The RNA-Binding Protein BoRHON1 Positively Regulates the Accumulation of Aliphatic Glucosinolates in Cabbage
Abstract
:1. Introduction
2. Results
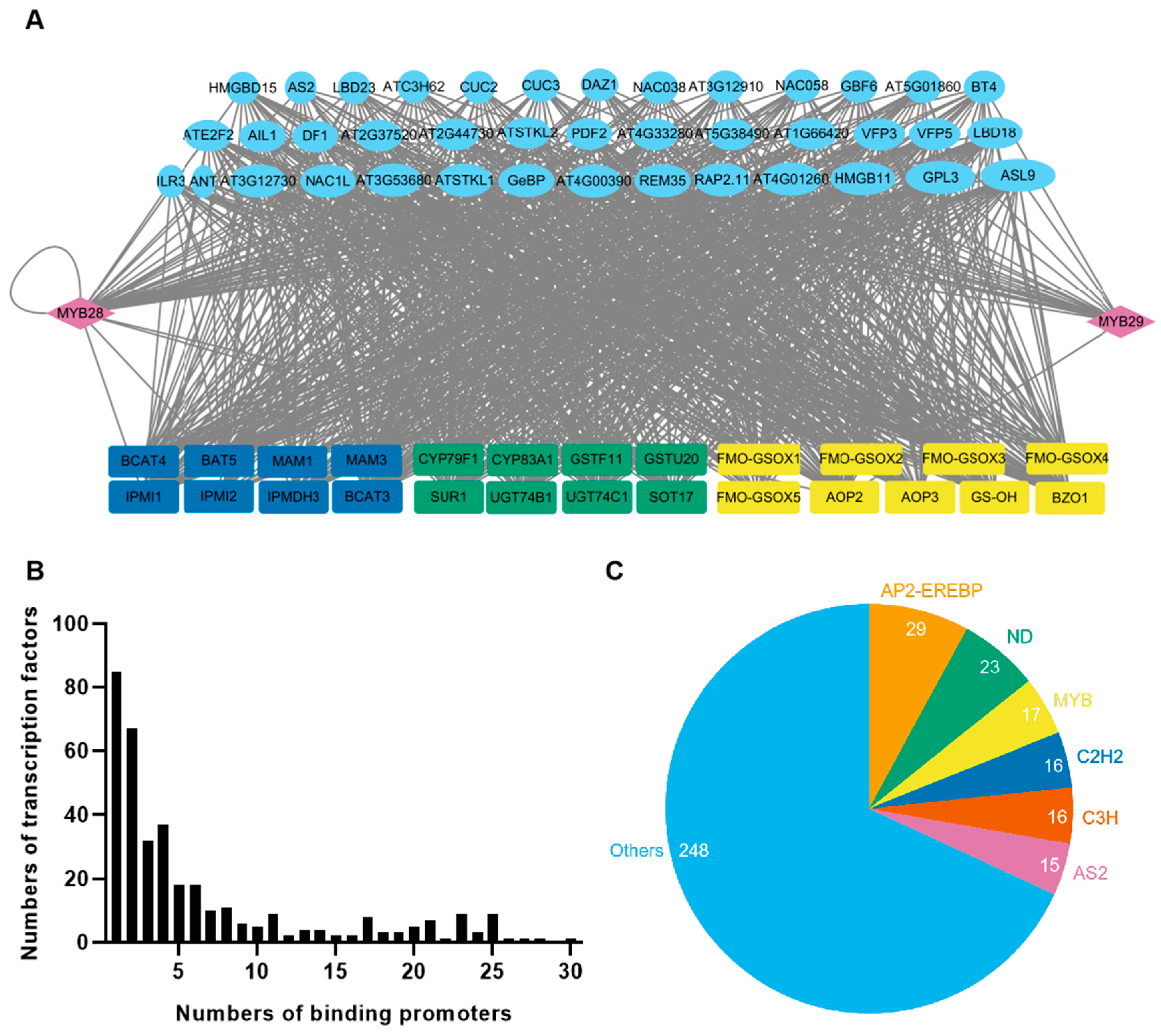
2.1. Transcriptional Regulatory Networks of MYB28 and MYB29 in Arabidopsis
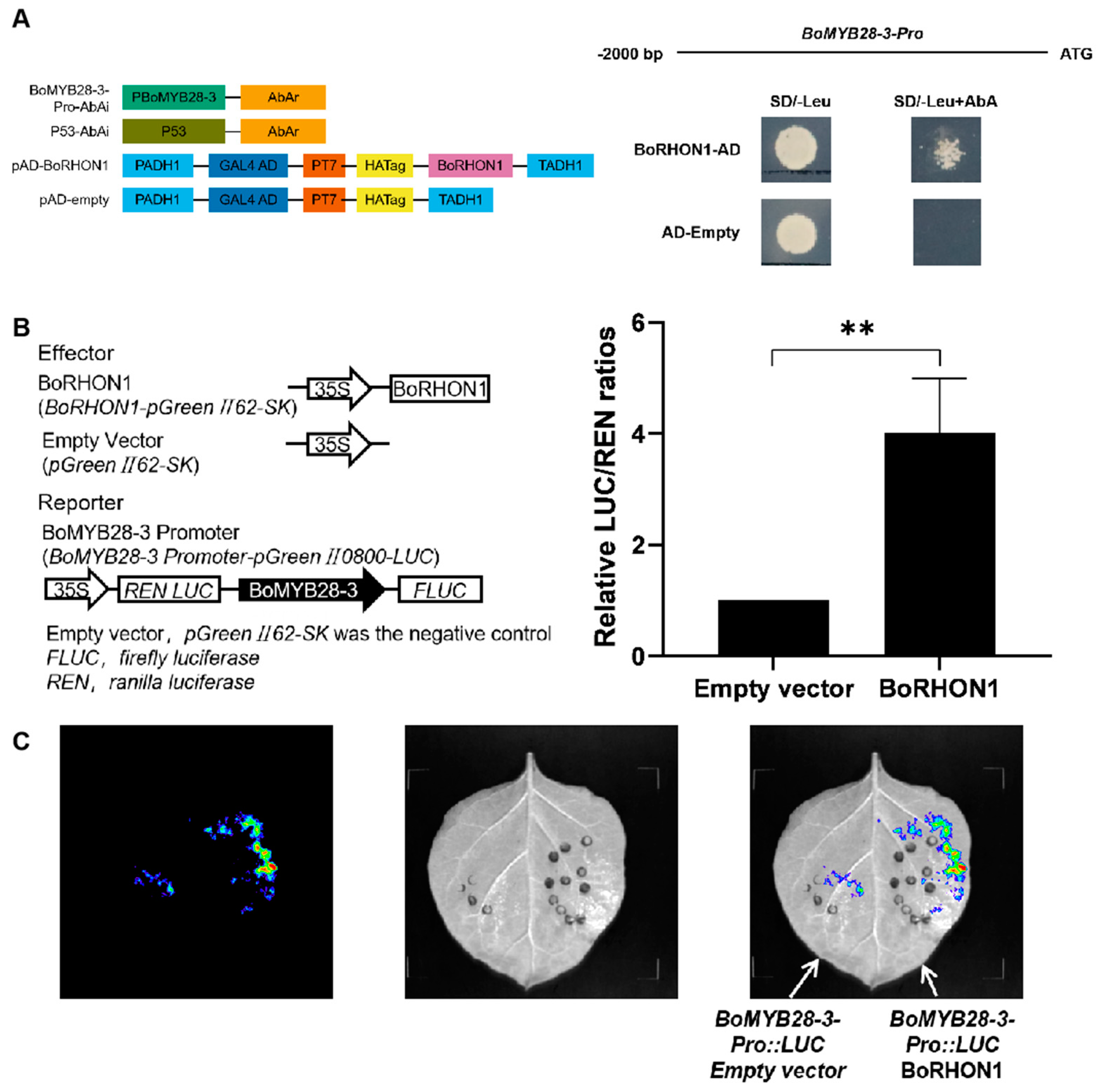
2.2. Identification of Upstream Regulators of BoMYB28s in Cabbage
2.3. BoRHON1 as a Novel Candidate Regulator of the Aliphatic Glucosinolates Pathway for Functional Validation in Cabbage
2.4. Sequence Analyses of BoRHON1
2.5. Expression Patterns and Sub-Cellular Localization of BoRHON1
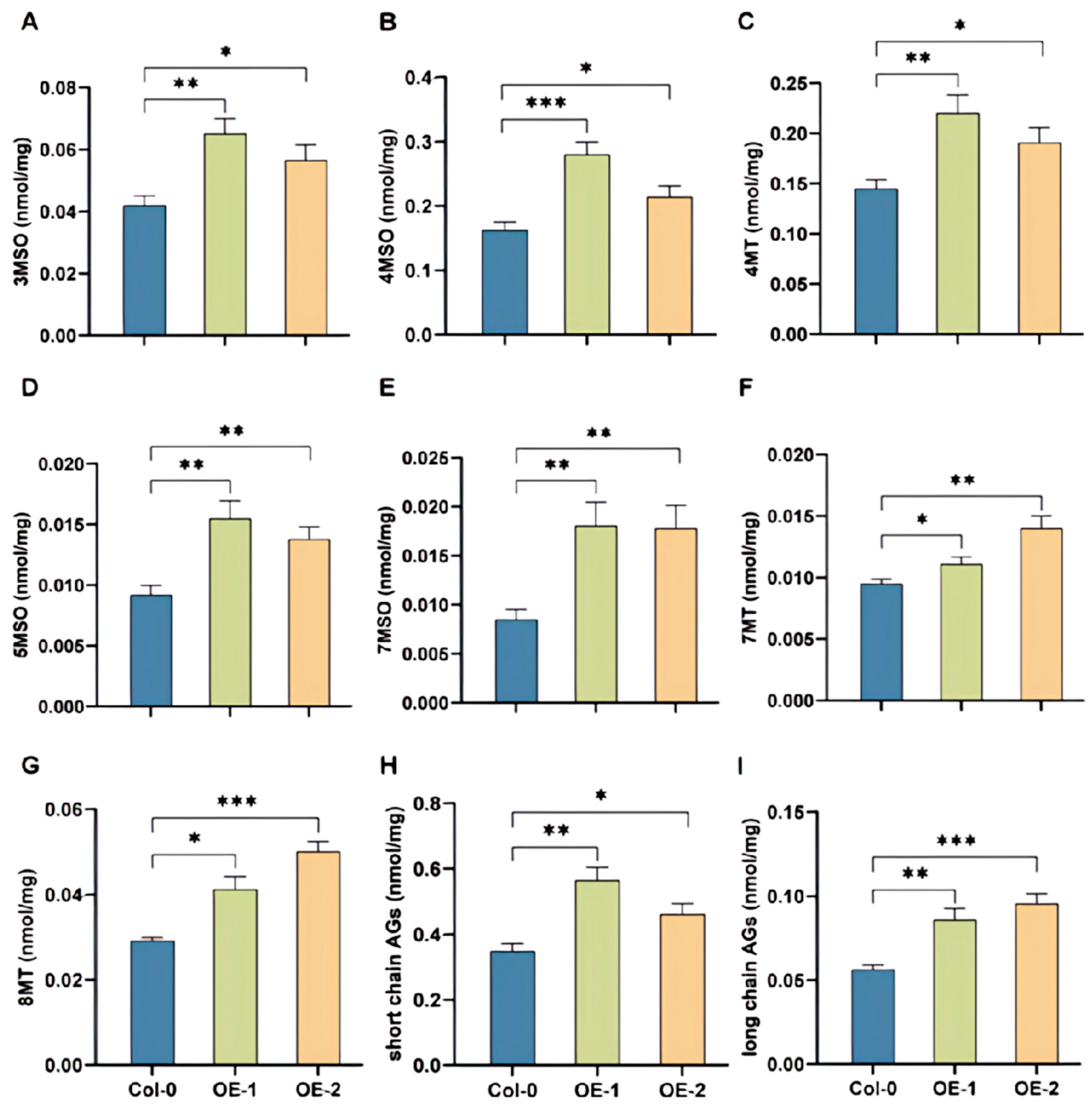
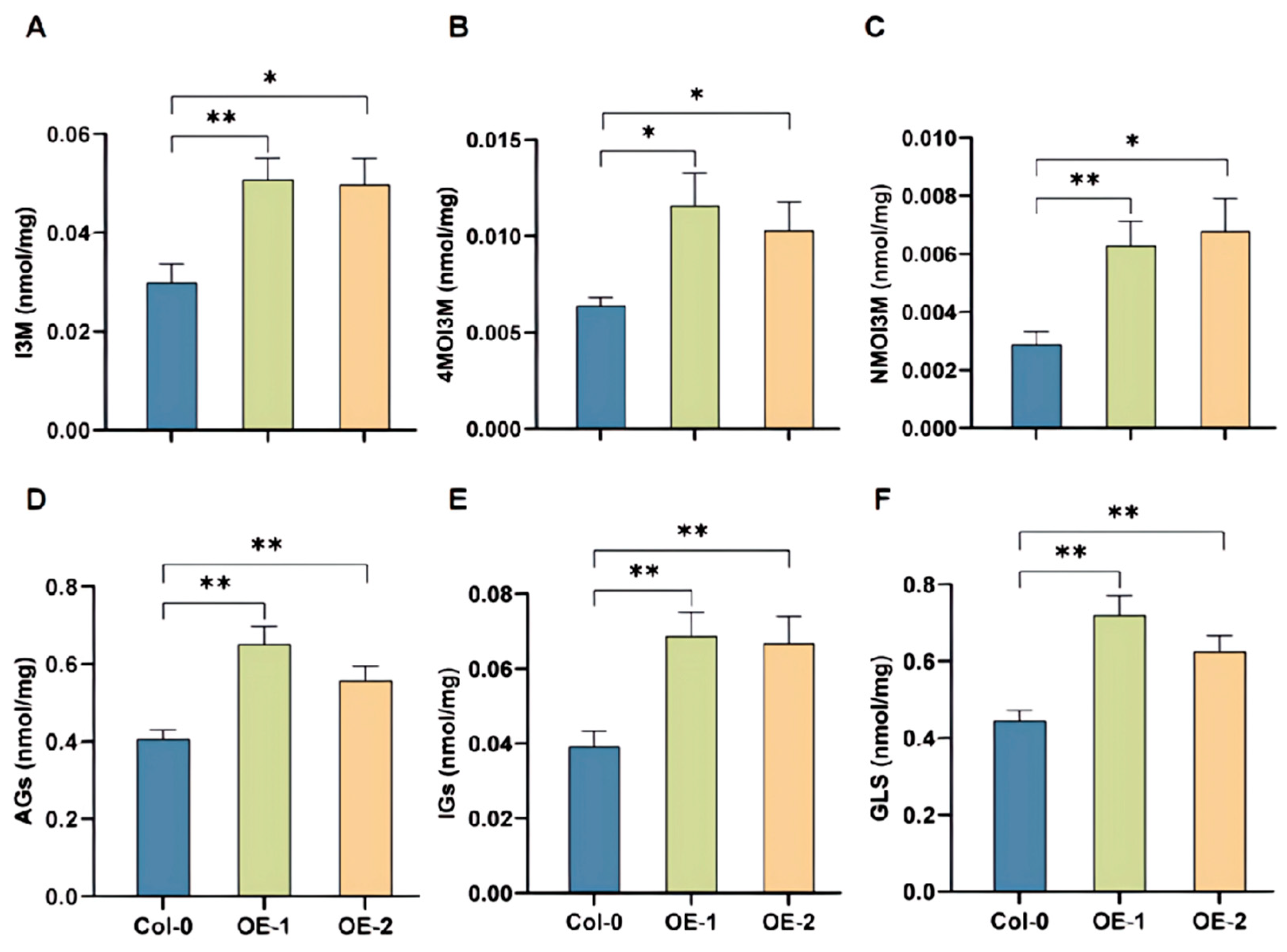
2.6. BoRHON1 Could Induce the Biosynthesis of Both Aliphatic and Indolic Glucosinolates
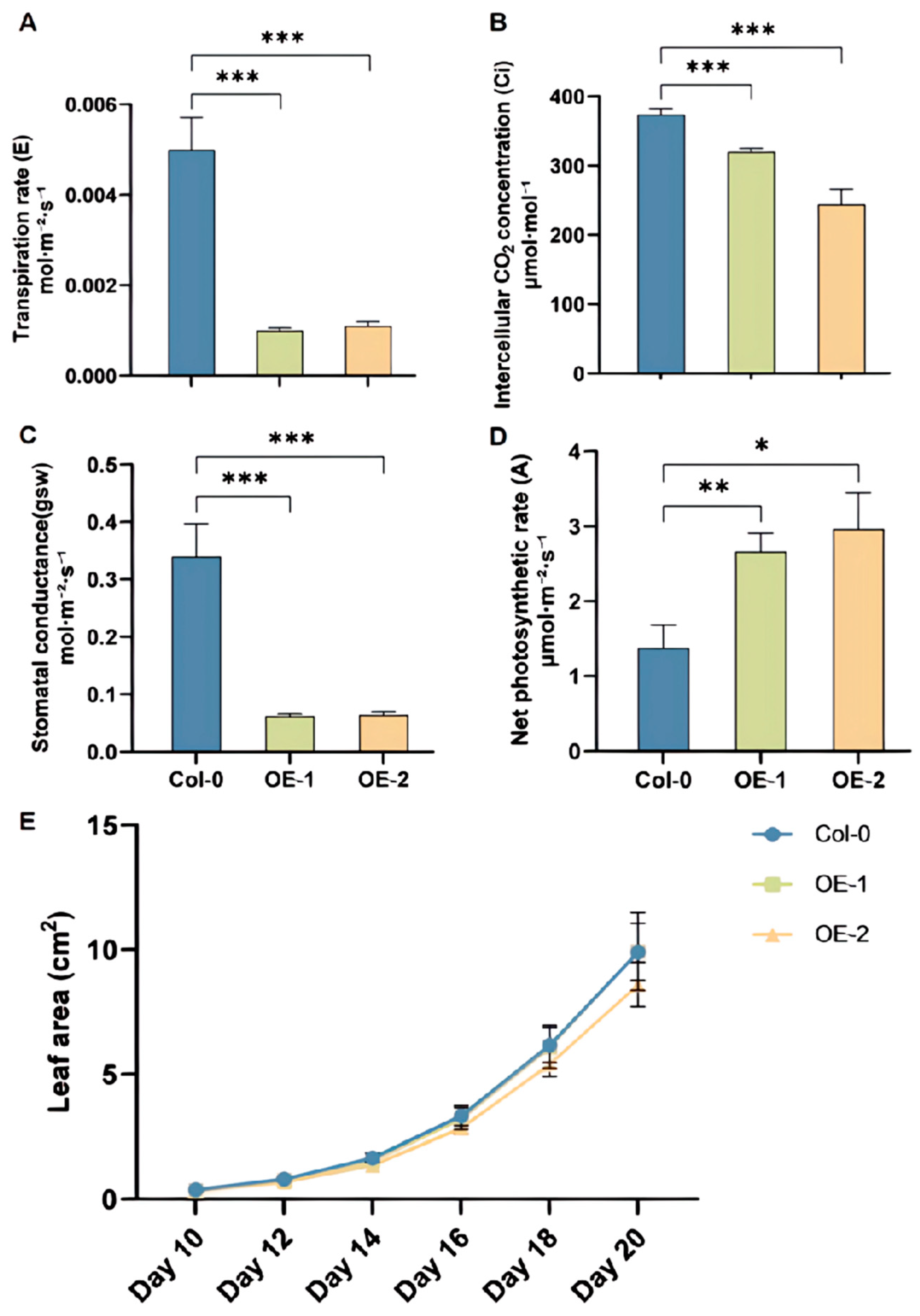
2.7. BoRHON1 Increased the Photosynthesis Rate
3. Discussion
3.1. The Gene-Regulatory Networks of Plant-Specialized Metabolisms
3.2. The Regulatory Roles of BoRHON1 in Modulating Glucosinolate Biosynthesis
3.3. The Interplay between Plant Growth and Defenses
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Yeast One-Hybrid Assay
4.3. Transient Expression Assay in N. benthamiana Leaves
4.4. Bioinformatic Analysis
4.5. Transcriptional Activation Activity Assay
4.6. Subcellular Localization Analysis
4.7. Quantitative Real-Time RT-PCR Analysis
4.8. Generation of Transgenic Arabidopsis Plants
4.9. Glucosinolate Extraction and Analysis
4.10. Determination of Photosynthetic Parameters
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Erb, M.; Kliebenstein, D.J. Plant secondary metabolites as defenses, regulators, and primary metabolites: The blurred functional trichotomy. Plant Physiol. 2020, 184, 39–52. [Google Scholar] [CrossRef]
- Wink, M. Plant breeding: Importance of plant secondary metabolites for protection against pathogens and herbivores. Theor. Appl. Genet. 1988, 75, 225–233. [Google Scholar] [CrossRef]
- Kliebenstein, D.J.; Osbourn, A. Making new molecules—Evolution of pathways for novel metabolites in plants. Curr. Opin. Plant Biol. 2012, 15, 415–423. [Google Scholar] [CrossRef]
- Kliebenstein, D.J. New synthesis—Regulatory evolution, the veiled world of chemical diversification. J. Chem. Ecol. 2013, 39, 349. [Google Scholar] [CrossRef] [PubMed]
- Soltis, N.E.; Kliebenstein, D.J. Natural variation of plant metabolism: Genetic mechanisms, interpretive caveats, and evolutionary and mechanistic insights. Plant Physiol. 2015, 169, 1456–1468. [Google Scholar] [CrossRef]
- Fernie, A.R.; Tohge, T. The genetics of plant metabolism. Annu. Rev. Genet. 2017, 51, 287–310. [Google Scholar] [CrossRef] [PubMed]
- Liou, C.S.; Sirk, S.J.; Diaz, C.A.C.; Klein, A.P.; Fischer, C.R.; Higginbottom, S.K.; Erez, A.; Donia, M.S.; Sonnenburg, J.L.; Sattely, E.S. A metabolic pathway for activation of dietary glucosinolates by a human gut symbiont. Cell 2020, 180, 717–728.e19. [Google Scholar] [CrossRef]
- Kliebenstein, D.J. Specificity and breadth of plant specialized metabolite-microbe interactions. Curr. Opin. Plant Biol. 2024, 77, 102459. [Google Scholar] [CrossRef]
- Halkier, B.A.; Gershenzon, J. Biology and biochemistry of glucosinolates. Annu. Rev. Plant Biol. 2006, 57, 303–333. [Google Scholar] [CrossRef]
- Marino, M.; Martini, D.; Venturi, S.; Tucci, M.; Porrini, M.; Riso, P.; Del Bo, C. An overview of registered clinical trials on glucosinolates and human health: The current situation. Front. Nutr. 2021, 8, 730906. [Google Scholar] [CrossRef]
- Costa-Perez, A.; Nunez-Gomez, V.; Baenas, N.; Di Pede, G.; Achour, M.; Manach, C.; Mena, P.; Del Rio, D.; Garcia-Viguera, C.; Moreno, D.A.; et al. Systematic review on the metabolic interest of glucosinolates and their bioactive derivatives for human health. Nutrients 2023, 15, 1424. [Google Scholar] [CrossRef]
- Barnum, C.R.; Cho, M.J.; Markel, K.; Shih, P.M. Engineering Brassica crops to optimize delivery of bioactive products postcooking. ACS Synth. Biol. 2024, 13, 736–744. [Google Scholar] [CrossRef]
- Chen, L.; Zeng, Q.; Zhang, J.; Li, C.; Bai, X.; Sun, F.; Kliebenstein, D.J.; Li, B. Large-scale identification of novel transcriptional regulators of the aliphatic glucosinolate pathway in Arabidopsis. J. Exp. Bot. 2024, 75, 300–315. [Google Scholar] [CrossRef]
- The Arabidopsis Genome Initiative. Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 2000, 408, 796–815. [Google Scholar] [CrossRef]
- Sønderby, I.E.; Geu-Flores, F.; Halkier, B.A. Biosynthesis of glucosinolates—Gene discovery and beyond. Trends Plant Sci. 2010, 15, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Colinas, M.; Goossens, A. Combinatorial transcriptional control of plant specialized metabolism. Trends Plant Sci. 2018, 23, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Lacchini, E.; Goossens, A. Combinatorial control of plant specialized metabolism: Mechanisms, functions, and consequences. Annu. Rev. Cell Dev. Biol. 2020, 36, 291–313. [Google Scholar] [CrossRef] [PubMed]
- Gigolashvili, T.; Yatusevich, R.; Berger, B.; Müller, C.; Flügge, U.I. The R2R3-MYB transcription factor HAG1/MYB28 is a regulator of methionine-derived glucosinolate biosynthesis in Arabidopsis thaliana. Plant J. 2007, 51, 247–261. [Google Scholar] [CrossRef]
- Hirai, M.Y.; Sugiyama, K.; Sawada, Y.; Tohge, T.; Obayashi, T.; Suzuki, A.; Araki, R.; Sakurai, N.; Suzuki, H.; Aoki, K. Omics-based identification of Arabidopsis Myb transcription factors regulating aliphatic glucosinolate biosynthesis. Proc. Natl. Acad. Sci. USA 2007, 104, 6478–6483. [Google Scholar] [CrossRef]
- Sønderby, I.E.; Hansen, B.G.; Bjarnholt, N.; Ticconi, C.; Halkier, B.A.; Kliebenstein, D.J. A systems biology approach identifies a R2R3 MYB gene subfamily with distinct and overlapping functions in regulation of aliphatic glucosinolates. PLoS ONE 2007, 2, e1322. [Google Scholar] [CrossRef]
- Bender, J.; Fink, G.R. A Myb homologue, ATR1, activates tryptophan gene expression in Arabidopsis. Proc. Natl. Acad. Sci. USA 1998, 95, 5655–5660. [Google Scholar] [CrossRef] [PubMed]
- Celenza, J.L.; Quiel, J.A.; Smolen, G.A.; Merrikh, H.; Silvestro, A.R.; Normanly, J.; Bender, J. The Arabidopsis ATR1 Myb transcription factor controls indolic glucosinolate homeostasis. Plant Physiol. 2005, 137, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Gigolashvili, T.; Berger, B.; Mock, H.P.; Müller, C.; Weisshaar, B.; Flügge, U.I. The transcription factor HIG1/MYB51 regulates indolic glucosinolate biosynthesis in Arabidopsis thaliana. Plant J. 2007, 50, 886–901. [Google Scholar] [CrossRef] [PubMed]
- Frerigmann, H.; Gigolashvili, T. MYB34, MYB51 and MYB122 distinctly regulate indolic glucosinolate biosynthesis in Arabidopsis thaliana. Mol. Plant 2014, 7, 814–828. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Yuan, W.; Miao, H.; Deng, M.; Wang, M.; Lin, J.; Zeng, W.; Wang, Q. Functional characterization of BoaMYB51s as central regulators of indole glucosinolate biosynthesis in Brassica oleracea var. alboglabra Bailey. Front. Plant Sci. 2018, 9, 1599. [Google Scholar] [CrossRef]
- Schweizer, F.; Fernandez-Calvo, P.; Zander, M.; Diez-Diaz, M.; Fonseca, S.; Glauser, G.; Lewsey, M.G.; Ecker, J.R.; Solano, R.; Reymond, P. Arabidopsis basic helix-loop-helix transcription factors MYC2, MYC3, and MYC4 regulate glucosinolate biosynthesis, insect performance, and feeding behavior. Plant Cell 2013, 25, 3117–3132. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Ma, Y.; Zhou, Y.; Zhang, H.; Duan, L.; Chen, H.; Zeng, J.; Zhou, Q.; Wang, S.; Gu, W.; et al. Biosynthesis, regulation, and domestication of bitterness in cucumber. Science 2014, 346, 1084–1088. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Perez, R.; Pavan, S.; Mazzeo, R.; Moldovan, C.; Aiese Cigliano, R.; Del Cueto, J.; Ricciardi, F.; Lotti, C.; Ricciardi, L.; Dicenta, F.; et al. Mutation of a bHLH transcription factor allowed almond domestication. Science 2019, 364, 1095–1098. [Google Scholar] [CrossRef]
- Wang, H.; Ren, J.; Zhou, S.; Duan, Y.; Zhu, C.; Chen, C.; Liu, Z.; Zheng, Q.; Xiang, S.; Xie, Z.; et al. Molecular regulation of oil gland development and biosynthesis of essential oils in Citrus spp. Science 2024, 383, 659–666. [Google Scholar] [CrossRef]
- Li, B.; Gaudinier, A.; Tang, M.; Taylor-Teeples, M.; Nham, N.T.; Ghaffari, C.; Benson, D.S.; Steinmann, M.; Gray, J.A.; Brady, S.M.; et al. Promoter-based integration in plant defense regulation. Plant Physiol. 2014, 166, 1803–1820. [Google Scholar] [CrossRef]
- Li, B.; Tang, M.; Nelson, A.; Caligagan, H.; Zhou, X.; Clark-Wiest, C.; Ngo, R.; Brady, S.M.; Kliebenstein, D.J. Network-guided discovery of extensive epistasis between transcription factors involved in aliphatic glucosinolate biosynthesis. Plant Cell 2018, 30, 178–195. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Tang, M.; Caseys, C.; Nelson, A.; Zhou, M.; Zhou, X.; Brady, S.M.; Kliebenstein, D.J. Epistatic transcription factor networks differentially modulate Arabidopsis growth and defense. Genetics 2020, 214, 529–541. [Google Scholar] [CrossRef]
- Tang, M.; Li, B.; Zhou, X.; Bolt, T.; Li, J.J.; Cruz, N.; Gaudinier, A.; Ngo, R.; Clark-Wiest, C.; Kliebenstein, D.J.; et al. A genome-scale TF-DNA interaction network of transcriptional regulation of Arabidopsis primary and specialized metabolism. Mol. Syst. Biol. 2021, 17, e10625. [Google Scholar] [CrossRef]
- Liu, S.; Liu, Y.; Yang, X.; Tong, C.; Edwards, D.; Parkin, I.A.; Zhao, M.; Ma, J.; Yu, J.; Huang, S.; et al. The Brassica oleracea genome reveals the asymmetrical evolution of polyploid genomes. Nat. Commun. 2014, 5, 3930. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, Y.; Cai, C.; Ji, J.; Han, F.; Zhang, L.; Chen, S.; Zhang, L.; Yang, Y.; Tang, Q.; et al. Large-scale gene expression alterations introduced by structural variation drive morphotype diversification in Brassica oleracea. Nat. Genet. 2024, 56, 517–529. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Cao, W.; Yang, L.; Zhang, Y.; Fang, Z.; Zhuang, M.; Lv, H.; Wang, Y.; Cheng, S.; Ji, J. Glucosinolate biosynthetic genes of cabbage: Genome-wide identification, evolution, and expression analysis. Genes 2023, 14, 476. [Google Scholar] [CrossRef] [PubMed]
- Stoppel, R.; Manavski, N.; Schein, A.; Schuster, G.; Teubner, M.; Schmitz-Linneweber, C.; Meurer, J. RHON1 is a novel ribonucleic acid-binding protein that supports RNase E function in the Arabidopsis chloroplast. Nucleic Acids Res. 2012, 40, 8593–8606. [Google Scholar] [CrossRef] [PubMed]
- Chi, W.; He, B.; Manavski, N.; Mao, J.; Ji, D.; Lu, C.; Rochaix, J.D.; Meurer, J.; Zhang, L. RHON1 mediates a Rho-like activity for transcription termination in plastids of Arabidopsis thaliana. Plant Cell 2014, 26, 4918–4932. [Google Scholar] [CrossRef]
- Yang, Z.; Li, M.; Sun, Q. RHON1 co-transcriptionally resolves R-loops for Arabidopsis chloroplast genome maintenance. Cell Rep. 2020, 30, 243–256.e5. [Google Scholar] [CrossRef]
- Bekaert, M.; Edger, P.P.; Hudson, C.M.; Pires, J.C.; Conant, G.C. Metabolic and evolutionary costs of herbivory defense: Systems biology of glucosinolate synthesis. New Phytol. 2012, 196, 596–605. [Google Scholar] [CrossRef] [PubMed]
- Kliebenstein, D.J. Is specialized metabolite regulation specialized? J. Exp. Bot. 2023, 74, 4942–4948. [Google Scholar] [CrossRef]
- Kliebenstein, D.J. False idolatry of the mythical growth versus immunity tradeoff in molecular systems plant pathology. Physiol. Mol. Plant Pathol. 2016, 95, 55–59. [Google Scholar] [CrossRef]
- Ahmed, S.S.; Gong, Z.H.; Ji, J.J.; Yin, Y.X.; Xiao, H.J.; Khan, M.A.; Rehman, A.; Ahmad, I. Construction of the intermediate vector pVBG2307 by incorporating vital elements of expression vectors pBI121 and pBI221. Genet. Mol. Res. 2012, 11, 3091–3104. [Google Scholar] [CrossRef] [PubMed]
- Kliebenstein, D.J.; Kroymann, J.; Brown, P.; Figuth, A.; Pedersen, D.; Gershenzon, J.; Mitchell-Olds, T. Genetic control of natural variation in Arabidopsis glucosinolate accumulation. Plant Physiol. 2001, 126, 811–825. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, X.; Zhang, R.; Zeng, Q.; Yang, W.; Fang, F.; Sun, Q.; Yan, C.; Li, F.; Liu, X.; Li, B. The RNA-Binding Protein BoRHON1 Positively Regulates the Accumulation of Aliphatic Glucosinolates in Cabbage. Int. J. Mol. Sci. 2024, 25, 5314. https://doi.org/10.3390/ijms25105314
Bai X, Zhang R, Zeng Q, Yang W, Fang F, Sun Q, Yan C, Li F, Liu X, Li B. The RNA-Binding Protein BoRHON1 Positively Regulates the Accumulation of Aliphatic Glucosinolates in Cabbage. International Journal of Molecular Sciences. 2024; 25(10):5314. https://doi.org/10.3390/ijms25105314
Chicago/Turabian StyleBai, Xue, Ruixing Zhang, Qi Zeng, Wenjing Yang, Fang Fang, Qingguo Sun, Chengtai Yan, Fangguan Li, Xifan Liu, and Baohua Li. 2024. "The RNA-Binding Protein BoRHON1 Positively Regulates the Accumulation of Aliphatic Glucosinolates in Cabbage" International Journal of Molecular Sciences 25, no. 10: 5314. https://doi.org/10.3390/ijms25105314




