Nicotinamide Mononucleotide Supplementation Alleviates Doxorubicin-Induced Multi-Organ Fibrosis
Abstract
:1. Introduction
2. Results
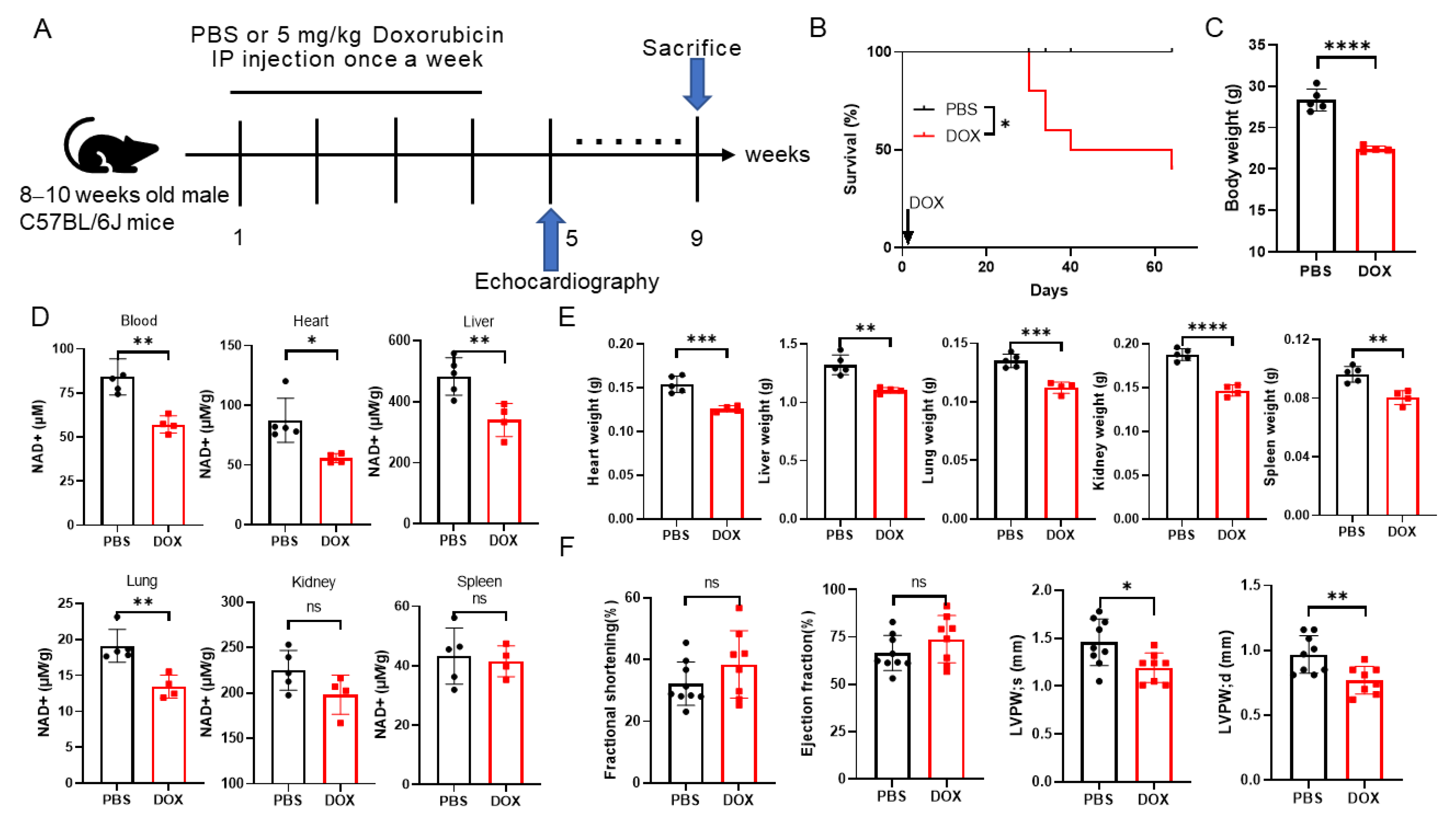
2.1. Doxorubicin Administration Leads to NAD+ Levels Decreasing and Multi-Organ Injury in Mice
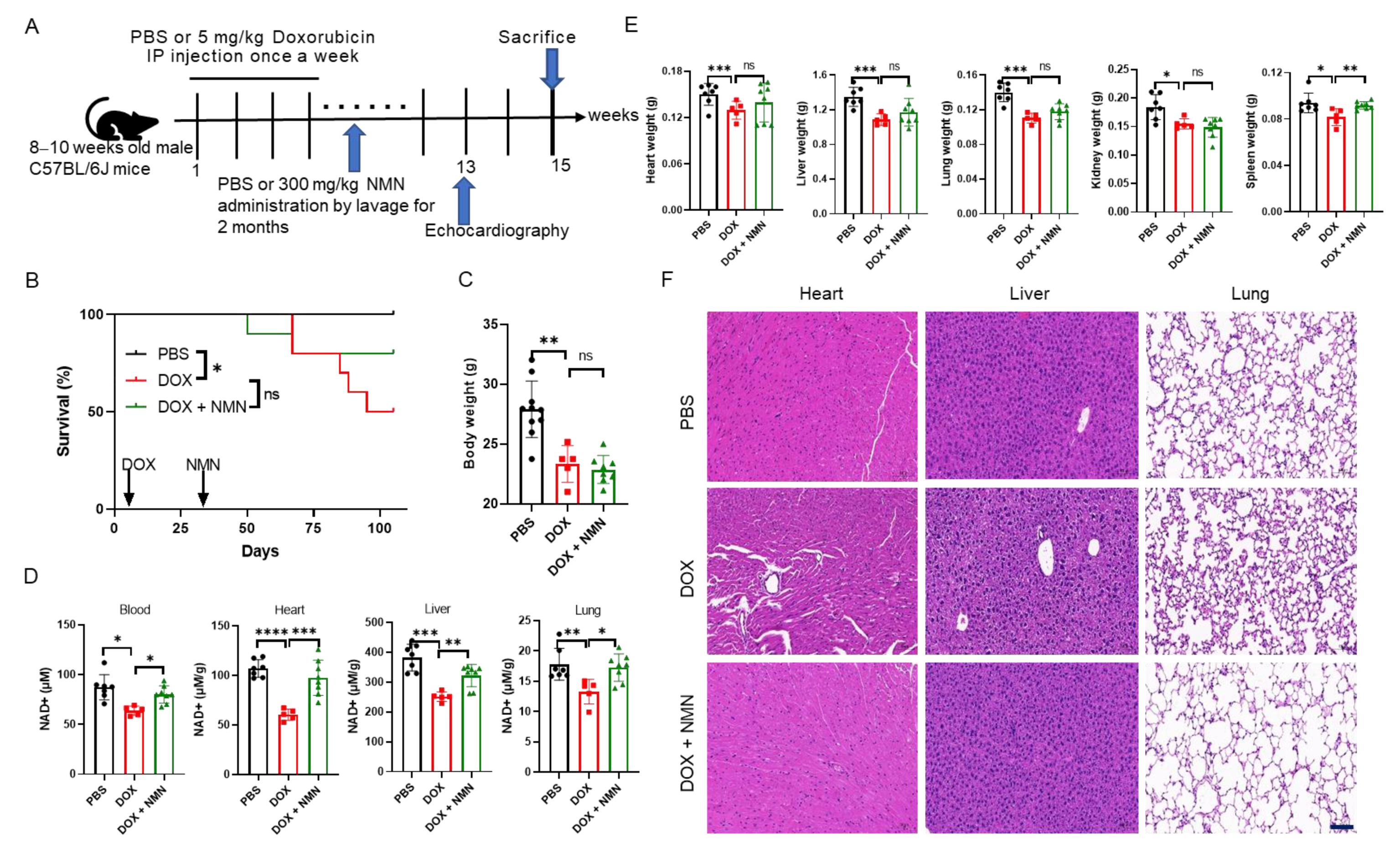
2.2. NMN Supplementation Attenuates Doxorubicin Toxicity to Multiple Organs and Promotes Mice’s Survival by Elevating NAD+ Levels
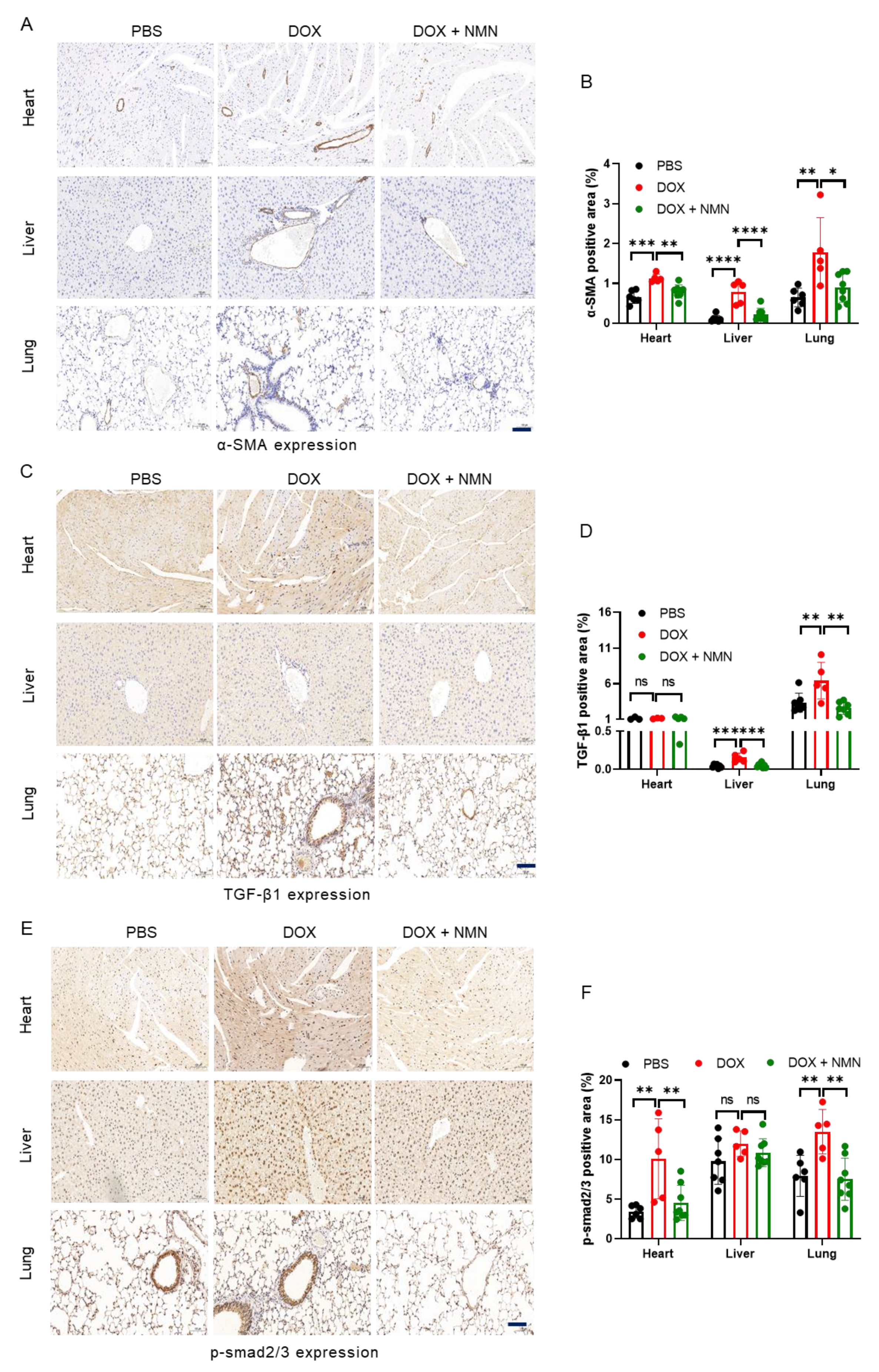
2.3. NMN Supplementation Alleviates Fibrosis of Heart, Liver and Lungs in Mice
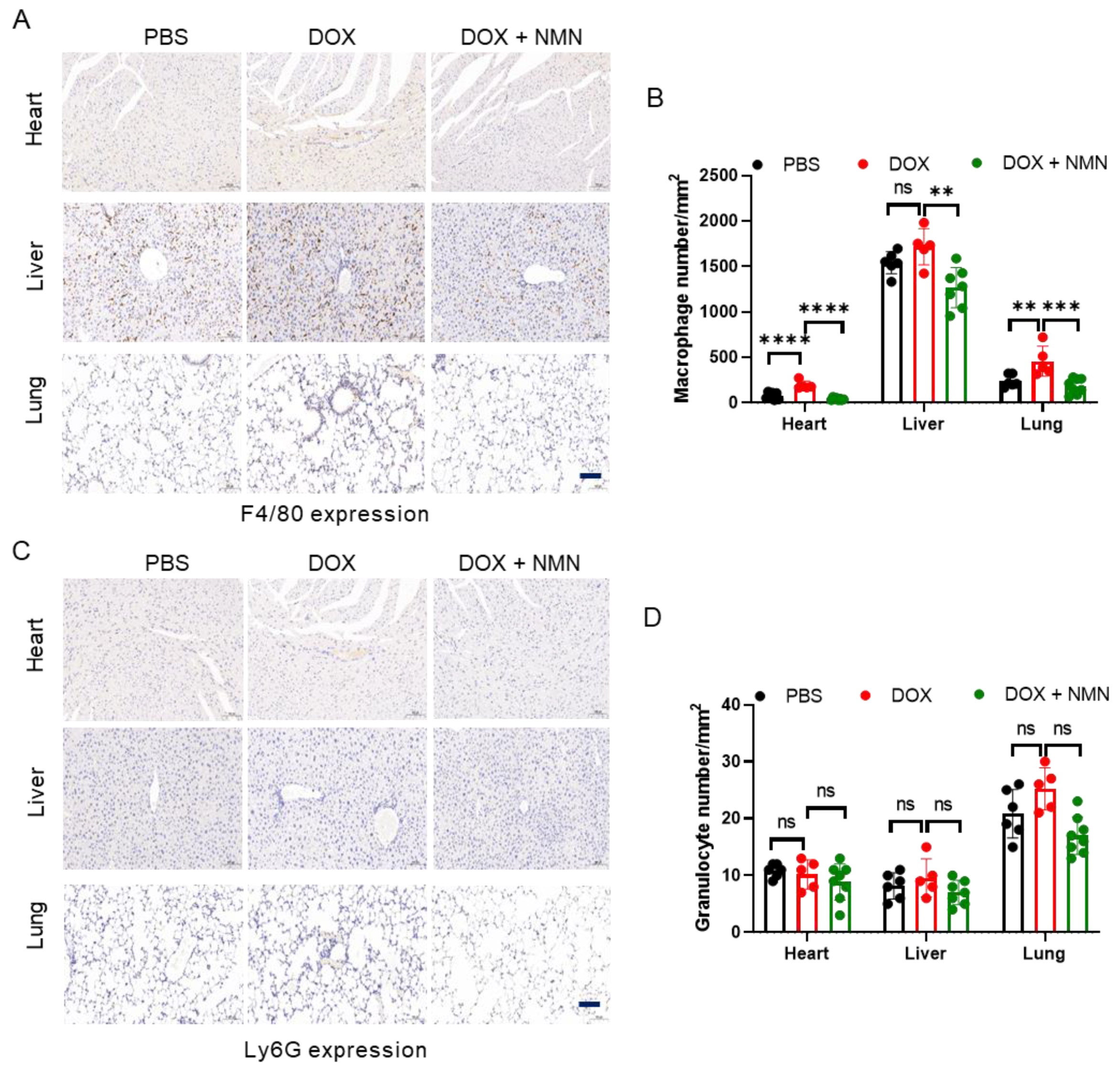
2.4. NMN Treatment Reduces Macrophage Infiltration in Mice
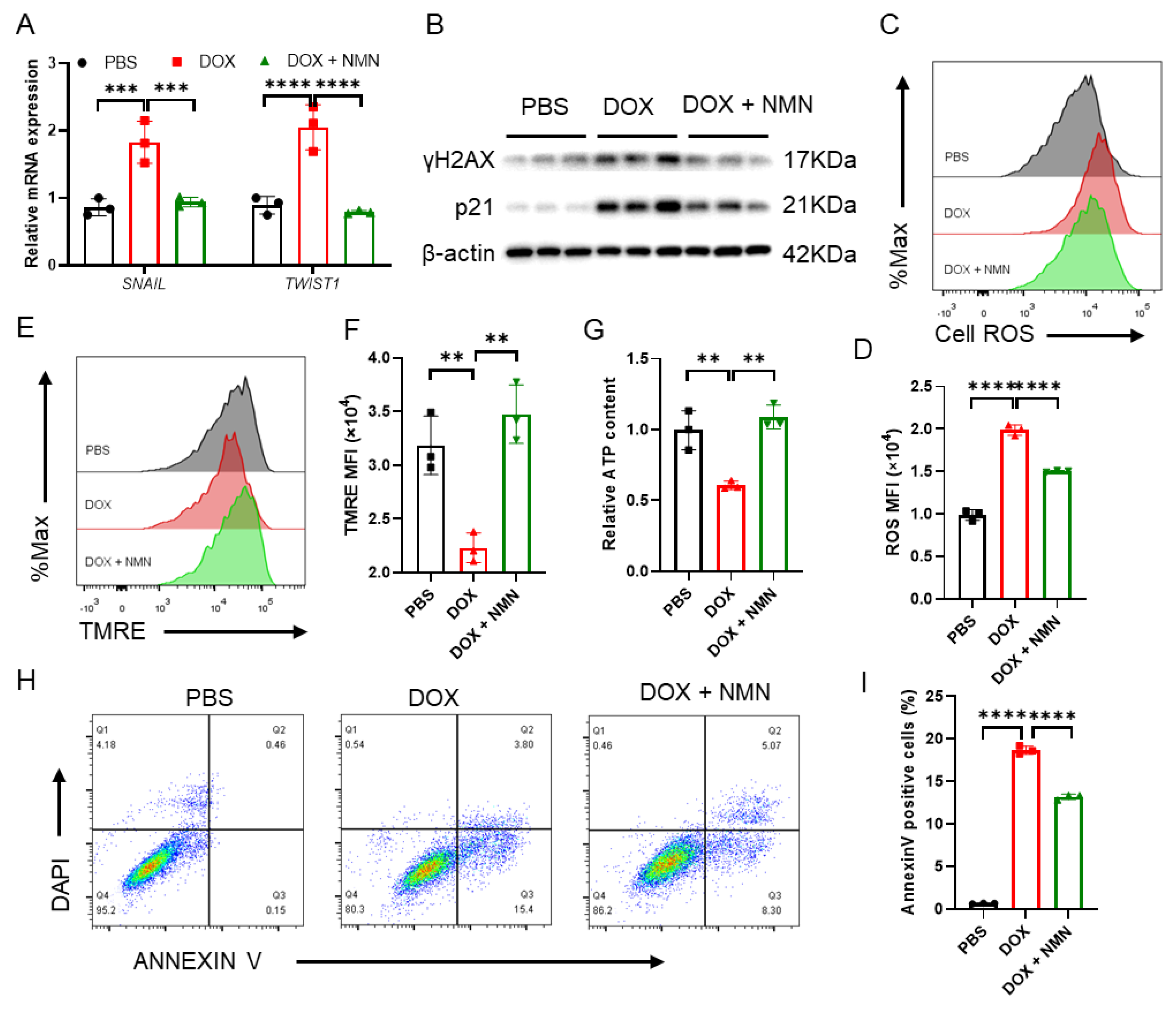
2.5. Boosting NAD+ Reduces Cellular Damage and Suppresses EMT Inducers’ Expression
3. Discussion
4. Materials and Methods
4.1. Animal Studies
4.2. Echocardiography
4.3. Histology
4.4. Immunohistochemical Analysis
4.5. NAD+ Extraction and Measurement
4.6. Flow Cytometry
4.7. Cell Culture and Drug Treatment
4.8. RNA Purification and Real-Time PCR
4.9. Analysis of the Cell Apoptosis
4.10. Western Blot Analysis
4.11. Cell ROS and Mitochondrial Transmembrane Potential Detection
4.12. ATP Content
4.13. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arcamone, F.; Cassinelli, G.; Fantini, G.; Grein, A.; Orezzi, P.; Pol, C.; Spalla, C. Adriamycin, 14-hydroxydaunomycin, a new antitumor antibiotic from S. peucetius var. caesius. Biotechnol. Bioeng. 1969, 11, 1101–1110. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.M.; Whaley, F.S.; Ewer, M.S. Congestive heart failure in patients treated with doxorubicin: A retrospective analysis of three trials. Cancer-Am. Cancer Soc. 2003, 97, 2869–2879. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.J.; Tang, Y.; Zhang, J.; Wang, B.J.; Xiao, M.; Lu, G.; Li, J.; Liu, Q.; Guo, Y.; Gu, J. Cardiac SIRT1 ameliorates doxorubicin-induced cardiotoxicity by targeting sestrin 2. Redox Biol. 2022, 52, 102310. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, X.; Bawa-Khalfe, T.; Lu, L.S.; Lyu, Y.L.; Liu, L.F.; Yeh, E.T. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat. Med. 2012, 18, 1639–1642. [Google Scholar] [CrossRef]
- Sansbury, B.E.; Jones, S.P.; Riggs, D.W.; Darley-Usmar, V.M.; Hill, B.G. Bioenergetic function in cardiovascular cells: The importance of the reserve capacity and its biological regulation. Chem. Biol. Interact. 2011, 191, 288–295. [Google Scholar] [CrossRef]
- Herrmann, J.; Lerman, A. An update on cardio-oncology. Trends Cardiovasc. Med. 2014, 24, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Wallace, K.B.; Sardao, V.A.; Oliveira, P.J. Mitochondrial Determinants of Doxorubicin-Induced Cardiomyopathy. Circ. Res. 2020, 126, 926–941. [Google Scholar] [CrossRef]
- Yang, S.; Shim, M.K.; Kim, W.J.; Choi, J.; Nam, G.H.; Kim, J.; Kim, J.; Moon, Y.; Kim, H.Y.; Park, J.; et al. Cancer-activated doxorubicin prodrug nanoparticles induce preferential immune response with minimal doxorubicin-related toxicity. Biomaterials 2021, 272, 120791. [Google Scholar] [CrossRef]
- Zhao, X.; Jin, Y.; Li, L.; Xu, L.; Tang, Z.; Qi, Y.; Yin, L.; Peng, J. MicroRNA-128-3p aggravates doxorubicin-induced liver injury by promoting oxidative stress via targeting Sirtuin-1. Pharmacol. Res. 2019, 146, 104276. [Google Scholar] [CrossRef]
- Fan, S.; Yan, Y.; Xia, Y.; Zhou, Z.; Luo, L.; Zhu, M.; Han, Y.; Yao, D.; Zhang, L.; Fang, M.; et al. Pregnane X receptor agonist nomilin extends lifespan and healthspan in preclinical models through detoxification functions. Nat. Commun. 2023, 14, 3368. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Q.; Qi, H.; Wang, C.; Wang, C.; Zhang, J.; Dong, L. Doxorubicin-Induced Systemic Inflammation Is Driven by Upregulation of Toll-Like Receptor TLR4 and Endotoxin Leakage. Cancer Res. 2016, 76, 6631–6642. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.; Rajesh, M.; Batkai, S.; Patel, V.; Kashiwaya, Y.; Liaudet, L.; Evgenov, O.V.; Mackie, K.; Hasko, G.; Pacher, P. CB1 cannabinoid receptors promote oxidative stress and cell death in murine models of doxorubicin-induced cardiomyopathy and in human cardiomyocytes. Cardiovasc. Res. 2010, 85, 773–784. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Y.; Qi, Y.; Xu, L.; Song, S.; Yin, L.; Tao, X.; Zhen, Y.; Han, X.; Ma, X.; et al. Protective effects of dioscin against doxorubicin-induced nephrotoxicity via adjusting FXR-mediated oxidative stress and inflammation. Toxicology 2017, 378, 53–64. [Google Scholar] [CrossRef]
- AlAsmari, A.F.; Ali, N.; Alharbi, M.; Alqahtani, F.; Alasmari, F.; Almoqbel, D.; AlSwayyed, M.; Alshammari, A.; Alanazi, M.M.; Alhoshani, A.; et al. Geraniol Ameliorates Doxorubicin-Mediated Kidney Injury through Alteration of Antioxidant Status, Inflammation, and Apoptosis: Potential Roles of NF-kappaB and Nrf2/Ho-1. Nutrients 2022, 14, 1620. [Google Scholar] [CrossRef]
- Ichikawa, Y.; Ghanefar, M.; Bayeva, M.; Wu, R.; Khechaduri, A.; Naga, P.S.; Mutharasan, R.K.; Naik, T.J.; Ardehali, H. Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation. J. Clin. Investig. 2014, 124, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Hasinoff, B.B.; Patel, D.; Wu, X. A QSAR study that compares the ability of bisdioxopiperazine analogs of the doxorubicin cardioprotective agent dexrazoxane (ICRF-187) to protect myocytes with DNA topoisomerase II inhibition. Toxicol. Appl. Pharmacol. 2020, 399, 115038. [Google Scholar] [CrossRef] [PubMed]
- Tebbi, C.K.; London, W.B.; Friedman, D.; Villaluna, D.; De Alarcon, P.A.; Constine, L.S.; Mendenhall, N.P.; Sposto, R.; Chauvenet, A.; Schwartz, C.L. Dexrazoxane-associated risk for acute myeloid leukemia/myelodysplastic syndrome and other secondary malignancies in pediatric Hodgkin’s disease. J. Clin. Oncol. 2007, 25, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Mantawy, E.M.; El-Bakly, W.M.; Esmat, A.; Badr, A.M.; El-Demerdash, E. Chrysin alleviates acute doxorubicin cardiotoxicity in rats via suppression of oxidative stress, inflammation and apoptosis. Eur. J. Pharmacol. 2014, 728, 107–118. [Google Scholar] [CrossRef]
- Sun, Z.; Yan, B.; Yu, W.Y.; Yao, X.; Ma, X.; Sheng, G.; Ma, Q. Vitexin attenuates acute doxorubicin cardiotoxicity in rats via the suppression of oxidative stress, inflammation and apoptosis and the activation of FOXO3a. Exp. Ther. Med. 2016, 12, 1879–1884. [Google Scholar] [CrossRef]
- Croft, T.; Venkatakrishnan, P.; Lin, S.J. NAD(+) Metabolism and Regulation: Lessons From Yeast. Biomolecules 2020, 10, 330. [Google Scholar] [CrossRef]
- Podyacheva, E.; Semenova, N.; Zinserling, V.; Mukhametdinova, D.; Goncharova, I.; Zelinskaya, I.; Sviridov, E.; Martynov, M.; Osipova, S.; Toropova, Y. Intravenous Nicotinamide Riboside Administration Has a Cardioprotective Effect in Chronic Doxorubicin-Induced Cardiomyopathy. Int. J. Mol. Sci. 2022, 23, 13096. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Liang, B.; Gao, Y.; Ye, T.; Li, M.; Zhang, Y.; Lu, Q.; Hu, X.; Li, H.; Yuan, Y.; et al. Nicotinic Acid Riboside Regulates Nrf-2/P62-Related Oxidative Stress and Autophagy to Attenuate Doxorubicin-Induced Cardiomyocyte Injury. Biomed Res. Int. 2022, 2022, 6293329. [Google Scholar] [CrossRef] [PubMed]
- Alano, C.C.; Garnier, P.; Ying, W.; Higashi, Y.; Kauppinen, T.M.; Swanson, R.A. NAD+ depletion is necessary and sufficient for poly(ADP-ribose) polymerase-1-mediated neuronal death. J. Neurosci. 2010, 30, 2967–2978. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Ma, Y.; Kong, X.; Ding, X.; Gu, H.; Chu, T.; Ying, W. NAD(+) administration decreases doxorubicin-induced liver damage of mice by enhancing antioxidation capacity and decreasing DNA damage. Chem. Biol. Interact. 2014, 212, 65–71. [Google Scholar] [CrossRef]
- Rajman, L.; Chwalek, K.; Sinclair, D.A. Therapeutic Potential of NAD-Boosting Molecules: The In Vivo Evidence. Cell Metab. 2018, 27, 529–547. [Google Scholar] [CrossRef] [PubMed]
- Margier, M.; Kuehnemann, C.; Hulo, N.; Morales, J.; Ashok, K.P.; Cros, C.; Cannelle, H.; Charmetant, J.; Verdin, E.; Canault, M.; et al. Nicotinamide Mononucleotide Administration Prevents Doxorubicin-Induced Cardiotoxicity and Loss in Physical Activity in Mice. Cells 2022, 12, 108. [Google Scholar] [CrossRef] [PubMed]
- Serrano, A.; Palou, A.; Bonet, M.L.; Ribot, J. Nicotinamide Riboside Supplementation to Suckling Male Mice Improves Lipid and Energy Metabolism in Skeletal Muscle and Liver in Adulthood. Nutrients 2022, 14, 2259. [Google Scholar] [CrossRef] [PubMed]
- Cobb, M.S.; Tao, S.; Shortt, K.; Girgis, M.; Hauptman, J.; Schriewer, J.; Chin, Z.; Dorfman, E.; Campbell, K.; Heruth, D.P.; et al. Smad3 promotes adverse cardiovascular remodeling and dysfunction in doxorubicin-treated hearts. Am. J. Physiol. Heart Circ. Physiol. 2022, 323, H1091–H1107. [Google Scholar] [CrossRef] [PubMed]
- Perry, J.M.; Tao, F.; Roy, A.; Lin, T.; He, X.C.; Chen, S.; Lu, X.; Nemechek, J.; Ruan, L.; Yu, X.; et al. Overcoming Wnt-beta-catenin dependent anticancer therapy resistance in leukaemia stem cells. Nat. Cell Biol. 2020, 22, 689–700. [Google Scholar] [CrossRef]
- Koyama, Y.; Brenner, D.A. Liver inflammation and fibrosis. J. Clin. Investig. 2017, 127, 55–64. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Y.; Huang, X.; Liu, K.; Wang, Q.D.; Chen, A.F.; Sun, K.; Lui, K.O.; Zhou, B. Seamless Genetic Recording of Transiently Activated Mesenchymal Gene Expression in Endothelial Cells During Cardiac Fibrosis. Circulation 2021, 144, 2004–2020. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Joyce, J.; Margulies, K.B.; Tsuda, T. Enhanced bioactive myocardial transforming growth factor-beta in advanced human heart failure. Circ. J. 2014, 78, 2711–2718. [Google Scholar] [CrossRef]
- Petrov, V.V.; Fagard, R.H.; Lijnen, P.J. Stimulation of collagen production by transforming growth factor-beta1 during differentiation of cardiac fibroblasts to myofibroblasts. Hypertension 2002, 39, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Anscher, M.S. Targeting the TGF-beta1 pathway to prevent normal tissue injury after cancer therapy. Oncologist 2010, 15, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Baldock, R.A.; Day, M.; Wilkinson, O.J.; Cloney, R.; Jeggo, P.A.; Oliver, A.W.; Watts, F.Z.; Pearl, L.H. ATM Localization and Heterochromatin Repair Depend on Direct Interaction of the 53BP1-BRCT2 Domain with gammaH2AX. Cell Rep. 2015, 13, 2081–2089. [Google Scholar] [CrossRef] [PubMed]
- Aix, E.; Gutierrez-Gutierrez, O.; Sanchez-Ferrer, C.; Aguado, T.; Flores, I. Postnatal telomere dysfunction induces cardiomyocyte cell-cycle arrest through p21 activation. J. Cell Biol. 2016, 213, 571–583. [Google Scholar] [CrossRef] [PubMed]
- Lovisa, S.; LeBleu, V.S.; Tampe, B.; Sugimoto, H.; Vadnagara, K.; Carstens, J.L.; Wu, C.C.; Hagos, Y.; Burckhardt, B.C.; Pentcheva-Hoang, T.; et al. Epithelial-to-mesenchymal transition induces cell cycle arrest and parenchymal damage in renal fibrosis. Nat. Med. 2015, 21, 998–1009. [Google Scholar] [CrossRef] [PubMed]
- Kremer, L.C.; van der Pal, H.J.; Offringa, M.; van Dalen, E.C.; Voute, P.A. Frequency and risk factors of subclinical cardiotoxicity after anthracycline therapy in children: A systematic review. Ann. Oncol. 2002, 13, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Broder, H.; Gottlieb, R.A.; Lepor, N.E. Chemotherapy and cardiotoxicity. Rev. Cardiovasc. Med. 2008, 9, 75–83. [Google Scholar]
- Liu, L.; Zhang, X.; Qian, B.; Min, X.; Gao, X.; Li, C.; Cheng, Y.; Huang, J. Over-expression of heat shock protein 27 attenuates doxorubicin-induced cardiac dysfunction in mice. Eur. J. Heart Fail. 2007, 9, 762–769. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Y.; Zhu, Z.; Liu, H.; Guo, H.; Xiong, C.; Xie, K.; Zhang, X.; Su, S. Protective effect of berberine on doxorubicin-induced acute hepatorenal toxicity in rats. Mol. Med. Rep. 2016, 13, 3953–3960. [Google Scholar] [CrossRef]
- Gupta, S.K.; Garg, A.; Bar, C.; Chatterjee, S.; Foinquinos, A.; Milting, H.; Streckfuss-Bomeke, K.; Fiedler, J.; Thum, T. Quaking Inhibits Doxorubicin-Mediated Cardiotoxicity Through Regulation of Cardiac Circular RNA Expression. Circ. Res. 2018, 122, 246–254. [Google Scholar] [CrossRef]
- Ma, Y.; Yang, L.; Ma, J.; Lu, L.; Wang, X.; Ren, J.; Yang, J. Rutin attenuates doxorubicin-induced cardiotoxicity via regulating autophagy and apoptosis. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1904–1911. [Google Scholar] [CrossRef] [PubMed]
- Ni, C.; Ma, P.; Wang, R.; Lou, X.; Liu, X.; Qin, Y.; Xue, R.; Blasig, I.; Erben, U.; Qin, Z. Doxorubicin-induced cardiotoxicity involves IFNgamma-mediated metabolic reprogramming in cardiomyocytes. J. Pathol. 2019, 247, 320–332. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, P.Y.; Long, N.A.; Zhuang, J.; Springer, D.A.; Zou, J.; Lin, Y.; Bleck, C.; Park, J.H.; Kang, J.G.; et al. p53 prevents doxorubicin cardiotoxicity independently of its prototypical tumor suppressor activities. Proc. Natl. Acad. Sci. USA 2019, 116, 19626–19634. [Google Scholar] [CrossRef]
- Owumi, S.E.; Lewu, D.O.; Arunsi, U.O.; Oyelere, A.K. Luteolin attenuates doxorubicin-induced derangements of liver and kidney by reducing oxidative and inflammatory stress to suppress apoptosis. Hum. Exp. Toxicol. 2021, 40, 1656–1672. [Google Scholar] [CrossRef]
- Verdin, E. NAD(+) in aging, metabolism, and neurodegeneration. Science 2015, 350, 1208–1213. [Google Scholar] [CrossRef] [PubMed]
- Xie, N.; Zhang, L.; Gao, W.; Huang, C.; Huber, P.E.; Zhou, X.; Li, C.; Shen, G.; Zou, B. NAD(+) metabolism: Pathophysiologic mechanisms and therapeutic potential. Signal Transduct. Target. Ther. 2020, 5, 227. [Google Scholar] [CrossRef] [PubMed]
- Mills, K.F.; Yoshida, S.; Stein, L.R.; Grozio, A.; Kubota, S.; Sasaki, Y.; Redpath, P.; Migaud, M.E.; Apte, R.S.; Uchida, K.; et al. Long-Term Administration of Nicotinamide Mononucleotide Mitigates Age-Associated Physiological Decline in Mice. Cell Metab. 2016, 24, 795–806. [Google Scholar] [CrossRef]
- Yoshino, M.; Yoshino, J.; Kayser, B.D.; Patti, G.J.; Franczyk, M.P.; Mills, K.F.; Sindelar, M.; Pietka, T.; Patterson, B.W.; Imai, S.I.; et al. Nicotinamide mononucleotide increases muscle insulin sensitivity in prediabetic women. Science 2021, 372, 1224–1229. [Google Scholar] [CrossRef]
- Song, J.; Li, J.; Yang, F.; Ning, G.; Zhen, L.; Wu, L.; Zheng, Y.; Zhang, Q.; Lin, D.; Xie, C.; et al. Nicotinamide mononucleotide promotes osteogenesis and reduces adipogenesis by regulating mesenchymal stromal cells via the SIRT1 pathway in aged bone marrow. Cell Death Dis. 2019, 10, 336. [Google Scholar] [CrossRef]
- Galluzzi, L.; Buque, A.; Kepp, O.; Zitvogel, L.; Kroemer, G. Immunological Effects of Conventional Chemotherapy and Targeted Anticancer Agents. Cancer Cell 2015, 28, 690–714. [Google Scholar] [CrossRef] [PubMed]
- Covarrubias, A.J.; Kale, A.; Perrone, R.; Lopez-Dominguez, J.A.; Pisco, A.O.; Kasler, H.G.; Schmidt, M.S.; Heckenbach, I.; Kwok, R.; Wiley, C.D.; et al. Senescent cells promote tissue NAD(+) decline during ageing via the activation of CD38(+) macrophages. Nat. Metab. 2020, 2, 1265–1283. [Google Scholar] [CrossRef]
- Dhingra, R.; Margulets, V.; Chowdhury, S.R.; Thliveris, J.; Jassal, D.; Fernyhough, P.; Dorn, G.N.; Kirshenbaum, L.A. Bnip3 mediates doxorubicin-induced cardiac myocyte necrosis and mortality through changes in mitochondrial signaling. Proc. Natl. Acad. Sci. USA 2014, 111, E5537–E5544. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [PubMed]
- Alano, C.C.; Ying, W.; Swanson, R.A. Poly(ADP-ribose) polymerase-1-mediated cell death in astrocytes requires NAD+ depletion and mitochondrial permeability transition. J. Biol. Chem. 2004, 279, 18895–18902. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Zhang, Y.; Zheng, M.; Cao, T.; Wang, G.; Zhang, L.; Ni, R.; Brockman, J.; Zhong, H.; Fan, G.C.; et al. Nicotinamide riboside promotes autolysosome clearance in preventing doxorubicin-induced cardiotoxicity. Clin. Sci. 2019, 133, 1505–1521. [Google Scholar] [CrossRef] [PubMed]
- Danz, E.D.; Skramsted, J.; Henry, N.; Bennett, J.A.; Keller, R.S. Resveratrol prevents doxorubicin cardiotoxicity through mitochondrial stabilization and the Sirt1 pathway. Free Radic. Biol. Med. 2009, 46, 1589–1597. [Google Scholar] [CrossRef] [PubMed]
- Cappetta, D.; Esposito, G.; Piegari, E.; Russo, R.; Ciuffreda, L.P.; Rivellino, A.; Berrino, L.; Rossi, F.; De Angelis, A.; Urbanek, K. SIRT1 activation attenuates diastolic dysfunction by reducing cardiac fibrosis in a model of anthracycline cardiomyopathy. Int. J. Cardiol. 2016, 205, 99–110. [Google Scholar] [CrossRef]
- Dos, S.A.; Lopez-Granero, C.; Farina, M.; Rocha, J.; Bowman, A.B.; Aschner, M. Oxidative stress, caspase-3 activation and cleavage of ROCK-1 play an essential role in MeHg-induced cell death in primary astroglial cells. Food Chem. Toxicol. 2018, 113, 328–336. [Google Scholar] [CrossRef]
- Sturmlechner, I.; Zhang, C.; Sine, C.C.; van Deursen, E.J.; Jeganathan, K.B.; Hamada, N.; Grasic, J.; Friedman, D.; Stutchman, J.T.; Can, I.; et al. p21 produces a bioactive secretome that places stressed cells under immunosurveillance. Science 2021, 374, b3420. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, C.; Cimini, M.; Cheng, Z.; Benedict, C.; Wang, C.; Trungcao, M.; Mallaredy, V.; Rajan, S.; Garikipati, V.; Kishore, R. Role of circular RNA cdr1as in modulation of macrophage phenotype. Life Sci. 2022, 309, 121003. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.R.; Zhang, R.N.; Sun, R.R.; Li, Y.Y.; Zhang, B.; Jin, X.M.; Zhang, H.F.; Xiao, B.G.; Ma, C.G.; Fan, H.J.; et al. Efficacy and mechanism of Wuzi Yanzong pill on the prevention and treatment of EAE. Heliyon 2023, 9, e20621. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, F.; Xu, A.; Wei, W.; Yang, S.; Xi, Z.; Ge, Y.; Wu, S.; Ju, Z. Nicotinamide Mononucleotide Supplementation Alleviates Doxorubicin-Induced Multi-Organ Fibrosis. Int. J. Mol. Sci. 2024, 25, 5303. https://doi.org/10.3390/ijms25105303
Wen F, Xu A, Wei W, Yang S, Xi Z, Ge Y, Wu S, Ju Z. Nicotinamide Mononucleotide Supplementation Alleviates Doxorubicin-Induced Multi-Organ Fibrosis. International Journal of Molecular Sciences. 2024; 25(10):5303. https://doi.org/10.3390/ijms25105303
Chicago/Turabian StyleWen, Fei, Anhua Xu, Wenjing Wei, Shenglong Yang, Zhiliang Xi, Yuanlong Ge, Shu Wu, and Zhenyu Ju. 2024. "Nicotinamide Mononucleotide Supplementation Alleviates Doxorubicin-Induced Multi-Organ Fibrosis" International Journal of Molecular Sciences 25, no. 10: 5303. https://doi.org/10.3390/ijms25105303



