Abstract
Parkinson’s disease (PD) is the second most common neurodegenerative disease worldwide. Given its prevalence, reliable biomarkers for early diagnosis are required. Exosomal proteins within extracellular nanovesicles are promising candidates for diagnostic, screening, prognostic, and disease monitoring purposes in neurological diseases such as PD. This review aims to evaluate the potential of extracellular vesicle proteins or miRNAs as biomarkers for PD. A comprehensive literature search until January 2024 was conducted across multiple databases, including PubMed, EMBASE, Web of Science, and Cochrane Library, to identify relevant studies reporting exosome biomarkers in blood samples from PD patients. Out of 417 articles screened, 47 studies were selected for analysis. Among exosomal protein biomarkers, α-synuclein, tau, Amyloid β 1-42, and C-X-C motif chemokine ligand 12 (CXCL12) were identified as significant markers for PD. Concerning miRNA biomarkers, miRNA-24, miR-23b-3p, miR-195-3p, miR-29c, and mir-331-5p are promising across studies. α-synuclein exhibited increased levels in PD patients compared to control groups in twenty-one studies, while a decrease was observed in three studies. Our meta-analysis revealed a significant difference in total exosomal α-synuclein levels between PD patients and healthy controls (standardized mean difference [SMD] = 1.369, 95% confidence interval [CI] = 0.893 to 1.846, p < 0.001), although these results are limited by data availability. Furthermore, α-synuclein levels significantly differ between PD patients and healthy controls (SMD = 1.471, 95% CI = 0.941 to 2.002, p < 0.001). In conclusion, certain exosomal proteins and multiple miRNAs could serve as potential biomarkers for diagnosis, prognosis prediction, and assessment of disease progression in PD.
1. Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disorder after Alzheimer’s disease [1], affecting roughly 10 million people globally, with ~2% prevalence among those >80 years old [2]. PD is a disease in which parts of the brain are progressively damaged over several years, resulting in motor symptoms such as tremor, bradykinesia, rigidity, as well as physical and psychiatric symptoms such as depression, anxiety, anosmia, insomnia, memory problems, etc. [3]. The exact cause of PD remains largely unknown, with current research pointing to a mix of genetic and environmental factors [4]. PD is histologically characterized by the specific loss of dopamine-producing neurons, particularly in the substantia nigra pars compacta, accompanied by the presence of abnormal protein clumps called Lewy bodies (LB) and Lewy neurites, containing α-synuclein (α-syn) [5,6]. Lewy bodies are abnormal protein aggregates found inside neurons, primarily composed of alpha-synuclein, observed in neurodegenerative diseases such as PD. In contrast, Lewy neurites are abnormal accumulations of protein within the processes (neurites), predominantly containing alpha-synuclein as well. Both are indicative of neuronal dysfunction and degeneration, with Lewy neurites observed in the dendrites and axons of affected neurons, closely related to the formation of Lewy bodies.
Diagnosing PD, particularly in its early stages, presents significant challenges due to the lack of definitive diagnostic tests, resulting in confirmation in only 80–90% of cases post-mortem [7,8,9]. Initial diagnosis relies on hallmark “parkinsonism” symptoms, including slow movement, tremors, and stiffness, the first being crucial and requiring the presence of ≥1 of the other two symptoms [10]. While the aggregation and spread of toxic forms of α-syn are key features of PD, as a diverse disorder, it poses challenges for developing and identifying useful biomarkers for diagnosis and disease progression, as well as for successfully translating new treatments to the clinic [11]. Therefore, there is an urgent need for reliable biomarkers to improve clinical diagnoses, treatment response assessments, and disease progression monitoring [12]. Additionally, identifying minimally invasive, reliable, repeatable, and cost-effective blood-based biomarkers is crucial for PD, where diagnostic criteria rely on analyzing proteins in cerebrospinal fluid and clinical/imaging assessments [13].
Recently, extracellular vesicles (EVs) have been proposed as biomarkers for diagnosing and predicting chronic neurodegenerative diseases [14]. EVs, comprising exosomes (50–150 nm), microvesicles (100–1000 nm), and apoptotic bodies (up to 5 μm), have been investigated for their potential as biomarkers in various neurological disorders, including PD [13,15]. Advances in exosome purification methods, such as size exclusion chromatography, have highlighted their potential as biomarker carriers [16,17]. Further, as mediators of intercellular communication, exosomes have been implicated in transmitting misfolded proteins between neurons, which explains their potential as biomarkers [18]. EVs are notably rich in non-coding RNAs, such as miRNAs, lncRNAs, and circRNAs, exhibiting broad distribution throughout both the brain and peripheral systems. They serve as crucial mediators linking normal neuronal function with disease pathology [19]. Particularly noteworthy is their capacity to facilitate the transportation and delivery of various miRNAs, among which miR-21 stands out. While typically associated with microglial anti-inflammatory responses, miR-21 has also been implicated in inflammatory contexts and highlighted as a potential novel biomarker for PD [20]. Several key categories of exosome biomarkers have been identified for PD: (i) α-synuclein-related markers: alpha-synuclein, Lewy body, etc.; (ii) neurotransmitter-related markers: dopaminergic neuron, dopamine transporter, etc.; (iii) inflammation- and immune system-related markers: TNF alpha, cytokine, etc. [2]; (iv) Alzheimer’s disease-related markers: tau, presenilin, etc. [21]; (v) miRNA biomarkers: downregulation of miR-1, miR-22, and miR-29a, as well as the upregulation of miR-16-2, miR-26a-2, and miR-30a, in PD patients [22].
Therefore, our aim is to explore the potential of exosomal proteins and miRNAs as biomarkers for PD, and to evaluate a candidate exosomal protein in peripheral blood as a potential PD biomarker through systematic reviews and meta-analyses.
2. Materials and Methods
2.1. Literature Search Strategy
This review collected studies published until January 2024 by searching four databases including PubMed, Embase, Web of Science, and Cochrane Library. The review was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (http://www.prisma-statement.org) (accessed on 2 January 2024). The following keywords were used for this research: (exosome OR exosomal OR exosomes [MeSH]) AND (Parkinson OR Parkinson disease [MeSH]) AND (blood OR blood [MeSH] OR plasma OR plasma [MeSH] OR serum OR serum [MeSH]) AND (biomarker OR biomarkers [MeSH] OR “biological marker”). Two authors (KYK and KAC) independently conducted searches and extracted articles after analyzing the title, abstract, and full text. In case of discrepancies, all three authors engaged in discussions.
2.2. Inclusion and Exclusion Criteria
The PICOS (population, intervention, comparison, outcome, and study design) framework was used to define the eligibility criteria. The inclusion criteria were as follows. (1) Participants included all PD patients diagnosed with PD regardless of factors such as stage, or presence of dementia. (2) The interventions reviewed in this study included evaluations of protein or miRNA PD biomarkers in exosomes in blood samples. (3) The comparator to PD patients was healthy controls. (4) The outcome was assessed by the levels of biomarkers. (5) Study designs included randomized controlled trials, epidemiological observational studies including cross-sectional, case–control, and cohort studies. The exclusion criteria were as follows: (1) studies including patients with other diseases such as Alzheimer’s disease, multiple system atrophy, progressive supranuclear palsy, and rapid eye movement sleep behavior disorder; (2) studies including biomarkers analyzed in CSF, tissue, cell, or animal samples; (3) studies not including healthy controls; (4) studies not related to blood exosomes; studies not related to protein or miRNA biomarkers; and (5) studies in form of letter, editorial, commentary, conference abstract, or systematic, scoping, umbrella, or literature reviews.
2.3. Data Extraction and Analysis
From the selected final studies, the following data were extracted: authors and publication years, study country, sample size, sex distribution, or age in PD and control groups, sample characteristics, statistically significant biomarkers reported in the extracted papers, and origin of exosomes. For the meta-analysis, we examined the standardized mean difference (SMD) in total exosomal or neuron-derived exosomal α-syn levels between PD and healthy control groups using the Comprehensive Meta-Analysis software version 4 (Biostats Inc., Englewood, NJ, USA). We used a random-effects model following the examination of the Q statistic and I2 method to assess heterogeneity. Statistical significance was determined at a p < 0.05. Furthermore, the articles included in this review underwent quality assessment using the Critical Appraisal Skills Programme (CASP, Oxford, UK, https://casp-uk.net/casp-tools-checklists/, accessed on 2 January 2024) checklists for randomized controlled trial, case–control study, and cohort study. The CASP tool comprises 11 questions across three sections: “Are the results of the study valid? (Section A)”, “What are the results? (Section B)”, and “Will the results help locally? (Section C)”.
3. Results and Discussion
3.1. Literature Search
Figure 1 illustrates the process for literature search and selection. For this systematic review, a total of 417 articles, including 124 from PubMed, 225 from Embase, 63 from Web of Science, and 5 from the Cochrane Library, were screened according to the inclusion and exclusion criteria. Using EndNote and manual screening, 187 articles, including duplicates and irrelevant studies, were removed, resulting in 284 articles being extracted. Title analysis served to extract 170 articles, and abstract analysis to extract 89 articles. Finally, after a close review of the full text, 47 articles were used for this systematic review. These selected studies included blood exosomal biomarkers that showed significant changes in PD patients compared to healthy control groups, with significance determined based on the statistical significance presented in each paper. Furthermore, the quality assessment scores of the included studies were evaluated using the CAST checklist for case–control study, ranging from 5 to 10 points (Supplementary Table S1).
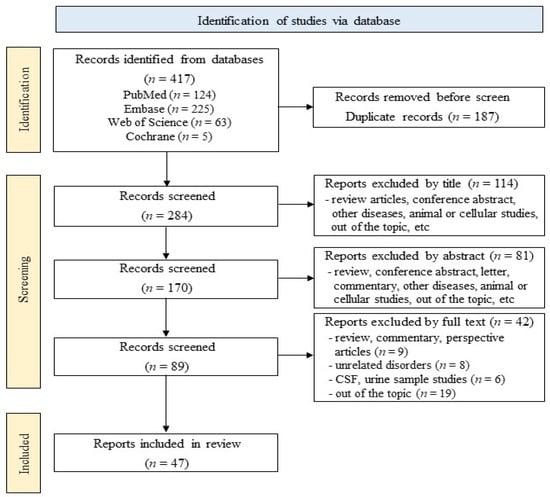
Figure 1.
Flowchart of the literature search.
Table 1 and Table 2 present the general characteristics of the studies included in this systematic review. We included studies published between 2014 and 2023 and conducted in Italy Taiwan, China, USA, UK, Germany, New Zealand, Japan, South Korea, Turkey, and India. The study groups included PD patients and healthy controls. Sex and age are presented according to study group. Human blood samples included plasma and serum. Exosomes were derived from total, neuron, oligodendroglia, astrocyte, blood cell, epithelial cell, platelet, and vascular smooth muscle cells. Table 1 shows the characteristics of 34 exosomal protein biomarkers in PD, while Table 2 illustrates the characteristics of 14 exosomal miRNA biomarkers in PD. One study included both protein and miRNA biomarkers.

Table 1.
General characteristics of studies on potential exosomal protein biomarkers.

Table 2.
General characteristics of studies on potential exosomal miRNA biomarkers.
3.2. Potentially Important Exosomal Biomarkers in PD
Table 3 presents the exosomal PD biomarkers present in ≥2 studies among those considered significant in each study. Among them, α-syn levels increased in PD patients compared to the control group in twenty studies and decreased in two studies. Furthermore, tau, Amyloid β 1-42, and C-X-C motif chemokine ligand 12 (CXCL12) were identified as significant exosomal protein PD biomarkers in two studies.

Table 3.
Potentially important exosomal biomarkers in PD.
First, plasma exosomal Aβ and tau are potential diagnostic candidates for PD. While the primary pathognomonic protein of PD is α-syn, other proteins like tau and Aβ have also been detected [66]. Clinicopathologic evidence suggests that a combined metric of LB, Aβ, and tau pathologies best correlates with dementia in PD [24,67,68]. The formation of α-syn oligomers leads to the generation of Aβ sheet fibrils, which aggregate into LB [13]. Cognitive dysfunction is a common nonmotor feature of PD, involving executive functions, attentional and visuospatial function, and memory. Approximately 15% of PD patients exhibit mild cognitive impairment at diagnosis [69], and many progress to dementia in the long term [70,71]. Early diagnosis and intervention are crucial for improving the prognosis and quality of life for PD patients with cognitive impairment [3]. Cognitive decline in PD results not only from the loss of dopaminergic neurons but also from involvement of serotonergic, glutaminergic, and cholinergic neurons in the subcortex and cortex [72]. Pathologically, cognitive dysfunction in PD is strongly associated with a combination of LB and Alzheimer’s pathology, involving both α-syn and Aβ [73,74]. Despite understanding the pathological roles of these proteins in PD, their application as biomarkers remains challenging. Studies have investigated the use of α-syn, Aβ, and tau as PD biomarkers [75].
Second, plasma exosomal CXCL12 can also be a potential diagnostic candidate for PD. Chemokines can be categorized into four subfamilies: the CXC subfamily, characterized by cysteine residues separated by a single amino acid; the CC chemokines, featuring two adjacent cysteine residues; the XC chemokines, which contain a single cysteine residue in the amino terminus; and the CX3C subfamily, with three amino acid residues separating the cysteine tandem [76]. The regulation of chemokine activity involves a network of feedback loops and mechanisms responsible for their suppression and/or stimulation [77]. The levels of chemokines in the extracellular fluid regulate inflammation, infection, immunological responses, tissue injury reactions, apoptosis, and immune cell trafficking [77,78]. Among homeostatic chemokines, CXCL12, also known as stromal-derived factor 1 (SDF-1), is one of the most evolutionarily conserved chemokines, binding to the CXCR4 receptor. CXCL12 and CXCR4 are widely expressed in the central nervous system (CNS), primarily on astrocytes and microglia in the normal CNS, as well as on neurons in the adult brain [79]. CXCL12 has attracted attention as a potential therapeutic target for promoting nerve regeneration, and emerging evidence suggests its involvement in regulating autophagy [80]. With respect to miRNAs, miRNA-24, miR-23b-3p, miR-195-3p, miR-29c, and mir-331-5p were identified as significant exosomal biomarkers in ≥2 studies. Plasma exosomal miRNAs (e.g., miR-24, miR-23b-3p, miR-29c, miR-195-3p, and miR-331-5p) stand as potential diagnostic candidates for PD. Within neurodegenerative disorders, the intricate pathophysiological landscape is notably influenced by miRNA gene regulation [81]. These small RNAs, typically around 22 nucleotides in length, exert post-transcriptional control over gene expression by forming base pairs with target mRNAs [82]. Evidence from multiple studies underscores the varied expression patterns of miRNAs within the human brain, some modulating genes implicated in neurodegeneration [83]. Specifically, exosomal miRNAs have garnered attention as promising circulating biomarkers due to their resilience against endogenous RNase degradation, stable presence, and detectability even in minute concentrations [84]. A wealth of research highlights the pivotal roles played by exosomal miRNAs in disease progression and their potential clinical utility as diagnostic indicators [58]. Noteworthy findings are as follows: (i) upregulation of miR-24 in PD patients, with potential associations to PD-like phenotypes [12,55,63]; (ii) identification of miR-23b-3p as a novel circulating miRNA linked to PD, known for its role in mitigating neuroinflammation and neuronal apoptosis [56,85,86]; (iii) significantly increased miR-29c expression in PD patients compared to controls [60]; (iv) miR-195 upregulation in PD patients [12,60,87]; and (v) elevated miR-331-5p expression in both serum exosomes and CSF exosomes of PD patients [63,64].
3.3. Meta-Analysis on Total Exosomal and Neuron-Derived Exosomal α-Synuclein
Figure 2 presents the results of a meta-analysis on the potential of total exosomal and neuron-derived exosomal α-syn as exosomal biomarker in PD. As shown in Figure 2A, the meta-analysis of total exosomal α-syn showed that patients with PD had a significant difference to heathy controls (standardized mean difference [SMD] = 1.369, 95% confidence interval [CI] = 0.893 to 1.846, p < 0.001). Moreover, neuron-derived exosomal α-syn showed that PD had significant difference compared to healthy control (SMD = 1.471, 95% CI = 0.941 to 2.002, p < 0.001). We discuss the potential of plasma exosomal α-syn as a diagnostic tool for PD. Exosomes, nano-sized EVs, are released into the extracellular matrix by various cell types and are abundant in body fluids such as plasma, urine, and cerebrospinal fluid [88]. Their cargo can reflect the intracellular environment of the originating cells and participate in intracellular communication under different physiological and pathological conditions [89]. In PD, exosomes may accelerate α-syn aggregation; in fact, there is evidence on their involvement in disease progression via prion-like spread of pathogenic misfolded α-syn [90]. α-syn is a 140-amino-acid protein mainly found in the brain, particularly in presynaptic areas, although it is also detectable in the nucleus of brain cells and peripheral organs [91]. It regulates synaptic vesicle dynamics at nerve terminals and dopamine neurotransmission, directly influencing mitochondrial physiology, potentially linking mitochondrial dysfunction to PD pathogenesis [91,92]. Mitochondrial defects may contribute to LB formation, a pathological hallmark of PD [93]. α-syn is readily secreted into extracellular spaces, and its levels can be measured in cerebrospinal fluid (CSF), plasma/serum, red blood cells, and saliva [94]. Despite variations in total α-syn levels in PD [91], our findings indicate significantly higher levels of α-syn in PD patients compared to healthy controls. Additionally, several studies have demonstrated elevated levels of α-syn in plasma neuronal-derived exosomes from PD patients compared to healthy controls [32,38,51,95]. It was reported that the interaction between α-syn and membranes plays a significant role in the conformational shifts of α-syn, potentially impacting protein functionality and contributing to aggregation in PD progression [2]. Furthermore, the aggregated and harmful variant of the protein, oligomeric α-syn, has been observed to engage with lipids, inducing structural alterations in lipid membranes. This interaction leads to various effects, including membrane disruption, thinning, pore formation, and lipid clustering [96]. Additionally, oligomeric α-syn appears to be influenced by post-translational modifications (PTMs) like phosphorylation, nitration, and dopamine (DA) modification, as noted in studies [97].
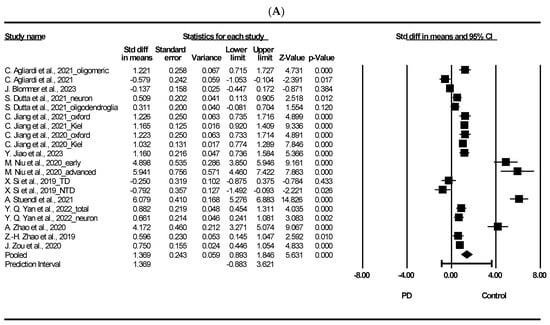
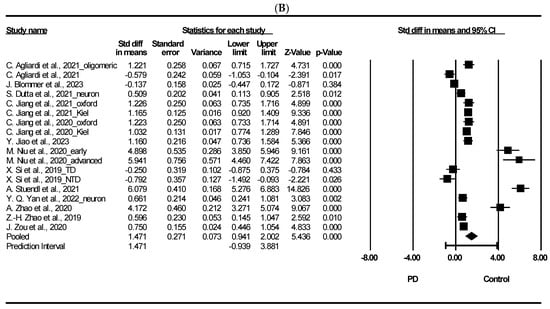
Figure 2.
Forest plots of exosomal and neuron-derived exosomal α-synuclein. (A) Exosomal α-synuclein, (B) neuron-derived exosomal α-synuclein. Std diff: standard difference, CI: confidence interval. Individual study effect is represented by a square, while the pooled effect across studies is represented by a diamond [13,24,31,33,34,35,38,44,45,49,50,51,53].
4. Conclusions
Here, we reviewed the potential of exosomal proteins such as α-syn, amyloid-β (Aβ), tau, and C-X-C motif chemokine ligand 12 (CXCL12), as well as miRNAs (miR-24, miR-23b-3p, miR-29c, miR-195-3p, and miR-331-5p) as biomarkers for PD. A systematic review and meta-analysis suggest that α-syn could be an effective exosomal biomarker protein.
First, we conclude that plasma exosomal α-syn could serve as an effective biomarker for PD, because our findings indicate significantly higher levels of α-syn in PD patients compared to healthy controls. Second, we suggest that plasma exosomal Aβ and tau may also serve as effective biomarkers for PD, particularly concerning cognitive function, because it has been shown that the annual changes in plasma EV tau and Aβ1-42 levels significantly differ between PD patients and healthy controls [27]. Third, we conclude that plasma exosomal CXCL12 may serve as an effective biomarker for PD, because previous studies have indicated that peripheral blood levels of CXCL12 correlate with pathological processes and disease progression in PD, making them potential diagnostic and prognostic markers [98,99]. In addition, CXCL12 levels have been associated with nonmotor symptoms such as autonomic dysfunction, while α-syn levels in plasma neuronal exosomes have been linked to clinical stage, motor symptoms, and nonmotor symptoms [34]. Fourth, we suggest that plasma exosomal miRNAs, including miR-24, miR-23b-3p, miR-29c, miR-195-3p, and miR-331-5p, hold promise as effective biomarkers for PD, because circulating miRNAs contribute significantly to the pathogenesis of numerous chronic conditions, including PD [100]. However, this study has several limitations. First, our findings are constrained by the data sourced from the literature included in this study. Since our study was limited to including both PD and healthy control groups, certain exosomal biomarkers in PD patients may not have been fully represented. Additionally, the presence of repeated data from the same researcher could potentially introduce bias into the results. Second, our analysis included both control and PD groups without stratification by PD stage. While analyzing PD stages is crucial, it was challenging in this study due to many included studies not providing information on the stages of the subjects. Consequently, further research is warranted to examine the specific stages of PD. Third, while studies with various exosomal biomarkers were included in this research, studies with analyzable data from two or more sources were limited. Therefore, only α-syn was included in the meta-analysis. Continuous research is necessary for potential exosomal biomarkers. Nonetheless, the significant alterations observed in exosomal α-syn levels among PD patients underscore the potential of exosomal proteins such as Aβ, tau, and CXCL12, along with various miRNAs as promising biomarkers for PD.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms25105307/s1.
Author Contributions
Conceptualization, K.-A.C. and K.Y.K.; formal analysis, K.-A.C. and K.Y.K.; investigation, K.Y.S.; writing—original draft, K.Y.S. and K.Y.K.; writing—review and editing, K.Y.S. and K.-A.C.; supervision, K.-A.C.; project administration, K.-A.C.; funding acquisition, K.-A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Gachon University research fund of 2022 (GCU202206140001) and the Basic Science Research Program through the National Research Foundation of Korea, funded by the Ministry of Education (NRF-2022R1A2C1092597). The funders had no role in the study design, data collection and analysis, decision to publish, or manuscript preparation.
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- de Lau, L.M.; Breteler, M.M. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006, 5, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Gualerzi, A.; Picciolini, S.; Bedoni, M.; Guerini, F.R.; Clerici, M.; Agliardi, C. Extracellular Vesicles as Biomarkers for Parkinson’s Disease: How Far from Clinical Translation? Int. J. Mol. Sci. 2024, 25, 1136. [Google Scholar] [CrossRef] [PubMed]
- Leng, B.; Sun, H.R.; Zhao, J.W.; Liu, Y.Y.; Shen, T.Q.; Liu, W.J.; Liu, X.; Tan, M.X.; Li, F.; Zhang, J.B.; et al. Plasma exosomal prion protein levels are correlated with cognitive decline in PD patients. Neurosci. Lett. 2020, 723, 134866. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.J.; Dan, X.L.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef] [PubMed]
- Schapira, A.H.V. Pathogenesis of Parkinson’s disease. Bailliere Clin. Neurol. 1997, 6, 15–36. [Google Scholar]
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Beach, T.G.; Adler, C.H. Importance of low diagnostic Accuracy for early Parkinson’s disease. Mov. Disord. 2018, 33, 1551–1554. [Google Scholar] [CrossRef] [PubMed]
- Gualerzi, A.; Picciolini, S.; Carlomagno, C.; Terenzi, F.; Ramat, S.; Sorbi, S.; Bedoni, M. Raman profiling of circulating extracellular vesicles for the stratification of Parkinson’s patients. Nanomed. Nanotechnol. Biol. Med. 2019, 22, 102097. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.C.; Wang, J.W.; Li, M.; Fang, J.Y.; Ding, H.R.; Meng, J.Y.; Zhang, J.W.; Fang, X.; Liu, H.C.; Ma, C.; et al. CircSV2b participates in oxidative stress regulation through miR-5107-5p-Foxk1-Akt1 axis in Parkinson’s disease. Redox Biol. 2022, 56, 102430. [Google Scholar] [CrossRef]
- Kitamura, Y.; Kojima, M.; Kurosawa, T.; Sasaki, R.; Ichihara, S.; Hiraku, Y.; Tomimoto, H.; Murata, M.; Oikawa, S. Proteomic Profiling of Exosomal Proteins for Blood-based Biomarkers in Parkinson’s Disease. Neuroscience 2018, 392, 121–128. [Google Scholar] [CrossRef]
- Lamontagne-Proulx, J.; St-Amour, I.; Labib, R.; Pilon, J.; Denis, H.L.; Cloutier, N.; Roux-Dalvai, F.; Vincent, A.T.; Mason, S.L.; Williams-Gray, C.; et al. Portrait of blood-derived extracellular vesicles in patients with Parkinson’s disease. Neurobiol. Dis. 2019, 124, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.Y.; Lu, J.M.; Zhao, Z.Q.; Li, M.C.; Lu, T.; An, X.S.; Xue, L.J. MicroRNA biomarkers of Parkinson’s disease in serum exosome-like microvesicles. Neurosci. Lett. 2017, 644, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Agliardi, C.; Meloni, M.; Guerini, F.R.; Zanzottera, M.; Bolognesi, E.; Baglio, F.; Clerici, M. Oligomeric α-Syn and SNARE complex proteins in peripheral extracellular vesicles of neural origin are biomarkers for Parkinson’s disease. Neurobiol. Dis. 2021, 148, 105185. [Google Scholar] [CrossRef] [PubMed]
- Quek, C.; Hill, A.F. The role of extracellular vesicles in neurodegenerative diseases. Biochem. Biophys. Res. Commun. 2017, 483, 1178–1186. [Google Scholar] [CrossRef] [PubMed]
- Latifkar, A.; Hur, Y.H.; Sanchez, J.C.; Cerione, R.A.; Antonyak, M.A. New insights into extracellular vesicle biogenesis and function. J. Cell Sci. 2019, 132, jcs222406. [Google Scholar] [CrossRef] [PubMed]
- Boing, A.N.; van der Pol, E.; Grootemaat, A.E.; Coumans, F.A.; Sturk, A.; Nieuwland, R. Single-step isolation of extracellular vesicles by size-exclusion chromatography. J. Extracell Vesicles 2014, 3, 23430. [Google Scholar] [CrossRef] [PubMed]
- Baranyai, T.; Herczeg, K.; Onódi, Z.; Voszka, I.; Módos, K.; Marton, N.; Nagy, G.; Mäger, I.; Wood, M.J.; El Andaloussi, S.; et al. Isolation of Exosomes from Blood Plasma: Qualitative and Quantitative Comparison of Ultracentrifugation and Size Exclusion Chromatography Methods. PLoS ONE 2015, 10, e0145686. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Kang, W.; Liu, Z.; Zhu, X. The role of exosomes in the diagnosis of Parkinson’s disease. Heliyon 2023, 9, e20595. [Google Scholar] [CrossRef]
- Zhang, P.; Rasheed, M.; Liang, J.; Wang, C.; Feng, L.; Chen, Z. Emerging Potential of Exosomal Non-coding RNA in Parkinson’s Disease: A Review. Front. Aging Neurosci. 2022, 14, 819836. [Google Scholar] [CrossRef]
- Keighron, C.N.; Avazzadeh, S.; Goljanek-Whysall, K.; McDonagh, B.; Howard, L.; Ritter, T.; Quinlan, L.R. Extracellular Vesicles, Cell-Penetrating Peptides and miRNAs as Future Novel Therapeutic Interventions for Parkinson’s and Alzheimer’s Disease. Biomedicines 2023, 11, 728. [Google Scholar] [CrossRef]
- Hook, V.; Podvin, S.; Mosier, C.; Boyarko, B.; Seyffert, L.; Stringer, H.; Rissman, R.A. Emerging evidence for dysregulated proteome cargoes of tau-propagating extracellular vesicles driven by familial mutations of tau and presenilin. Extracell Vesicles Circ. Nucl. Acids 2023, 4, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Margis, R.; Margis, R.; Rieder, C.R. Identification of blood microRNAs associated to Parkinsonis disease. J. Biotechnol. 2011, 152, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Anastasi, F.; Masciandaro, S.M.; Carratore, R.D.; Dell’Anno, M.T.; Signore, G.; Falleni, A.; McDonnell, L.A.; Bongioanni, P. Proteomics Profiling of Neuron-Derived Small Extracellular Vesicles from Human Plasma: Enabling Single-Subject Analysis. Int. J. Mol. Sci. 2021, 22, 2951. [Google Scholar] [CrossRef] [PubMed]
- Blommer, J.; Pitcher, T.; Mustapic, M.; Eren, E.; Yao, P.J.; Vreones, M.P.; Pucha, K.A.; Dalrymple-Alford, J.; Shoorangiz, R.; Meissner, W.G.; et al. Extracellular vesicle biomarkers for cognitive impairment in Parkinson’s disease. Brain 2023, 146, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Cerri, S.; Ghezzi, C.; Sampieri, M.; Siani, F.; Avenali, M.; Dornini, G.; Zangaglia, R.; Minafra, B.; Blandini, F. The Exosomal/Total alpha-Synuclein Ratio in Plasma Is Associated With Glucocerebrosidase Activity and Correlates With Measures of Disease Severity in PD Patients. Front. Cell. Neurosci. 2018, 12, 125. [Google Scholar] [CrossRef]
- Chan, L.; Chung, C.C.; Chen, J.H.; Yu, R.C.; Hong, C.T. Cytokine profile in plasma extracellular vesicles of parkinson’s disease and the association with cognitive function. Cells 2021, 10, 604. [Google Scholar] [CrossRef]
- Chan, L.; Chung, C.C.; Hsieh, Y.C.; Wu, R.M.; Hong, C.T. Plasma extracellular vesicle tau, beta-amyloid, and alpha-synuclein and the progression of Parkinson’s disease: A follow-up study. Ther. Adv. Neurol. Disord. 2023, 16, 17562864221150329. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.T.; Pan, C.Z.; Ruan, X.L.; Lei, L.P.; Lin, S.M.; Wang, Y.Z.; Zhao, Z.H. Evaluation of ferritin and TfR level in plasma neural-derived exosomes as potential markers of Parkinson’s disease. Front. Aging Neurosci. 2023, 15, 1216905. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.Y.; Chan, L.; Chung, C.C.; Chiu, J.Y.; Hsieh, Y.C.; Hong, C.T. Altered Insulin Receptor Substrate 1 Phosphorylation in Blood Neuron-Derived Extracellular Vesicles From Patients With Parkinson’s Disease. Front. Cell Dev. Biol. 2020, 8, 564641. [Google Scholar] [CrossRef]
- Chung, C.C.; Chan, L.; Chen, J.H.; Bamodu, O.A.; Chiu, H.W.; Hong, C.T. Plasma extracellular vesicles tau and β-amyloid as biomarkers of cognitive dysfunction of Parkinson’s disease. FASEB J. 2021, 35, e21895. [Google Scholar] [CrossRef]
- Dutta, S.; Hornung, S.; Kruayatidee, A.; Maina, K.N.; del Rosario, I.; Paul, K.C.; Wong, D.Y.; Duarte Folle, A.; Markovic, D.; Palma, J.A.; et al. α-Synuclein in blood exosomes immunoprecipitated using neuronal and oligodendroglial markers distinguishes Parkinson’s disease from multiple system atrophy. Acta Neuropathol. 2021, 142, 495–511. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Jiang, C.; Tofaris, G.K.; Davis, J.J. Facile Impedimetric Analysis of Neuronal Exosome Markers in Parkinson’s Disease Diagnostics. Anal. Chem. 2020, 92, 13647–13651. [Google Scholar] [CrossRef]
- Jiang, C.; Hopfner, F.; Berg, D.; Hu, M.T.; Pilotto, A.; Borroni, B.; Davis, J.J.; Tofaris, G.K. Validation of α-Synuclein in L1CAM-Immunocaptured Exosomes as a Biomarker for the Stratification of Parkinsonian Syndromes. Mov. Disord. 2021, 36, 2663–2669. [Google Scholar] [CrossRef]
- Jiang, C.; Hopfner, F.; Katsikoudi, A.; Hein, R.; Catli, C.; Evetts, S.; Huang, Y.; Wang, H.; Ryder, J.W.; Kuhlenbaeumer, G.; et al. Serum neuronal exosomes predict and differentiate Parkinson’s disease from atypical parkinsonism. J. Neurol. Neurosurg. Psychiatry 2020, 91, 720–729. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Zhu, X.; Zhou, X.; Li, Y.; Zhou, L.; Zhao, A.; Luo, N.; Niu, M.; Liu, J. Collaborative plasma biomarkers for Parkinson disease development and progression: A cross-sectional and longitudinal study. Eur. J. Neurol. 2023, 30, 3090–3097. [Google Scholar] [CrossRef] [PubMed]
- Kluge, A.; Bunk, J.; Schaeffer, E.; Drobny, A.; Xiang, W.; Knacke, H.; Bub, S.; Lückstädt, W.; Arnold, P.; Lucius, R.; et al. Detection of neuron-derived pathological α-synuclein in blood. Brain 2022, 145, 3058–3071. [Google Scholar] [CrossRef] [PubMed]
- Lucien, F.; Benarroch, E.E.; Mullan, A.; Ali, F.; Boeve, B.F.; Mielke, M.M.; Petersen, R.C.; Kim, Y.; Stang, C.; Camerucci, E.; et al. Poly (ADP-Ribose) and α–synuclein extracellular vesicles in patients with Parkinson disease: A possible biomarker of disease severity. PLoS ONE 2022, 17, e0264446. [Google Scholar] [CrossRef] [PubMed]
- Niu, M.; Li, Y.; Li, G.; Zhou, L.; Luo, N.; Yao, M.; Kang, W.; Liu, J. A longitudinal study on α-synuclein in plasma neuronal exosomes as a biomarker for Parkinson’s disease development and progression. Eur. J. Neurol. 2020, 27, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Picca, A.; Guerra, F.; Calvani, R.; Marini, F.; Biancolillo, A.; Landi, G.; Beli, R.; Landi, F.; Bernabei, R.; Bentivoglio, A.R.; et al. Mitochondrial Signatures in Circulating Extracellular Vesicles of Older Adults with Parkinson’s Disease: Results from the EXosomes in PArkiNson’s Disease (EXPAND) Study. J. Clin. Med. 2020, 9, 504. [Google Scholar] [CrossRef]
- Sharafeldin, M.; Yan, S.J.; Jiang, C.; Tofaris, G.K.; Davis, J.J. Alternating Magnetic Field-Promoted Nanoparticle Mixing: The On-Chip Immunocapture of Serum Neuronal Exosomes for Parkinson’s Disease Diagnostics. Anal. Chem. 2023, 95, 7906–7913. [Google Scholar] [CrossRef]
- Shi, M.; Liu, C.Q.; Cook, T.J.; Bullock, K.M.; Zhao, Y.C.; Ginghina, C.; Li, Y.F.; Aro, P.; Dator, R.; He, C.M.; et al. Plasma exosomal α-synuclein is likely CNS-derived and increased in Parkinson’s disease. Acta Neuropathol. 2014, 128, 639–650. [Google Scholar] [CrossRef]
- Shi, M.; Kovac, A.; Korff, A.; Cook, T.J.; Ginghina, C.; Bullock, K.M.; Yang, L.; Stewart, T.; Zheng, D.; Aro, P.; et al. CNS tau efflux via exosomes is likely increased in Parkinson’s disease but not in Alzheimer’s disease. Alzheimers Dement. 2016, 12, 1125–1131. [Google Scholar] [CrossRef]
- Shim, K.H.; Go, H.G.; Bae, H.; Jeong, D.E.; Kim, D.; Youn, Y.C.; Kim, S.; An, S.S.A.; Kang, M.J. Decreased Exosomal Acetylcholinesterase Activity in the Plasma of Patients With Parkinson’s Disease. Front. Aging Neurosci. 2021, 13, 665400. [Google Scholar] [CrossRef]
- Si, X.L.; Tian, J.; Chen, Y.X.; Yan, Y.P.; Pu, J.L.; Zhang, B.R. Central Nervous System-Derived Exosomal Alpha-Synuclein in Serum May Be a Biomarker in Parkinson’s Disease. Neuroscience 2019, 413, 308–316. [Google Scholar] [CrossRef]
- Stuendl, A.; Kraus, T.; Chatterjee, M.; Zapke, B.; Sadowski, B.; Moebius, W.; Hobert, M.A.; Deuschle, C.; Brockmann, K.; Maetzler, W.; et al. α-Synuclein in Plasma-Derived Extracellular Vesicles Is a Potential Biomarker of Parkinson’s Disease. Mov. Disord. 2021, 36, 2508–2518. [Google Scholar] [CrossRef]
- Wang, P.; Lan, G.; Xu, B.; Yu, Z.; Tian, C.; Lei, X.; Meissner, W.G.; Feng, T.; Yang, Y.; Zhang, J. α-Synuclein-carrying astrocytic extracellular vesicles in Parkinson pathogenesis and diagnosis. Transl. Neurodegener. 2023, 12, 40. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, Y.; Cai, H.; Yang, C.; Li, S.; Lv, H.; Feng, T.; Yu, Z. Aβ1-42-containing platelet-derived extracellular vesicle is associated with cognitive decline in Parkinson’s disease. Front. Aging Neurosci. 2023, 15, 1170663. [Google Scholar] [CrossRef]
- Yan, S.; Jiang, C.; Janzen, A.; Barber, T.R.; Seger, A.; Sommerauer, M.; Davis, J.J.; Marek, K.; Hu, M.T.; Oertel, W.H.; et al. Neuronally Derived Extracellular Vesicle α-Synuclein as a Serum Biomarker for Individuals at Risk of Developing Parkinson Disease. JAMA Neurol. 2024, 81, 59–68. [Google Scholar] [CrossRef]
- Yan, Y.Q.; Pu, J.L.; Zheng, R.; Fang, Y.; Gu, L.Y.; Guo, T.; Si, X.L.; Zhou, C.; Chen, Y.; Liu, Y.; et al. Different patterns of exosomal α-synuclein between Parkinson’s disease and probable rapid eye movement sleep behavior disorder. Eur. J. Neurol. 2022, 29, 3590–3599. [Google Scholar] [CrossRef] [PubMed]
- Zhao, A.; Li, Y.; Niu, M.; Li, G.; Luo, N.; Zhou, L.; Kang, W.; Liu, J. SNCA Hypomethylation in Rapid Eye Movement Sleep Behavior Disorder Is a Potential Biomarker for Parkinson’s Disease. J. Park. Dis. 2020, 10, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.H.; Chen, Z.T.; Zhou, R.L.; Zhang, X.; Ye, Q.Y.; Wang, Y.Z. Increased DJ-1 and α-synuclein in plasma neural-derived exosomes as potential markers for Parkinson’s disease. Front. Aging Neurosci. 2019, 11, 438. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.X.; Xie, Z.H.; Zhang, X.R.; Mao, J.; Wang, M.Y.; Wei, S.J.; Fu, Y.W.; Zheng, H.; He, Y.; Chen, H.; et al. Investigation of α-Synuclein Species in Plasma Exosomes and the Oligomeric and Phosphorylated α-Synuclein as Potential Peripheral Biomarker of Parkinson’s Disease. Neuroscience 2021, 469, 79–90. [Google Scholar] [CrossRef]
- Zou, J.; Guo, Y.; Wei, L.; Yu, F.; Yu, B.; Xu, A. Long Noncoding RNA POU3F3 and α-Synuclein in Plasma L1CAM Exosomes Combined with β-Glucocerebrosidase Activity: Potential Predictors of Parkinson’s Disease. Neurotherapeutics 2020, 17, 1104–1119. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, M.A.; Ebanks, S.; Markus, H.; Lewis, M.M.; Midya, V.; Vrana, K.; Huang, X.; Hall, M.A.; Kawasawa, Y.I. Neuronally enriched microvesicle RNAs are differentially expressed in the serums of Parkinson’s patients. Front. Neurosci. 2023, 17, 1145923. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, C.; Mostile, G.; Baglieri, G.; Giunta, F.; Luca, A.; Raciti, L.; Zappia, M.; Purrello, M.; Ragusa, M.; Nicoletti, A. Specific Signatures of Serum miRNAs as Potential Biomarkers to Discriminate Clinically Similar Neurodegenerative and Vascular-Related Diseases. Cell. Mol. Neurobiol. 2020, 40, 531–546. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Chai, S.; Xiong, T.; Wei, J.; Mao, W.; Zhu, Y.; Li, X.; Wei, W.; Dai, X.; Yang, B.; et al. Aberrant Expression of Circulating MicroRNA Leads to the Dysregulation of Alpha-Synuclein and Other Pathogenic Genes in Parkinson’s Disease. Front. Cell Dev. Biol. 2021, 9, 695007. [Google Scholar] [CrossRef]
- Citterio, L.A.; Mancuso, R.; Agostini, S.; Meloni, M.; Clerici, M. Serum and Exosomal miR-7-1-5p and miR-223-3p as Possible Biomarkers for Parkinson’s Disease. Biomolecules 2023, 13, 865. [Google Scholar] [CrossRef] [PubMed]
- Manna, I.; Quattrone, A.; De Benedittis, S.; Vescio, B.; Iaccino, E.; Quattrone, A. Exosomal miRNA as peripheral biomarkers in Parkinson’s disease and progressive supranuclear palsy: A pilot study. Park. Relat. Disord. 2021, 93, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Nie, C.; Sun, Y.Z.; Zhen, H.F.; Guo, M.; Ye, J.Y.; Liu, Z.L.; Yang, Y.; Zhang, X.Q. Differential Expression of Plasma Exo-miRNA in Neurodegenerative Diseases by Next-Generation Sequencing. Front. Neurosci. 2020, 14, 438. [Google Scholar] [CrossRef]
- Ozdilek, B.; Demircan, B. Serum microRNA expression levels in Turkish patients with Parkinson’s disease. Int. J. Neurosci. 2021, 131, 1181–1189. [Google Scholar] [CrossRef]
- Rai, S.; Bharti, P.S.; Singh, R.; Rastogi, S.; Rani, K.; Sharma, V.; Gorai, P.K.; Rani, N.; Verma, B.K.; Reddy, T.J.; et al. Circulating plasma miR-23b-3p as a biomarker target for idiopathic Parkinson’s disease: Comparison with small extracellular vesicle miRNA. Front. Neurosci. 2023, 17, 1174951. [Google Scholar] [CrossRef]
- Sproviero, D.; Gagliardi, S.; Zucca, S.; Arigoni, M.; Giannini, M.; Garofalo, M.; Olivero, M.; Dell’orco, M.; Pansarasa, O.; Bernuzzi, S.; et al. Different mirna profiles in plasma derived small and large extracellular vesicles from patients with neurodegenerative diseases. Int. J. Mol. Sci. 2021, 22, 2737. [Google Scholar] [CrossRef]
- Tong, G.A.; Zhang, P.P.; Hu, W.B.; Zhang, K.; Chen, X.W. Diagnostic Test to Identify Parkinson’s Disease from the Blood Sera of Chinese Population: A Cross-Sectional Study. Park. Dis. 2022, 2022, 8683877. [Google Scholar] [CrossRef]
- Yao, Y.F.; Qu, M.W.; Li, G.C.; Zhang, F.B.; Rui, H.C. Circulating exosomal miRNAs as diagnostic biomarkers in Parkinson’s disease. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 5278–5283. [Google Scholar]
- Zhang, X.; Yang, R.; Hu, B.L.; Lu, P.C.; Zhou, L.L.; He, Z.Y.; Wu, H.M.; Zhu, J.H. Reduced Circulating Levels of miR-433 and miR-133b Are Potential Biomarkers for Parkinson’s Disease. Front. Cell. Neurosci. 2017, 11, 170. [Google Scholar] [CrossRef]
- Irwin, D.J.; Lee, V.M.; Trojanowski, J.Q. Parkinson’s disease dementia: Convergence of alpha-synuclein, tau and amyloid-beta pathologies. Nat. Rev. Neurosci. 2013, 14, 626–636. [Google Scholar] [CrossRef]
- Jellinger, K.; Braak, H.; Braak, E.; Fischer, P. Alzheimer lesions in the entorhinal region and isocortex in Parkinson’s and Alzheimer’s diseases. Ann. N. Y. Acad. Sci. 1991, 640, 203–209. [Google Scholar] [CrossRef]
- Irwin, D.J.; Hurtig, H.I. The Contribution of Tau, Amyloid-Beta and Alpha-Synuclein Pathology to Dementia in Lewy Body Disorders. J. Alzheimers Dis. Park. 2018, 8, 4. [Google Scholar] [CrossRef]
- Monastero, R.; Cicero, C.E.; Baschi, R.; Davi, M.; Luca, A.; Restivo, V.; Zangara, C.; Fierro, B.; Zappia, M.; Nicoletti, A. Mild cognitive impairment in Parkinson’s disease: The Parkinson’s disease cognitive study (PACOS). J. Neurol. 2018, 265, 1050–1058. [Google Scholar] [CrossRef]
- Aarsland, D.; Andersen, K.; Larsen, J.P.; Lolk, A.; Nielsen, H.; Kragh-Sorensen, P. Risk of dementia in Parkinson’s disease: A community-based, prospective study. Neurology 2001, 56, 730–736. [Google Scholar] [CrossRef]
- Hely, M.A.; Reid, W.G.; Adena, M.A.; Halliday, G.M.; Morris, J.G. The Sydney multicenter study of Parkinson’s disease: The inevitability of dementia at 20 years. Mov. Disord. 2008, 23, 837–844. [Google Scholar] [CrossRef]
- Fang, C.; Lv, L.; Mao, S.; Dong, H.; Liu, B. Cognition Deficits in Parkinson’s Disease: Mechanisms and Treatment. Park. Dis. 2020, 2020, 2076942. [Google Scholar] [CrossRef] [PubMed]
- Stav, A.L.; Aarsland, D.; Johansen, K.K.; Hessen, E.; Auning, E.; Fladby, T. Amyloid-beta and alpha-synuclein cerebrospinal fluid biomarkers and cognition in early Parkinson’s disease. Park. Relat. Disord 2015, 21, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Svenningsson, P.; Westman, E.; Ballard, C.; Aarsland, D. Cognitive impairment in patients with Parkinson’s disease: Diagnosis, biomarkers, and treatment. Lancet Neurol. 2012, 11, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Parnetti, L.; Gaetani, L.; Eusebi, P.; Paciotti, S.; Hansson, O.; El-Agnaf, O.; Mollenhauer, B.; Blennow, K.; Calabresi, P. CSF and blood biomarkers for Parkinson’s disease. Lancet Neurol. 2019, 18, 573–586. [Google Scholar] [CrossRef] [PubMed]
- Lazennec, G.; Richmond, A. Chemokines and chemokine receptors: New insights into cancer-related inflammation. Trends Mol. Med. 2010, 16, 133–144. [Google Scholar] [CrossRef]
- Groblewska, M.; Litman-Zawadzka, A.; Mroczko, B. The Role of Selected Chemokines and Their Receptors in the Development of Gliomas. Int. J. Mol. Sci. 2020, 21, 3704. [Google Scholar] [CrossRef] [PubMed]
- Grayson, M.H.; Holtzman, M.J. Chemokine signaling regulates apoptosis as well as immune cell traffic in host defense. Cell Cycle 2006, 5, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Bajetto, A.; Bonavia, R.; Barbero, S.; Piccioli, P.; Costa, A.; Florio, T.; Schettini, G. Glial and neuronal cells express functional chemokine receptor CXCR4 and its natural ligand stromal cell-derived factor 1. J. Neurochem. 1999, 73, 2348–2357. [Google Scholar] [CrossRef] [PubMed]
- Yon, D.K.; Kim, Y.J.; Park, D.C.; Jung, S.Y.; Kim, S.S.; Yeo, J.H.; Lee, J.; Lee, J.M.; Yeo, S.G. Induction of Autophagy and Its Role in Peripheral Nerve Regeneration after Peripheral Nerve Injury. Int. J. Mol. Sci. 2023, 24, 16219. [Google Scholar] [CrossRef]
- Jin, X.F.; Wu, N.; Wang, L.; Li, J. Circulating microRNAs: A novel class of potential biomarkers for diagnosing and prognosing central nervous system diseases. Cell. Mol. Neurobiol. 2013, 33, 601–613. [Google Scholar] [CrossRef]
- Satterlee, J.S.; Barbee, S.; Jin, P.; Krichevsky, A.; Salama, S.; Schratt, G.; Wu, D.Y. Noncoding RNAs in the brain. J. Neurosci. 2007, 27, 11856–11859. [Google Scholar] [CrossRef] [PubMed]
- Eacker, S.M.; Dawson, T.M.; Dawson, V.L. Understanding microRNAs in neurodegeneration. Nat. Rev. Neurosci. 2009, 10, 837–841. [Google Scholar] [CrossRef]
- Quattrone, A.; Antonini, A.; Vaillancourt, D.E.; Seppi, K.; Ceravolo, R.; Strafella, A.P.; Morelli, M.; Nigro, S.; Vescio, B.; Bianco, M.G.; et al. A New MRI Measure to Early Differentiate Progressive Supranuclear Palsy from De Novo Parkinson’s Disease in Clinical Practice: An International Study. Mov. Disord. 2021, 36, 681–689. [Google Scholar] [CrossRef]
- Chen, Q.; Xu, J.; Li, L.; Li, H.; Mao, S.; Zhang, F.; Zen, K.; Zhang, C.Y.; Zhang, Q. MicroRNA-23a/b and microRNA-27a/b suppress Apaf-1 protein and alleviate hypoxia-induced neuronal apoptosis. Cell Death Dis. 2014, 5, e1132. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Zhang, H.; Wang, B.; Ao, Q.; Shi, J.; He, Z. MicroRNA-23b alleviates neuroinflammation and brain injury in intracerebral hemorrhage by targeting inositol polyphosphate multikinase. Int. Immunopharmacol. 2019, 76, 105887. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Huang, Z.; Chen, M.; Wang, C.; Chen, X.; Chen, J.; Zhang, J. Identification of a panel of five serum miRNAs as a biomarker for Parkinson’s disease. Park. Relat. Disord. 2016, 22, 68–73. [Google Scholar] [CrossRef]
- Huang, J.; Yuan, X.; Chen, L.; Hu, B.; Wang, H.; Huang, W. The Biology, Pathological Roles of Exosomes and Their Clinical Application in Parkinson’s Disease. Neuroscience 2023, 531, 24–38. [Google Scholar] [CrossRef]
- Herman, M.; Randall, G.W.; Spiegel, J.L.; Maldonado, D.J.; Simoes, S. Endo-lysosomal dysfunction in neurodegenerative diseases: Opinion on current progress and future direction in the use of exosomes as biomarkers. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2024, 379, 20220387. [Google Scholar] [CrossRef]
- Cao, Z.; Wu, Y.; Liu, G.; Jiang, Y.; Wang, X.; Wang, Z.; Feng, T. alpha-Synuclein in salivary extracellular vesicles as a potential biomarker of Parkinson’s disease. Neurosci. Lett. 2019, 696, 114–120. [Google Scholar] [CrossRef]
- Chopra, A.; Outeiro, T.F. Aggregation and beyond: Alpha-synuclein-based biomarkers in synucleinopathies. Brain 2024, 147, 81–90. [Google Scholar] [CrossRef]
- Cole, N.B.; Dieuliis, D.; Leo, P.; Mitchell, D.C.; Nussbaum, R.L. Mitochondrial translocation of alpha-synuclein is promoted by intracellular acidification. Exp. Cell Res. 2008, 314, 2076–2089. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Shin, S.Y.; Choi, C.; Lee, Y.H.; Lee, S.J. Formation and removal of alpha-synuclein aggregates in cells exposed to mitochondrial inhibitors. J. Biol. Chem. 2002, 277, 5411–5417. [Google Scholar] [CrossRef]
- Atik, A.; Stewart, T.; Zhang, J. Alpha-Synuclein as a Biomarker for Parkinson’s Disease. Brain Pathol. 2016, 26, 410–418. [Google Scholar] [CrossRef]
- Bras, I.C.; Khani, M.H.; Vasili, E.; Mobius, W.; Riedel, D.; Parfentev, I.; Gerhardt, E.; Fahlbusch, C.; Urlaub, H.; Zweckstetter, M.; et al. Molecular Mechanisms Mediating the Transfer of Disease-Associated Proteins and Effects on Neuronal Activity. J. Park. Dis. 2022, 12, 2397–2422. [Google Scholar] [CrossRef]
- Zhang, S.; Eitan, E.; Wu, T.Y.; Mattson, M.P. Intercellular transfer of pathogenic α-synuclein by extracellular vesicles is induced by the lipid peroxidation product 4-hydroxynonenal. Neurobiol. Aging 2018, 61, 52–65. [Google Scholar] [CrossRef]
- Valencia, J.; Ferreira, M.; Merino-Torres, J.F.; Marcilla, A.; Soriano, J.M. The Potential Roles of Extracellular Vesicles as Biomarkers for Parkinson’s Disease: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 11508. [Google Scholar] [CrossRef]
- Bagheri, V.; Khorramdelazad, H.; Hassanshahi, G.; Moghadam-Ahmadi, A.; Vakilian, A. CXCL12 and CXCR4 in the Peripheral Blood of Patients with Parkinson’s Disease. Neuroimmunomodulation 2018, 25, 201–205. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Y.; Zhao, A.; Luo, N.; Niu, M.; Kang, W.; Xie, A.; Lu, H.; Chen, L.; Liu, J. Parkinson’s disease peripheral immune biomarker profile: A multicentre, cross-sectional and longitudinal study. J. Neuroinflamm. 2022, 19, 116. [Google Scholar] [CrossRef]
- Harischandra, D.S.; Ghaisas, S.; Rokad, D.; Zamanian, M.; Jin, H.; Anantharam, V.; Kimber, M.; Kanthasamy, A.; Kanthasamy, A.G. Environmental neurotoxicant manganese regulates exosome-mediated extracellular miRNAs in cell culture model of Parkinson’s disease: Relevance to alpha-synuclein misfolding in metal neurotoxicity. Neurotoxicology 2018, 64, 267–277. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).