | 302.2 ± 6.4 | 302.0 ± 6.5 | 302.9 ± 5.7 | 302.1 ± 3.5 |
| Time in center, s | 5.9 ± 1.8 | 5.7 ± 1.8 | 6.1 ± 2.2 | 7.2 ± 2.2 | 5.6 ± 1.4 |
| Zone transitions | 10.7 ± 1.9 | 12.9 ± 1.6 | 12.6 ± 2.3 | 12.6 ± 2.0 | 11.6 ± 1.7 |
| Distance, s | 1764 ± 92 | 1762 ± 112 | 1783 ± 105 | 1720 ± 94 | 1692 ± 135 |
| Days of Experiment | Speed. cm/s | Time. min | Track Slope. Degrees |
|---|---|---|---|
| 9 11 16 18 | 20 | 10 | 0 |
| 23 25 30 32 | 24 | 10 | 0 |
| 37 39 44 46 51 53 | 26 | 10 | 0 |
| 58 60 65 67 71 74 79 | 28 | 10 | 0 |
| 81 85 88 92 93 | 30 | 10 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sidorova, Y.S.; Petrov, N.A.; Markova, Y.M.; Kolobanov, A.I.; Zorin, S.N. The Influence of a High-Cholesterol Diet and Forced Training on Lipid Metabolism and Intestinal Microbiota in Male Wistar Rats. Int. J. Mol. Sci. 2024, 25, 5383. https://doi.org/10.3390/ijms25105383
Sidorova YS, Petrov NA, Markova YM, Kolobanov AI, Zorin SN. The Influence of a High-Cholesterol Diet and Forced Training on Lipid Metabolism and Intestinal Microbiota in Male Wistar Rats. International Journal of Molecular Sciences. 2024; 25(10):5383. https://doi.org/10.3390/ijms25105383
Chicago/Turabian StyleSidorova, Yuliya S., Nikita A. Petrov, Yuliya M. Markova, Alexey I. Kolobanov, and Sergey N. Zorin. 2024. "The Influence of a High-Cholesterol Diet and Forced Training on Lipid Metabolism and Intestinal Microbiota in Male Wistar Rats" International Journal of Molecular Sciences 25, no. 10: 5383. https://doi.org/10.3390/ijms25105383






