Proteomic Analysis of Umbilical Cord Mesenchymal Stem Cell-Derived Extracellular Vesicles: A Systematic Review
Abstract
1. Introduction
1.1. Umbilical Cord Mesenchymal Stem Cells in Regenerative Medicine
1.2. Extracellular Vesicles as Emerging Biotherapeutic Agent
1.3. Challenges of Therapeutics EVs in Clinical Translation
1.4. EV Proteomic Analysis as Future Standardization and Quality Control
2. Results
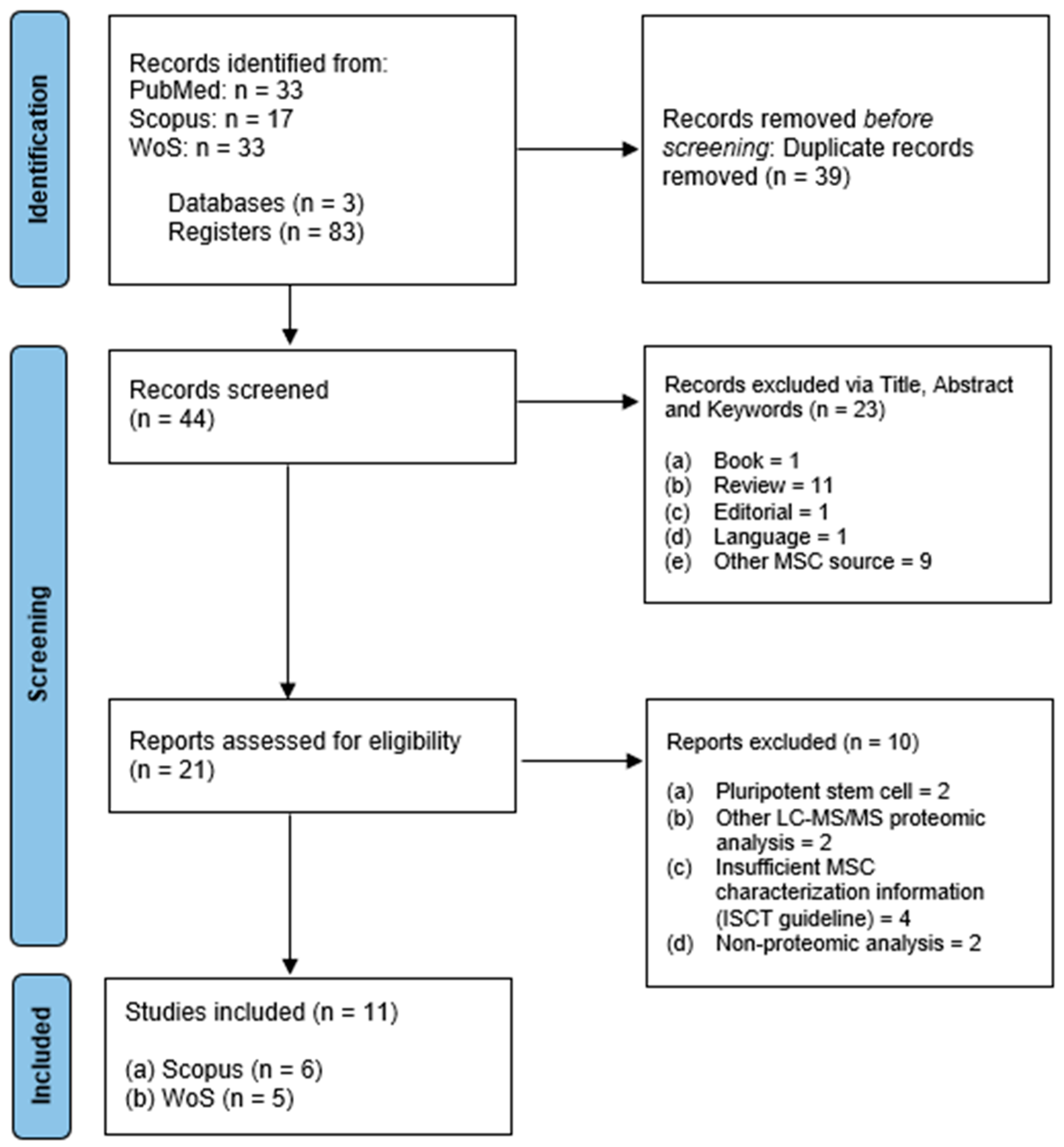
2.1. Search Results and Characteristics
2.2. Umbilical Cord Mesenchymal Stem Cell Characterization
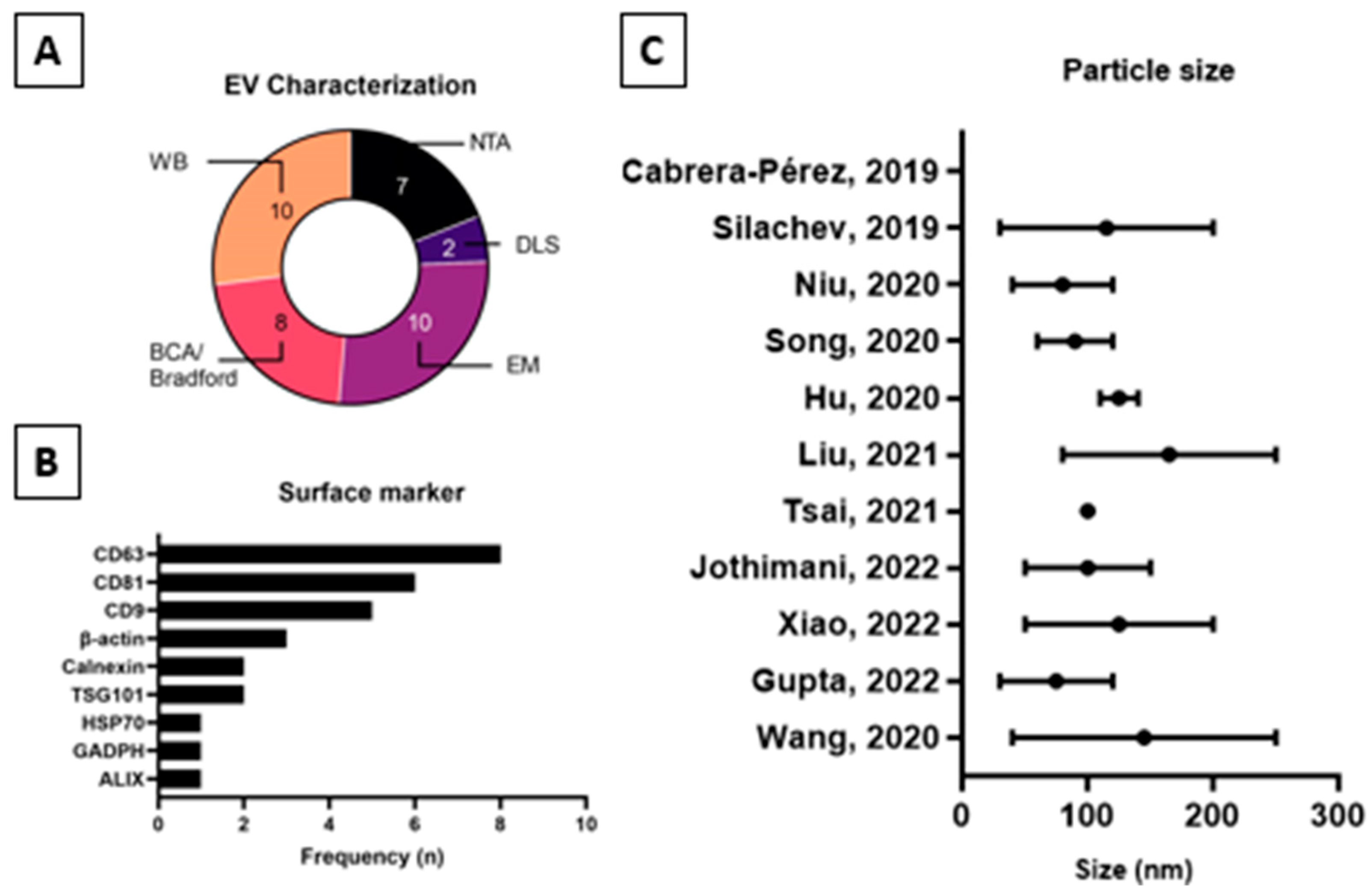
2.3. Extracellular Vesicles Characterization
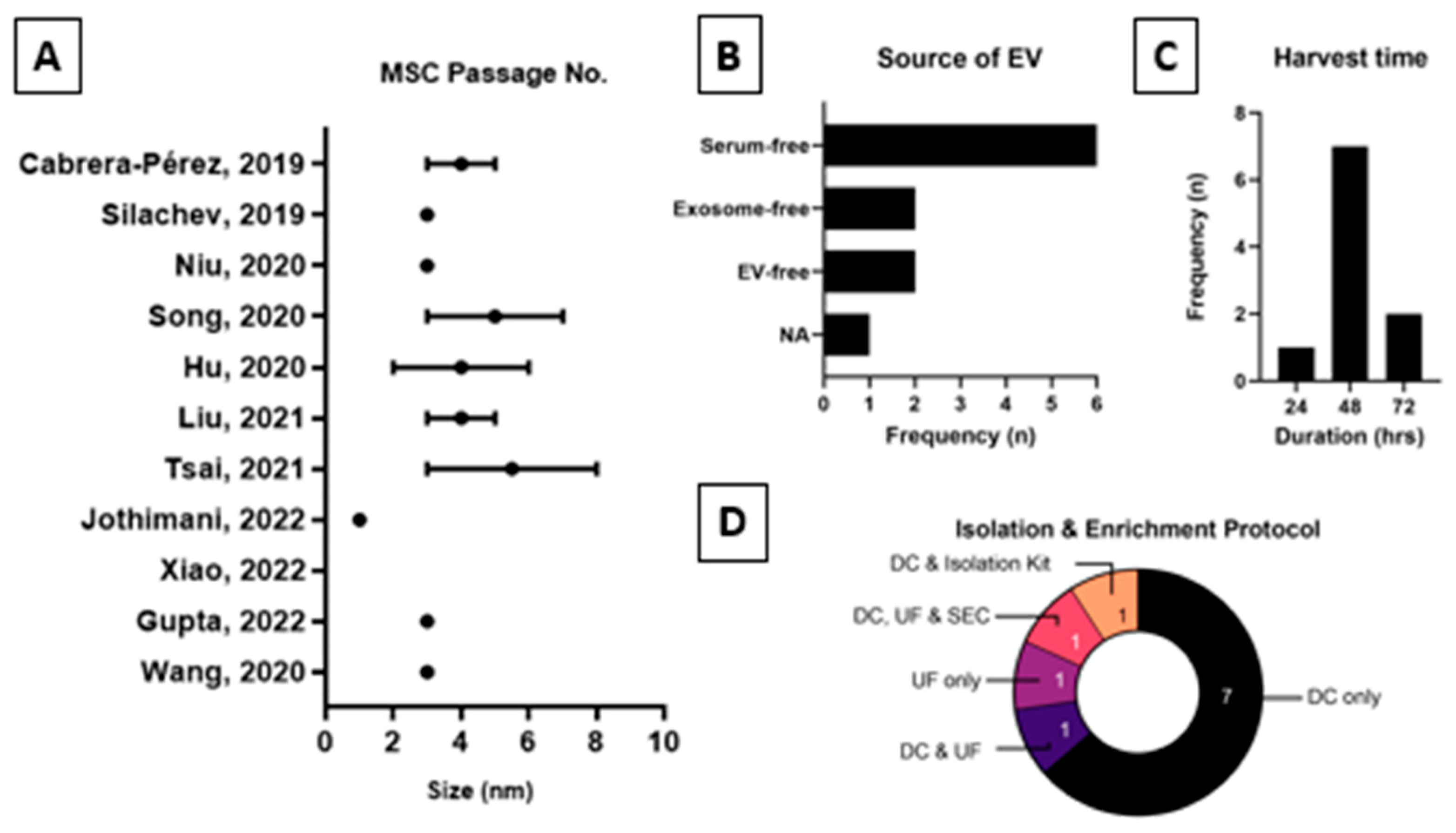
2.4. Extracellular Vesicles Production Process and Proteomics Analysis
3. Discussion
4. Materials and Methods
4.1. Search Strategy
4.2. Inclusion Criteria
4.3. Exclusion Criteria
4.4. Data Extraction and Management
4.5. Quality Assessment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Semenova, E.; Grudniak, M.P.; Machaj, E.K.; Bocian, K.; Chroscinska-Krawczyk, M.; Trochonowicz, M.; Stepaniec, I.M.; Murzyn, M.; E Zagorska, K.; Boruczkowski, D.; et al. Mesenchymal Stromal Cells from Different Parts of Umbilical Cord: Approach to Comparison & Characteristics. Stem Cell Rev. Rep. 2021, 17, 1780–1795. [Google Scholar] [CrossRef]
- Taghizadeh, R.; Cetrulo, K.; Cetrulo, C. Wharton’s Jelly stem cells: Future clinical applications. Placenta 2011, 32 (Suppl. S4), S311–S315. [Google Scholar] [CrossRef] [PubMed]
- Carlin, R.; Davis, D.; Weiss, M.; Schultz, B.; Troyer, D. Expression of early transcription factors Oct-4, Sox-2 and Nanog by porcine umbilical cord (PUC) matrix cells. Reprod. Biol. Endocrinol. 2006, 4, 8. [Google Scholar] [CrossRef] [PubMed]
- La Rocca, G.; Anzalone, R.; Corrao, S.; Magno, F.; Loria, T.; Lo Iacono, M.; Di Stefano, A.; Giannuzzi, P.; Marasà, L.; Cappello, F.; et al. Isolation and characterization of Oct-4+/HLA-G+ mesenchymal stem cells from human umbilical cord matrix: Differentiation potential and detection of new markers. Histochem. Cell Biol. 2009, 131, 267–282. [Google Scholar] [CrossRef]
- Fafián-Labora, J.A.; Morente-López, M.; Arufe, M.C. Effect of aging on behaviour of mesenchymal stem cells. World J. Stem Cells 2019, 11, 337–346. [Google Scholar] [CrossRef]
- Stolzing, A.; Jones, E.; McGonagle, D.; Scutt, A. Age-related changes in human bone marrow-derived mesenchymal stem cells: Consequences for cell therapies. Mech. Ageing Dev. 2008, 129, 163–173. [Google Scholar] [CrossRef]
- Baksh, D.; Yao, R.; Tuan, R.S. Comparison of Proliferative and Multilineage Differentiation Potential of Human Mesenchymal Stem Cells Derived from Umbilical Cord and Bone Marrow. Stem Cells 2007, 25, 1384–1392. [Google Scholar] [CrossRef]
- Liao, S.-K.; Cheng, H.; Ghetu, N.; Wallace, C.; Wei, F.-C. The Impact of Mesenchymal Stem Cell Source on Proliferation, Differentiation, Immunomodulation and Therapeutic Efficacy. J. Stem Cell Res. Ther. 2014, 4, 10. [Google Scholar]
- Pittenger, M.F.; Discher, D.E.; Péault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal stem cell perspective: Cell biology to clinical progress. NPJ Regen. Med. 2019, 4, 22. [Google Scholar] [CrossRef]
- Lanzoni, G.; Linetsky, E.; Correa, D.; Cayetano, S.M.; Alvarez, R.A.; Kouroupis, D.; Gil, A.A.; Poggioli, R.; Ruiz, P.; Marttos, A.C.; et al. Umbilical cord mesenchymal stem cells for COVID-19 acute respiratory distress syndrome: A double-blind, phase 1/2a, randomized controlled trial. Stem Cells Transl. Med. 2021, 10, 660–673. [Google Scholar] [CrossRef]
- Dilogo, I.H.; Aditianingsih, D.; Sugiarto, A.; Burhan, E.; Damayanti, T.; Sitompul, P.A.; Mariana, N.; Antarianto, R.D.; Liem, I.K.; Kispa, T.; et al. Umbilical Cord Mesenchymal Stromal Cells as Critical COVID-19 Adjuvant Therapy: A Randomized Controlled Trial. Stem Cells Transl. Med. 2021, 10, 1279–1287. [Google Scholar] [CrossRef]
- Wang, S.; Guo, L.; Ge, J.; Yu, L.; Cai, T.; Tian, R.; Jiang, Y.; Zhao, R.C.; Wu, Y. Excess Integrins Cause Lung Entrapment of Mesenchymal Stem Cells. Stem Cells 2015, 33, 3315–3326. [Google Scholar] [CrossRef]
- Fennema, E.M.; Tchang, L.A.; Yuan, H.; van Blitterswijk, C.A.; Martin, I.; Scherberich, A.; de Boer, J. Ectopic bone formation by aggregated mesenchymal stem cells from bone marrow and adipose tissue: A comparative study. J. Tissue Eng. Regen. Med. 2018, 12, e150–e158. [Google Scholar] [CrossRef]
- Jeong, J.O.; Han, J.W.; Kim, J.M.; Cho, H.J.; Park, C.; Lee, N.; Kim, D.W.; Yoon, Y.S. Malignant Tumor Formation after Transplantation of Short-Term Cultured Bone Marrow Mesenchymal Stem Cells in Experimental Myocardial Infarction and Diabetic Neuropathy. Circ. Res. 2011, 108, 1340–1347. [Google Scholar] [CrossRef]
- Riazifar, M.; Mohammadi, M.R.; Pone, E.J.; Yeri, A.; Lässer, C.; Segaliny, A.I.; McIntyre, L.L.; Shelke, G.V.; Hutchins, E.; Hamamoto, A.; et al. Stem Cell-Derived Exosomes as Nanotherapeutics for Autoimmune and Neurodegenerative Disorders. ACS Nano 2019, 13, 6670–6688. [Google Scholar] [CrossRef]
- Willis, G.R.; Fernandez-Gonzalez, A.; Anastas, J.; Vitali, S.H.; Liu, X.; Ericsson, M.; Kwong, A.; Mitsialis, S.A.; Kourembanas, S. Mesenchymal Stromal Cell Exosomes Ameliorate Experimental Bronchopulmonary Dysplasia and Restore Lung Function through Macrophage Immunomodulation. Am. J. Respir. Crit. Care Med. 2018, 197, 104–116. [Google Scholar] [CrossRef]
- Lener, T.; Gimona, M.; Aigner, L.; Börger, V.; Buzas, E.; Camussi, G.; Chaput, N.; Chatterjee, D.; Court, F.A.; del Portillo, H.A.; et al. Applying extracellular vesicles based therapeutics in clinical trials—An ISEV position paper. J. Extracell. Vesicles 2015, 4, 30087. [Google Scholar] [CrossRef]
- Ma, Z.-J.; Yang, J.-J.; Lu, Y.-B.; Liu, Z.-Y.; Wang, X.-X. Mesenchymal stem cell-derived exosomes: Toward cell-free therapeutic strategies in regenerative medicine. World J. Stem Cells 2020, 12, 814–840. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Lv, K.; Li, Q.; Zhang, L.; Wang, Y.; Zhong, Z.; Zhao, J.; Lin, X.; Wang, J.; Zhu, K.; Xiao, C.; et al. Incorporation of small extracellular vesicles in sodium alginate hydrogel as a novel therapeutic strategy for myocardial infarction. Theranostics 2019, 9, 7403–7416. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.-C.; Yuan, T.; Rui, B.-Y.; Zhu, Z.-Z.; Guo, S.-C.; Zhang, C.-Q. Exosomes derived from human platelet-rich plasma prevent apoptosis induced by glucocorticoid-associated endoplasmic reticulum stress in rat osteonecrosis of the femoral head via the Akt/Bad/Bcl-2 signal pathway. Theranostics 2017, 7, 733–750. [Google Scholar] [CrossRef]
- Jia, Y.; Zhu, Y.; Qiu, S.; Xu, J.; Chai, Y. Exosomes secreted by endothelial progenitor cells accelerate bone regeneration during distraction osteogenesis by stimulating angiogenesis. Stem Cell Res. Ther. 2019, 10, 12. [Google Scholar] [CrossRef]
- Yin, S.; Ji, C.; Wu, P.; Jin, C.; Qian, H. Human umbilical cord mesenchymal stem cells and exosomes: Bioactive ways of tissue injury repair. Am. J. Transl. Res. 2019, 11, 1230–1240. [Google Scholar]
- Sun, X.; Shan, A.; Wei, Z.; Xu, B. Intravenous mesenchymal stem cell-derived exosomes ameliorate myocardial inflammation in the dilated cardiomyopathy. Biochem. Biophys. Res. Commun. 2018, 503, 2611–2618. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Rao, S.-S.; Ren, L.; Hu, X.-K.; Tan, Y.-J.; Hu, Y.; Luo, J.; Liu, Y.-W.; Yin, H.; Huang, J.; et al. Exosomal DMBT1 from human urine-derived stem cells facilitates diabetic wound repair by promoting angiogenesis. Theranostics 2018, 8, 1607–1623. [Google Scholar] [CrossRef]
- Rani, S.; Ritter, T. The Exosome—A Naturally Secreted Nanoparticle and its Application to Wound Healing. Adv. Mater. 2015, 28, 5542–5552. [Google Scholar] [CrossRef]
- Gimona, M.; Pachler, K.; Laner-Plamberger, S.; Schallmoser, K.; Rohde, E. Manufacturing of Human Extracellular Vesicle-Based Therapeutics for Clinical Use. Int. J. Mol. Sci. 2017, 18, 1190. [Google Scholar] [CrossRef]
- Willis, G.R.; Kourembanas, S.; Mitsialis, S.A. Toward Exosome-Based Therapeutics: Isolation, Heterogeneity, and Fit-for-Purpose Potency. Front. Cardiovasc. Med. 2017, 4, 63. [Google Scholar] [CrossRef]
- Witwer, K.W.; Van Balkom, B.W.M.; Bruno, S.; Choo, A.; Dominici, M.; Gimona, M.; Hill, A.F.; De Kleijn, D.; Koh, M.; Lai, R.C.; et al. Defining mesenchymal stromal cell (MSC)-derived small extracellular vesicles for therapeutic applications. J. Extracell. Vesicles 2019, 8, 1609206. [Google Scholar] [CrossRef] [PubMed]
- Gimona, M.; Brizzi, M.F.; Choo, A.B.H.; Dominici, M.; Davidson, S.M.; Grillari, J.; Hermann, D.M.; Hill, A.F.; de Kleijn, D.; Lai, R.C.; et al. Critical considerations for the development of potency tests for therapeutic applications of mesenchymal stromal cell-derived small extracellular vesicles. Cytotherapy 2021, 23, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Rohde, E.; Pachler, K.; Gimona, M. Manufacturing and characterization of extracellular vesicles from umbilical cord–derived mesenchymal stromal cells for clinical testing. Cytotherapy 2019, 21, 581–592. [Google Scholar] [CrossRef]
- Phinney, D.G.; Pittenger, M.F. Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem Cells 2017, 35, 851–858. [Google Scholar] [CrossRef]
- Toh, W.S.; Lai, R.C.; Zhang, B.; Lim, S.K. MSC exosome works through a protein-based mechanism of action. Biochem. Soc. Trans. 2018, 46, 843–853. [Google Scholar] [CrossRef]
- Nikfarjam, S.; Rezaie, J.; Zolbanin, N.M.; Jafari, R. Mesenchymal stem cell derived-exosomes: A modern approach in translational medicine. J. Transl. Med. 2020, 18, 449. [Google Scholar] [CrossRef]
- Cloet, T.; Momenbeitollahi, N.; Li, H. Recent advances on protein-based quantification of extracellular vesicles. Anal. Biochem. 2021, 622, 114168. [Google Scholar] [CrossRef]
- van Balkom, B.W.M.; Gremmels, H.; Giebel, B.; Lim, S.K. Proteomic Signature of Mesenchymal Stromal Cell-Derived Small Extracellular Vesicles. Proteomics 2019, 19, e1800163. [Google Scholar] [CrossRef]
- Carrera, M.; Mateos, J. Shotgun Proteomics Methods and Protocols Preface. In Shotgun Proteomics: Methods and Protocols; Methods in Molecular, Biology; Carrera, M., Mateos, J., Eds.; Humana: Louisville, KY, USA, 2021. [Google Scholar]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Cabrera-Pérez, R.; Monguió-Tortajada, M.; Gámez-Valero, A.; Rojas-Márquez, R.; Borràs, F.E.; Roura, S.; Vives, J. Osteogenic commitment of Wharton’s jelly mesenchymal stromal cells: Mechanisms and implications for bioprocess development and clinical application. Stem Cell Res. Ther. 2019, 10, 356. [Google Scholar] [CrossRef]
- Silachev, D.N.; Goryunov, K.V.; Shpilyuk, M.A.; Beznoschenko, O.S.; Morozova, N.Y.; Kraevaya, E.E.; Popkov, V.A.; Pevzner, I.B.; Zorova, L.D.; Evtushenko, E.A.; et al. Effect of MSCs and MSC-Derived Extracellular Vesicles on Human Blood Coagulation. Cells 2019, 8, 258. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Wang, X.; Li, M.; Niu, B. Exosomes from human umbilical cord Mesenchymal stem cells attenuates stress-induced hippocampal dysfunctions. Metab. Brain Dis. 2020, 35, 1329–1340. [Google Scholar] [CrossRef] [PubMed]
- Song, A.; Wang, J.; Tong, Y.; Fang, J.; Zhang, Y.; Zhang, H.; Ruan, H.; Wang, K.; Liu, Y. BKCa channels regulate the immunomodulatory properties of WJ-MSCs by affecting the exosome protein profiles during the inflammatory response. Stem Cell Res. Ther. 2020, 11, 440. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, Y.; Ni, C.-Y.; Chen, C.-Y.; Rao, S.-S.; Yin, H.; Huang, J.; Tan, Y.-J.; Wang, Z.-X.; Cao, J.; et al. Human umbilical cord mesenchymal stromal cells-derived extracellular vesicles exert potent bone protective effects by CLEC11A-mediated regulation of bone metabolism. Theranostics 2020, 10, 2293–2308. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Qiao, G.; Cao, W.; Li, C.; Pan, S.; Wang, L.; Liu, Y.; Ma, L.; Cui, D. Proteomics analyses reveal functional differences between exosomes of mesenchymal stem cells derived from the umbilical cord and those derived from the adipose tissue. Cell J. 2021, 23, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.C.-S.; Yang, K.D.; Chang, K.-H.; Lin, F.C.-F.; Chou, R.-H.; Li, M.-C.; Cheng, C.-C.; Kao, C.-Y.; Chen, C.-P.; Lin, H.-C.; et al. Umbilical Cord Mesenchymal Stromal Cell-Derived Exosomes Rescue the Loss of Outer Hair Cells and Repair Cochlear Damage in Cisplatin-Injected Mice. Int. J. Mol. Sci. 2021, 22, 6664. [Google Scholar] [CrossRef]
- Jothimani, G.; Pathak, S.; Dutta, S.; Duttaroy, A.K.; Banerjee, A. A Comprehensive Cancer-Associated MicroRNA Expression Profiling and Proteomic Analysis of Human Umbilical Cord Mesenchymal Stem Cell-Derived Exosomes. Tissue Eng. Regen. Med. 2022, 19, 1013–1031. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yin, F.; Guo, M.; Gan, G.; Lin, G.; Wen, C.; Wang, J.; Song, P.; Wang, J.; Qi, Z.; et al. Quantitative proteomic analysis of exosomes from umbilical cord mesenchymal stem cells and rat bone marrow stem cells. Proteomics 2022, 23, e2200204. [Google Scholar] [CrossRef]
- Gupta, S.; Harshita, S.; Naina, S.; Pranshu, R.E.; Manu, D.; Alka, Y.; Nidhi, N.; Kumar, A.; Baibaswata, N.; Arup, B.; et al. Comparative Evaluation of Anti-Fibrotic Effect of Tissue Specific Mesenchymal Stem Cells Derived Extracellular Vesicles for the Amelioration of CCl4 Induced Chronic Liver Injury. Stem Cell Rev. Rep. 2022, 18, 1097–1112. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-G.; He, Z.-Y.; Liang, S.; Yang, Q.; Cheng, P.; Chen, A.-M. Comprehensive proteomic analysis of exosomes derived from human bone marrow, adipose tissue, and umbilical cord mesenchymal stem cells. Stem Cell Res. Ther. 2020, 11, 511. [Google Scholar] [CrossRef] [PubMed]
- Almeria, C.; Kreß, S.; Weber, V.; Egger, D.; Kasper, C. Heterogeneity of mesenchymal stem cell-derived extracellular vesicles is highly impacted by the tissue/cell source and culture conditions. Cell Biosci. 2022, 12, 51. [Google Scholar] [CrossRef]
- Qiu, G.; Zheng, G.; Ge, M.; Wang, J.; Huang, R.; Shu, Q.; Xu, J. Functional proteins of mesenchymal stem cell-derived extracellular vesicles. Stem Cell Res. Ther. 2019, 10, 359. [Google Scholar] [CrossRef]
- Wang, Y.; Yi, H.; Song, Y. The safety of MSC therapy over the past 15 years: A meta-analysis. Stem Cell Res. Ther. 2021, 12, 545. [Google Scholar] [CrossRef]
- Cortés-Ríos, J.; Zárate, A.M.; Figueroa, J.D.; Medina, J.; Fuentes-Lemus, E.; Rodríguez-Fernández, M.; Aliaga, M.; López-Alarcón, C. Protein quantification by bicinchoninic acid (BCA) assay follows complex kinetics and can be performed at short incubation times. Anal. Biochem. 2020, 608, 113904. [Google Scholar] [CrossRef]
- Kielkopf, C.L.; Bauer, W.; Urbatsch, I.L. Bradford Assay for Determining Protein Concentration. Cold Spring Harb. Protoc. 2020, 2020, 102269. [Google Scholar] [CrossRef]
- Webber, J.; Clayton, A. How pure are your vesicles? J. Extracell. Vesicles 2013, 2, 19861. [Google Scholar] [CrossRef]
- Kodam, S.P.; Ullah, M. Diagnostic and Therapeutic Potential of Extracellular Vesicles. Technol. Cancer Res. Treat. 2021, 20. [Google Scholar] [CrossRef]
- Bağcı, C.; Sever-Bahcekapili, M.; Belder, N.; Bennett, A.P.S.; Erdener, Ş.E.; Dalkara, T. Overview of extracellular vesicle characterization techniques and introduction to combined reflectance and fluorescence confocal microscopy to distinguish extracellular vesicle subpopulations. Neurophotonics 2022, 9, 021903. [Google Scholar] [CrossRef]
- Sormunen, A.; Koivulehto, E.; Alitalo, K.; Saksela, K.; Laham-Karam, N.; Ylä-Herttuala, S. Comparison of Automated and Traditional Western Blotting Methods. Methods Protoc. 2023, 6, 43. [Google Scholar] [CrossRef]
- Nik Mohamed Kamal, N.N.S.; Awang, R.A.R.; Mohamad, S.; Shahidan, W.N.S. Plasma- and Saliva Exosome Profile Reveals a Distinct MicroRNA Signature in Chronic Periodontitis. Front. Physiol. 2020, 11, 587381. [Google Scholar] [CrossRef]
- Cacciottolo, M.; Nice, J.B.; Li, Y.; LeClaire, M.J.; Twaddle, R.; Mora, C.L.; Adachi, S.Y.; Chin, E.R.; Young, M.; Angeles, J.; et al. Exosome-Based Multivalent Vaccine: Achieving Potent Immunization, Broadened Reactivity, and Strong T-Cell Responses with Nanograms of Proteins. Microbiol. Spectr. 2023, 11, e0050323. [Google Scholar] [CrossRef] [PubMed]
- Turaga, S.M.; Sardiu, M.E.; Vishwakarma, V.; Mitra, A.; Bantis, L.E.; Madan, R.; Merchant, M.L.; Klein, J.B.; Samuel, G.; Godwin, A.K. Identification of small extracellular vesicle protein biomarkers for pediatric Ewing Sarcoma. Front. Mol. Biosci. 2023, 10, 1138594. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Yuan, Z.; Weng, J.; Pei, D.; Du, X.; He, C.; Lai, P. Challenges and advances in clinical applications of mesenchymal stromal cells. J. Hematol. Oncol. 2021, 14, 24. [Google Scholar] [CrossRef]
- George, B. Regulations and guidelines governing stem cell based products: Clinical considerations. Perspect. Clin. Res. 2011, 2, 94–99. [Google Scholar] [CrossRef]
- Childs, P.G.; Reid, S.; Salmeron-Sanchez, M.; Dalby, M.J. Hurdles to uptake of mesenchymal stem cells and their progenitors in therapeutic products. Biochem. J. 2020, 477, 3349–3366. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Aromataris, E.; Fernandez, R.; Godfrey, C.M.; Holly, C.; Khalil, H.; Tungpunkom, P. Summarizing systematic reviews: Methodological development, conduct and reporting of an umbrella review approach. Int. J. Evid. Based Healthcare 2015, 13, 132–140. [Google Scholar] [CrossRef]
| Author, Year (Reference) | MSC Source | TDP | Minimal Criteria | Optional | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | C | O | Positive | Negative | Positive | Negative | ||||||||||
| CD73 | CD90 | CD105 | CD11b | CD14 | CD34 | CD45 | CD29 | CD44 | CD20 | CD31 | HLA-DR | |||||
| Cabera-Perez, R., 2019 [39] | FI | × | × | × | × | × | × | × | ||||||||
| Slachev, D.M., 2019 [40] | FI | × | × | × | × | × | × | × | ||||||||
| Niu, Y., 2020 [41] | FI | × | × | × | × | × | ||||||||||
| Song, A., 2020 [42] | FI | × | × | × | × | × | × | × | × | |||||||
| Hu, Y., 2020 [43] | FI | × | × | × | × | × | × | × | × | × | ||||||
| Liu, B., 2021 [44] | FI | × | × | × | × | × | × | |||||||||
| Tsai, S.C.S., 2021 [45] | FI | × | × | × | × | × | × | × | ||||||||
| Jothimani, G., 2022 [46] | FI | × | × | × | × | |||||||||||
| Xiao, X., 2022 [47] | FI | × | × | |||||||||||||
| Gupta, S., 2022 [48] | FI | × | × | × | × | × | × | × | × | × | ||||||
| Wang, Z.G., 2020 [49] | CL | × | × | × | × | × | × | × | × | × | × | |||||
| Author, Year (Reference) | Size (nm) | Particle Count | Protein Concentration | EM | WB |
|---|---|---|---|---|---|
| Cabera-Perez, R., 2019 [39] | × | ||||
| Silachev, D.M., 2019 [40] | 40–250 | NTA | × | × | TF and β-actin |
| Niu, Y., 2020 [41] | 30–120 | × | × | CD9, CD63, CD81, and β-actin | |
| Song, A., 2020 [42] | 50–200 | NTA | × | × | CD63 and CD81 |
| Hu, Y., 2020 [43] | 50–150 | DLS | × | × | CD9, CD63, CD81, TSG101 and GADPH |
| Liu, B., 2021 [44] | 100 | NTA | × | CD63 and β-actin | |
| Tsai, S.C.S., 2021 [45] | 80–250 | NTA | × | CD9, CD63, CD81 and HSP70 | |
| Jothimani, G., 2022 [46] | 110–140 | DLS | × | CD63 | |
| Xiao, X., 2022 [47] | 60–120 | NTA | × | × | CD9, CD63, CD81, Calnexin and β-actin |
| Gupta, S., 2022 [48] | 40–120 | NTA | × | × | CD63 and ALIX |
| Wang, Z.G., 2020 [49] | 30–200 | NTA | × | × | CD9, CD81, TSG101 and Calnexin |
| Author, Year (Ref.) | Culture Medium | MSC Passage | Harvest (h) | EV Source and Enrichment Protocol |
|---|---|---|---|---|
| Cabrera-Pérez, R., 2019 [39] | DMEM with Glu and 10% hserB | 3–5 | NA | EV-depleted media via DC, UF, and SEC:
|
| Silachev, D.N., 2019 [40] | DMEM/F12 (1:1) with Glu, AA, and 7% FBS | 3 | 24 | DC:
|
| Niu, Y., 2020 [41] | DMEM/F12 with 1% AA and 10% FBS | 3 | 48 | Serum-free media via DC:
|
| Song, A., 2020 [42] | αMEM with 1% AA and 10% FBS | 3–7 | 48 | Exosome-free FBS via DC:
|
| Hu, Y., 2020 [43] | DMEM/F12 with 10% FBS, 1% Glu, and AA. | 2–6 | 48 | EV-free FBS via DC and UF:
|
| Liu, B., 2021 [44] | MSC serum-free medium | 3–5 | 72 | Serum-free medium via DC:
|
| Tsai, S.C.S., 2021 [45] | LG DMEM with 10% FBS | 3–8 | 48 | Serum-free media via UF:
|
| Jothimani, G., 2022 [46] | LG DMEM with 20% FBS, Glu, 1% AA | 1 | 72 | Exosome-depleted medium via DC:
|
| Xiao, X., 2022 [47] | DMEM/F12 with 20% FBS | NA | 48 | Serum-free medium via DC:
|
| Gupta, S., 2022 [48] | LG DMEM with 10% FBS, Glu, 1% AA | 3 | 48 | Serum-free medium via DC and exosome isolation kit:
|
| Wang, Z.G., 2020 [49] | Specific culture media | 3 | 48 | Serum-free medium via DC:
|
| Author, Year (Ref.) | Search Algorithm | Proteomic Database | No. of Proteins | Bioinformatic Analysis | Result |
|---|---|---|---|---|---|
| Cabrera-Pérez, R., 2019 [39] | Mascot | SwissProt compared to EVpedia, ExoCarta, and Vesiclepedia | 119 | NA |
|
| Silachev, D.N., 2019 [40] | MaxQuant | SwissProt | 148 | GO (Perseus software v2.0.11) |
|
| Niu, Y., 2020 [41] | MaxQuant | Compared with Vesiclepedia | 942 | NA |
|
| Song, A., 2020 [42] | Spectronaut Pulsar | UniProt | 2196 | KEGG and GO |
|
| Hu, Y., 2020 [43] | MaxQuant | SwissProt | 5570 | GO (UniProt-GOA, InterPro) |
|
| Liu, B., 2021 [44] | Sequest HT and Proteome Discoverer | UniProt | 485 | STRING, GO, KEGG, ClueGO, and CluePedia |
|
| Tsai, S.C.S., 2021 [45] | NA | NA | >1500 | NA |
|
| Jothimani, G., 2022 [46] | Mascot | NA | 214 | STRING, GO |
|
| Xiao, X., 2022 [47] | ProteomeXchange | UniProtKB/SwissProt Compared with ExoCarta and Vesiclepedia | 4200 | David Bioinformatics Resources, KEGG, and STRING |
|
| Gupta, S., 2022 [48] | Paragon | ProteinPilot | NA | GO, Reactome, KEGG, and STRING |
|
| Wang, Z.G., 2020 [49] | MaxQuant | SwissProt Compared with ExoCarta | 431 | GO, KEGG, and KAAS |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krishnan, I.; Chan, A.M.L.; Law, J.X.; Ng, M.H.; Jayapalan, J.J.; Lokanathan, Y. Proteomic Analysis of Umbilical Cord Mesenchymal Stem Cell-Derived Extracellular Vesicles: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 5340. https://doi.org/10.3390/ijms25105340
Krishnan I, Chan AML, Law JX, Ng MH, Jayapalan JJ, Lokanathan Y. Proteomic Analysis of Umbilical Cord Mesenchymal Stem Cell-Derived Extracellular Vesicles: A Systematic Review. International Journal of Molecular Sciences. 2024; 25(10):5340. https://doi.org/10.3390/ijms25105340
Chicago/Turabian StyleKrishnan, Illayaraja, Alvin Man Lung Chan, Jia Xian Law, Min Hwei Ng, Jaime Jacqueline Jayapalan, and Yogeswaran Lokanathan. 2024. "Proteomic Analysis of Umbilical Cord Mesenchymal Stem Cell-Derived Extracellular Vesicles: A Systematic Review" International Journal of Molecular Sciences 25, no. 10: 5340. https://doi.org/10.3390/ijms25105340
APA StyleKrishnan, I., Chan, A. M. L., Law, J. X., Ng, M. H., Jayapalan, J. J., & Lokanathan, Y. (2024). Proteomic Analysis of Umbilical Cord Mesenchymal Stem Cell-Derived Extracellular Vesicles: A Systematic Review. International Journal of Molecular Sciences, 25(10), 5340. https://doi.org/10.3390/ijms25105340









