Anticancer Effects of the Novel Pyrazolyl-Urea GeGe-3
Abstract
:1. Introduction
2. Results and Discussion
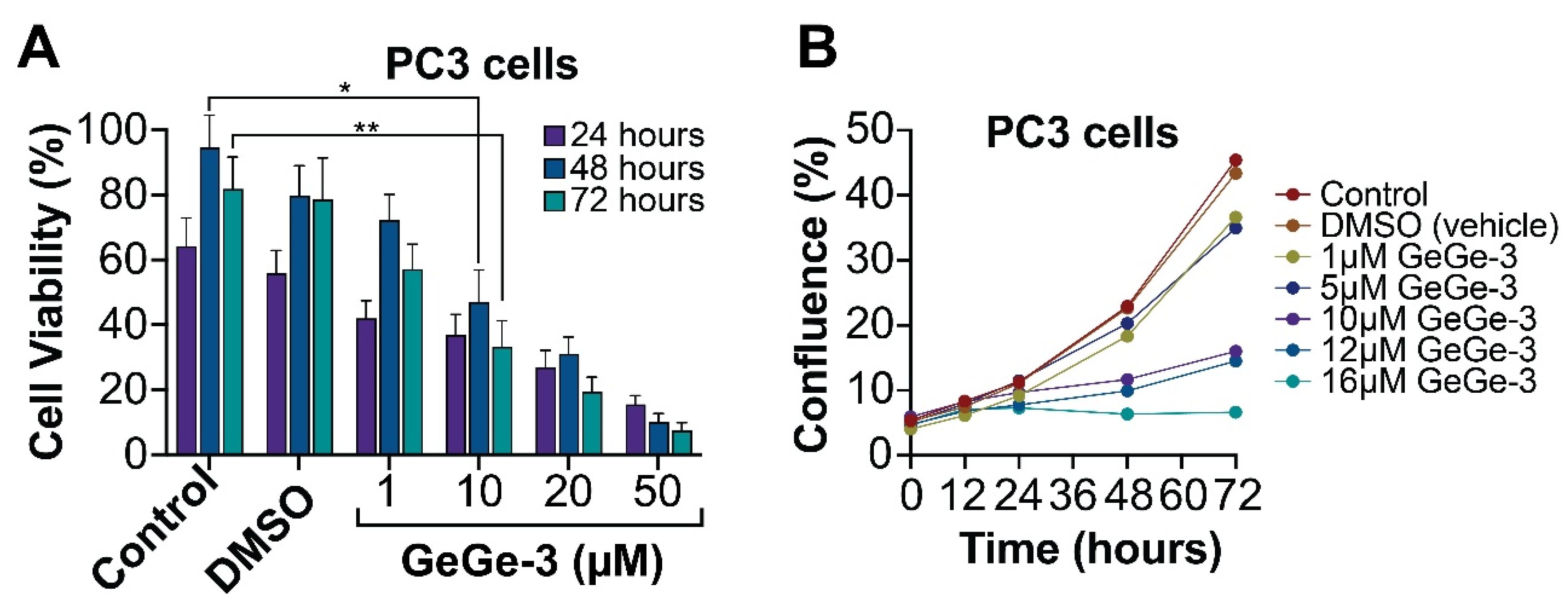
2.1. Effect of GeGe-3 on Cell Proliferation
2.2. Effect of GeGe-3 on Cell Metabolism
2.3. Effect of GeGe-3 on Cell Migration and Invasion
3. Materials and Methods
3.1. Cell Culture and Reagents
3.2. Trypan Blue Cell Viability Assay
3.3. MTT Assay
3.4. Cell Confluence Assay
3.5. Scratch Assay
3.6. Trans-Well Migration Assays
3.7. Statistical Analysis of Data
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lusardi, M.; Spallarossa, A.; Brullo, C. Amino-pyrazoles in medicinal chemistry: A review. Int. J. Mol. Sci. 2023, 24, 7834. [Google Scholar] [CrossRef] [PubMed]
- Güniz Küçükgüzel, Ş.; Şenkardeş, S. Recent advances in bioactive pyrazoles. Eur. J. Med. Chem. 2015, 97, 786–815. [Google Scholar] [CrossRef]
- Alam, M.J.; Alam, O.; Naim, M.J.; Nawaz, F.; Manaithiya, A.; Imran, M.; Thabet, H.K.; Aslhehri, S.; Ghoneim, M.M.; Alam, P.; et al. Recent advancement in drug design and discovery of pyrazole biomolecules as cancer and inflammation therapeutics. Molecules 2022, 27, 8708. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Thorn, C.F.; Bertagnolli, M.M.; Grosser, T.; Altman, R.B.; Klein, T.E. Celecoxib pathways: Pharmacokinetics and pharmacodynamics. Pharmacogenet. Genom. 2012, 22, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Maciejewska, N.; Olszewski, M.; Jurasz, J.; Serocki, M.; Dzierzynska, M.; Cekala, K.; Wieczerzak, E.; Baginski, M. Novel chalcone-derived pyrazoles as potential therapeutic agents for the treatment of non-small cell lung cancer. Sci. Rep. 2022, 12, 3703. [Google Scholar] [CrossRef] [PubMed]
- El-Gamal, M.I.; Zararei, S.O.; Madkour, M.M.; Anbar, H.S. Evaluation of substituted pyrazole-based kinase inhibitors in one decade (2011–2020): Current status and future prospects. Molecules 2022, 27, 330. [Google Scholar] [CrossRef] [PubMed]
- Bennai, F.E.; Doudach, L.; Cherrah, Y.; Ramli, Y.; Karrouchi, K.; Ansar, M.; Faouzi, M.E.A. Overview of the recent developments of pyrazole derivatives as anticancer agents in different cell lines. Bioorg. Chem. 2020, 97, 103470. [Google Scholar]
- Dube, Z.F.; Soremekun, O.S.; Ntombela, T.; Alahmdi, M.I.; Abo-Dya, N.E.; Sidhom, P.A.; Shawky, A.M.; Shibl, M.F.; Soliman, M.E. Inherent efficacies of pyrazole-based derivatives for cancer therapy: The interface between experiment and in silico. Future Med. Chem. 2023, 15, 1719–1738. [Google Scholar] [CrossRef]
- Nitulescu, G.M.; Stancov, G.; Seremet, O.C.; Nitulescu, G.; Mihai, D.P.; Duta-Brau, C.G.; Barbuceanu, S.F.; Olaru, O.T. The importance of the pyrazole scaffold in the design of protein kinase inhibitors as targeted anticancer therapies. Molecules 2023, 28, 5359. [Google Scholar] [CrossRef]
- Meta, E.; Brullo, C.; Sidibe, A.; Imhof, B.A.; Bruno, O. Design, synthesis and biological evaluation of new pyrazolyl-ureas and imidazopyrazolecarboxamides able to interfere with MAPK and PI3K upstream signaling involved in the angiogenesis. Eur. J. Med. Chem. 2017, 133, 24–35. [Google Scholar] [CrossRef]
- Meta, E.; Imhof, B.A.; Roprazb, P.; Fish, R.J.; Brullo, C.; Bruno, O.; Sidibé, A. The pyrazolyl-urea GeGe3 inhibits tumor angiogenesis and reveals dystrophia myotonica protein kinase (DMPK)1 as a novel angiogenesis target. Oncotarget 2017, 8, 108195–108212. [Google Scholar] [CrossRef] [PubMed]
- Morretta, E.; Belvedere, R.; Petrella, A.; Spallarossa, A.; Rapetti, F.; Bruno, O.; Brullo, C.; Monti, M.C. Novel insights on the molecular mechanism of action of the anti-angiogenic pyrazolylurea GeGe-3 by functional proteomics. Bioorg. Chem. 2021, 115, 105168. [Google Scholar] [CrossRef]
- Elíes, J.; Yáñez, M.; Pereira, T.M.C.; Gil-Longo, J.; MacDougall, D.A.; Campos-Toimil, M. An update to calcium binding proteins. Adv. Exp. Med. Biol. 2020, 1131, 183–213. [Google Scholar]
- Sun, J.; Mu, H.; Dai, K.; Yi, L. Calreticulin: A potential anti-cancer therapeutic target. Pharmazie 2017, 72, 503–510. [Google Scholar]
- Fucikova, J.; Spisek, R.; Kroemer, G.; Galluzzi, L. Calreticulin and cancer. Cell Res. 2021, 31, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Yumoto, F.; Ohki, S.; Yasunaga, T.; Tanokura, M.; Wakabayashi, T. Structural basis for Ca2+-regulated muscle relaxation at interaction sites of troponin with actin and tropomyosin. J. Mol. Biol. 2005, 352, 178–201. [Google Scholar] [CrossRef]
- Michalak, M.; Murphy-Ullrich, J.E. Calreticulin: Non-endoplasmic reticulum functions in physiology and disease. FASEB J. 2010, 24, 665–683. [Google Scholar]
- Zamanian, M.; Veerakumarasivam, A.; Abdullah, S.; Rosli, R. Calreticulin and cancer. Pathol. Oncol. Res. 2013, 19, 149–154. [Google Scholar] [CrossRef]
- Belvedere, R.; Morretta, E.; Novizio, N.; Morello, S.; Bruno, O.; Brullo, C.; Petrella, A. The pyrazolyl-urea GeGe3 inhibits the activity of ANXA1 in the angiogenesis induced by the pancreatic cancer derived EVs. Biomolecules 2021, 11, 1758. [Google Scholar] [CrossRef]
- D’Acunto, C.W.; Gbelcova, H.; Festa, M.; Rumi, T. The complex understanding of annexin A1 phosphorylation. Cell. Signal. 2014, 26, 173–178. [Google Scholar] [CrossRef]
- Keter, F.; Darkwa, J. Perspective: The potential of pyrazole-based compounds in medicine. Biometals 2012, 25, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Kasiotis, K.M.; Tzanetou, E.N.; Haroutounian, S.S. Pyrazoles as potential anti-angiogenesis agents: A contemporary overview. Front. Chem. 2014, 2, 78. [Google Scholar] [CrossRef] [PubMed]
- Ebenezer, O.; Shapi, M.; Tuszynski, J.A. A Review of the Recent Development in the Synthesis and Biological Evaluations of Pyrazole Derivatives. Biomedicines 2022, 10, 1124. [Google Scholar] [CrossRef]
- Karrouchi, K.; Radi, S.; Ramli, Y.; Taoufik, J.; Mabkhot, Y.N.; Al-aizari, F.A.; Ansar, M. Synthesis and Pharmacological Activities of Pyrazole Derivatives: A Review. Molecules 2018, 23, 134. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Huang, Y.-L.; Wang, M.-H.; Chen, C.-Y.; Chen, W.-M.; Wng, Y.-C.; Wu, P.-Y. Calreticulin regulates b1-integring mRNA stability in PC-3 prostate cancer cells. Biomedicines 2022, 10, 646. [Google Scholar] [CrossRef]
- Juan-Rivera, M.C.; Martinez-Ferrer, M. Integrin inhibitors in prostate cancer. Cancers 2018, 10, 44. [Google Scholar] [CrossRef]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Williams, A.; Cooper, E.; Clark, B.; Perry, L.; Ponassi, M.; Iervasi, E.; Brullo, C.; Greenhough, A.; Ladomery, M. Anticancer Effects of the Novel Pyrazolyl-Urea GeGe-3. Int. J. Mol. Sci. 2024, 25, 5380. https://doi.org/10.3390/ijms25105380
Williams A, Cooper E, Clark B, Perry L, Ponassi M, Iervasi E, Brullo C, Greenhough A, Ladomery M. Anticancer Effects of the Novel Pyrazolyl-Urea GeGe-3. International Journal of Molecular Sciences. 2024; 25(10):5380. https://doi.org/10.3390/ijms25105380
Chicago/Turabian StyleWilliams, Ashleigh, Emma Cooper, Bethany Clark, Laura Perry, Marco Ponassi, Erika Iervasi, Chiara Brullo, Alexander Greenhough, and Michael Ladomery. 2024. "Anticancer Effects of the Novel Pyrazolyl-Urea GeGe-3" International Journal of Molecular Sciences 25, no. 10: 5380. https://doi.org/10.3390/ijms25105380




