Extracellular Vesicles Generated by Mesenchymal Stem Cells in Stirred Suspension Bioreactors Promote Angiogenesis in Human-Brain-Derived Endothelial Cells
Abstract
:1. Introduction
2. Results
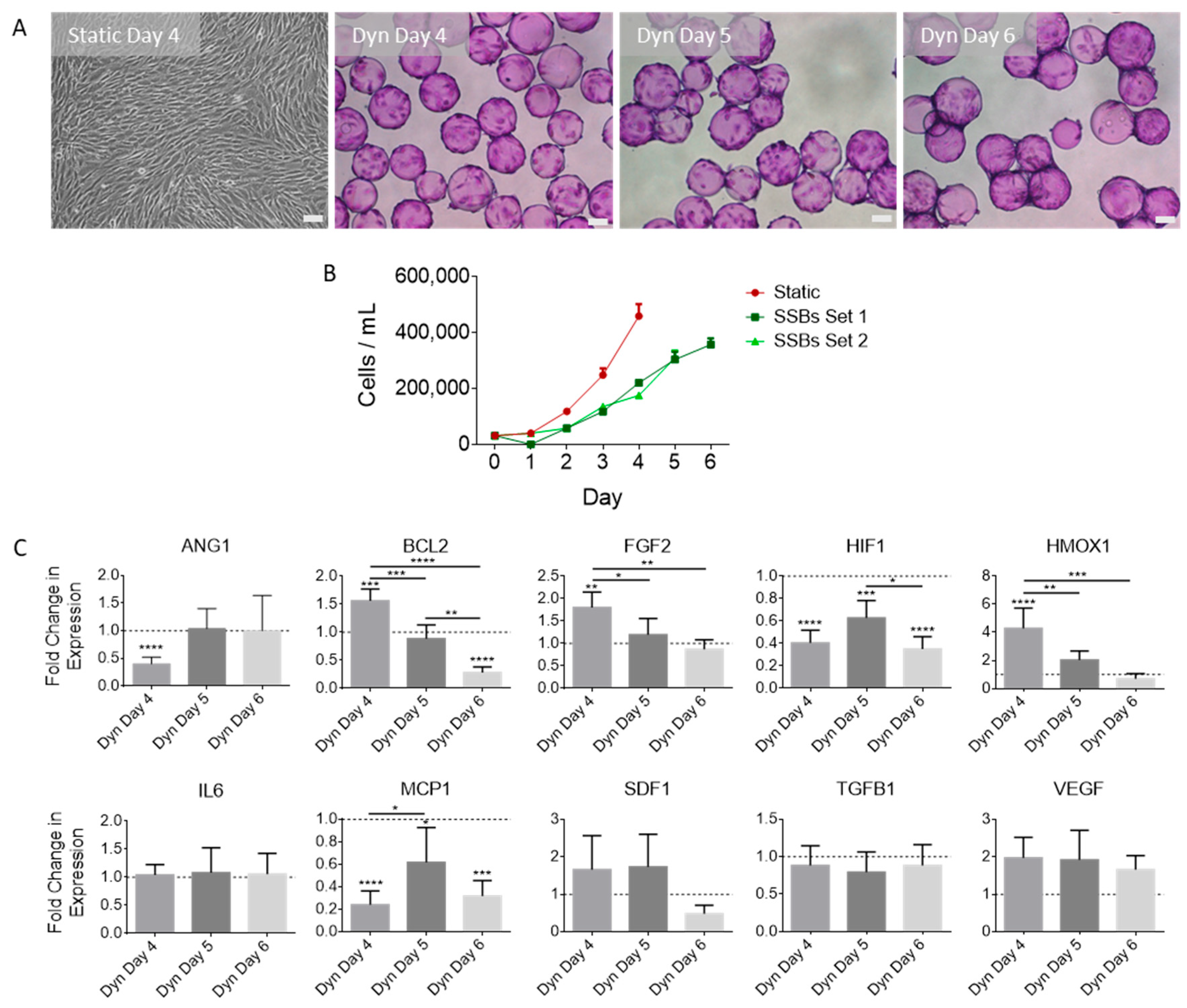
2.1. SSB Culture Affects MSC Proliferation and Gene Expression, with Dependence on Confluence
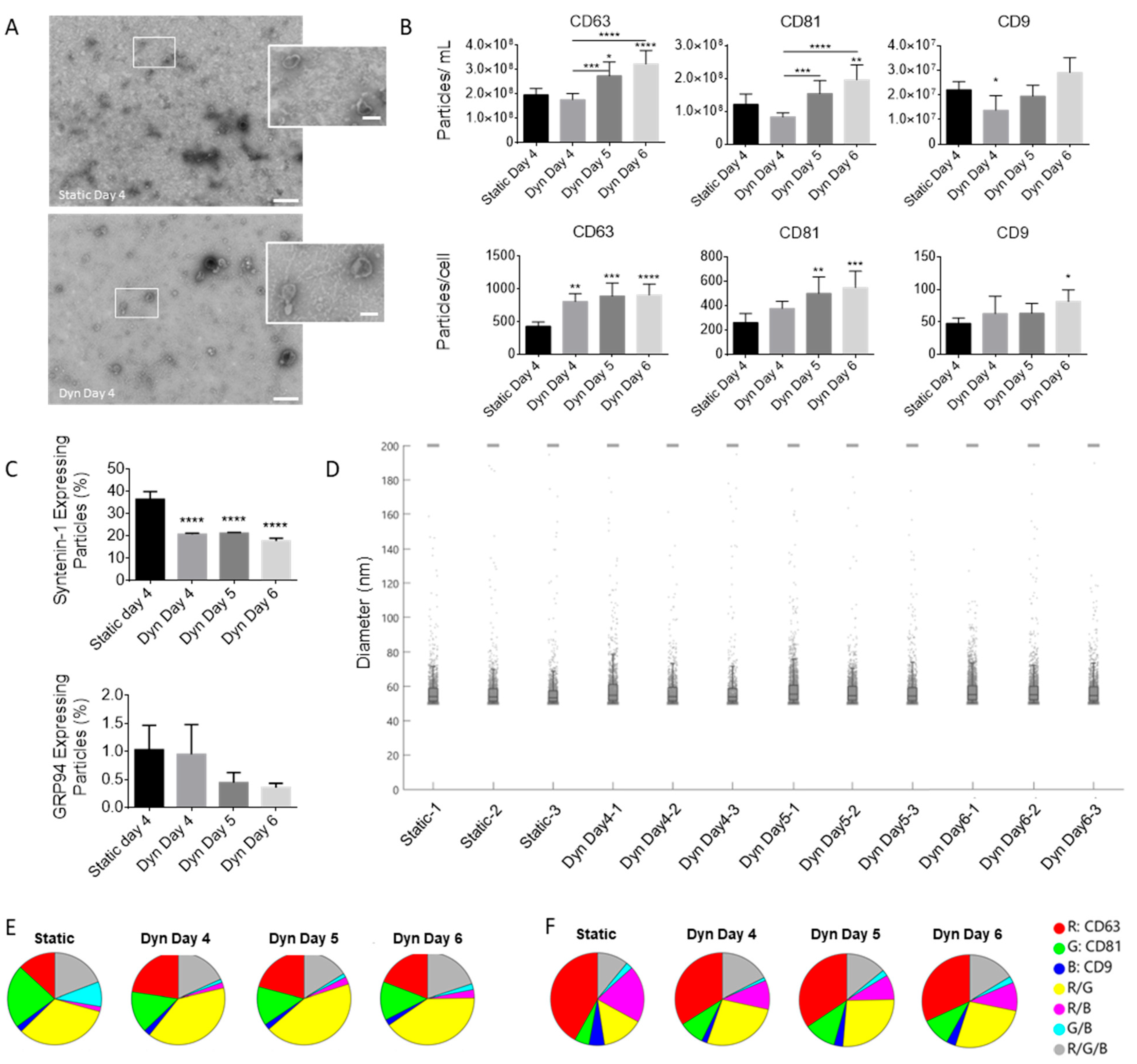
2.2. Dynamic Culture Conditions and Cell Confluency Alter EV Secretion and Composition
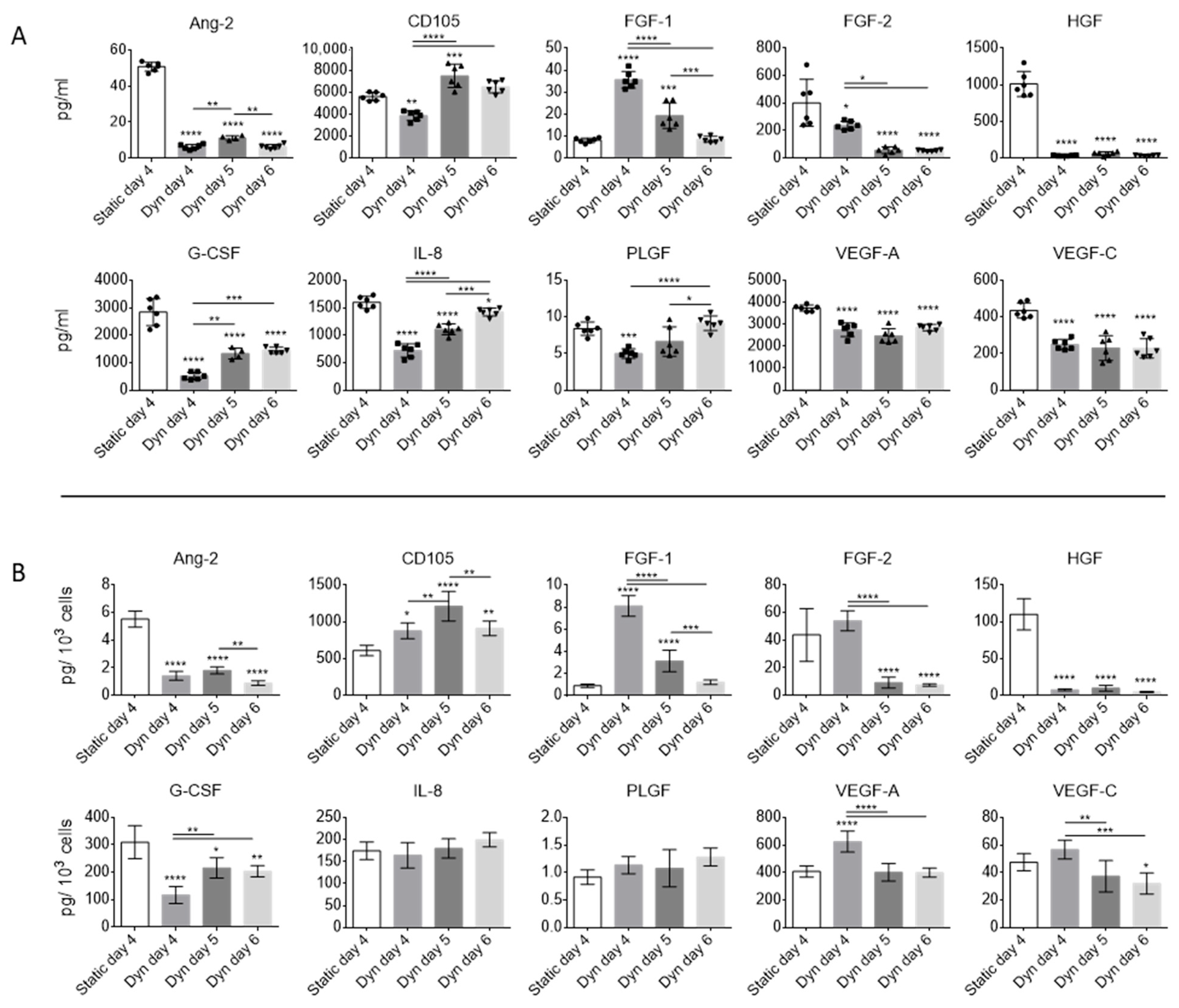
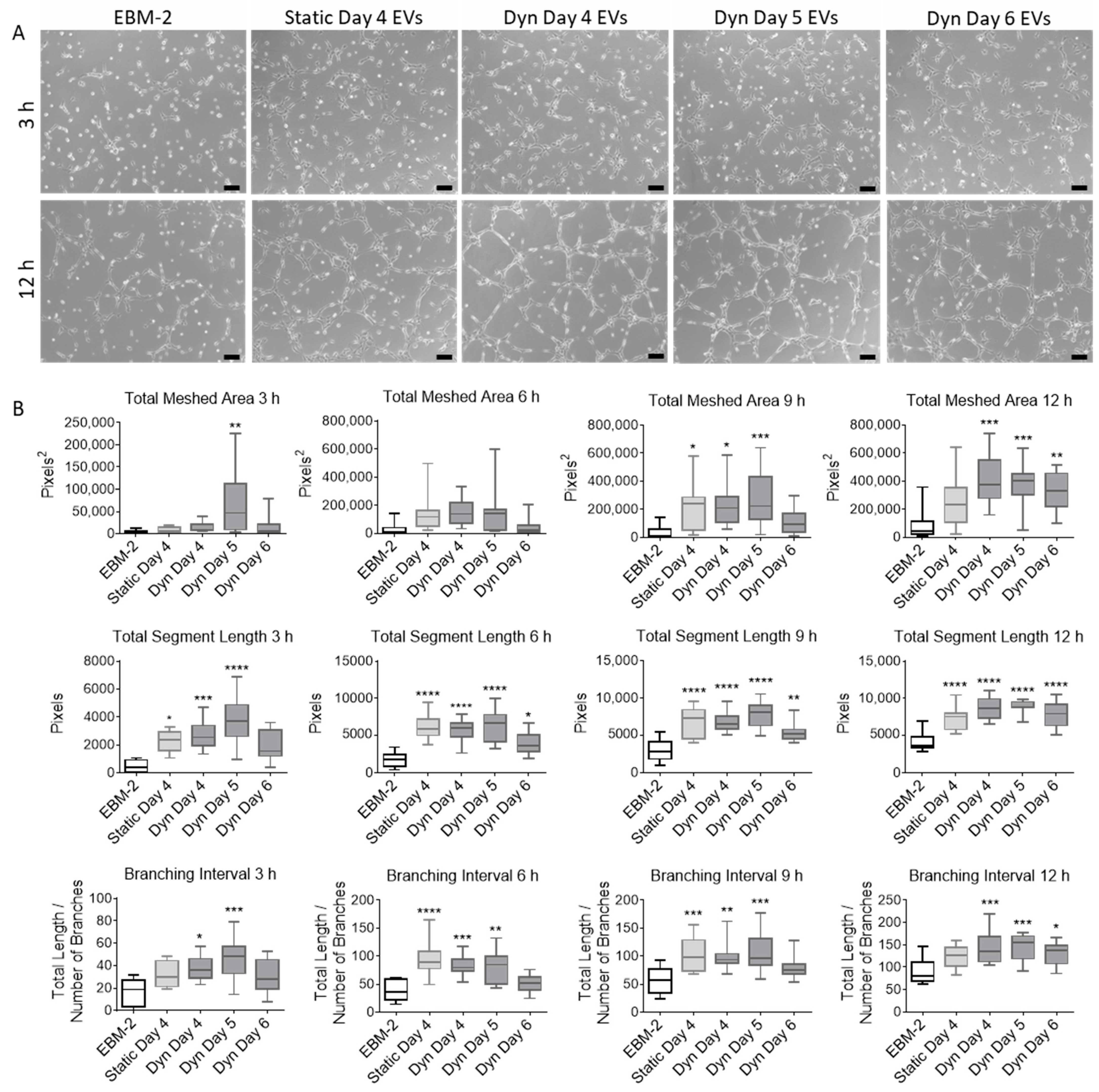
2.3. EVs Isolated from MSCs Cultured in SSBs Have Improved Angiogenic Properties
3. Discussion
4. Materials and Methods
4.1. MSC Culture
4.2. RT-qPCR Analysis of MSCs
4.3. EV Isolation
4.4. TEM
4.5. SP-IRIS
4.6. Luminex Multiplex Analysis
4.7. CMEC Culture
4.8. Proliferation Assay
4.9. Tube Formation Assay
4.10. Statistical Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clarke, D.D.; Sokoloff, L. Circulation and Energy Metabolism of the Brain. In Basic Neurochemistry: Molecular, Cellular and Medical Aspects, 6th ed.; Lippincott-Raven: Philadelphia, PA, USA, 1999; pp. 637–669. [Google Scholar]
- Moon, S.; Chang, M.-S.; Koh, S.-H.; Choi, Y.K. Repair Mechanisms of the Neurovascular Unit after Ischemic Stroke with a Focus on VEGF. Int. J. Mol. Sci. 2021, 22, 8543. [Google Scholar] [CrossRef] [PubMed]
- Ergul, A.; Alhusban, A.; Fagan, S.C. Angiogenesis: A harmonized target for recovery after stroke. Stroke 2012, 43, 2270–2274. [Google Scholar] [CrossRef] [PubMed]
- Navaratna, D.; Guo, S.; Arai, K.; Lo, E.H. Mechanisms and targets for angiogenic therapy after stroke. Cell Adhes. Migr. 2009, 3, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Blood-brain barrier drug targeting: The future of brain drug development. Mol. Interv. 2003, 3, 90–105. [Google Scholar] [CrossRef] [PubMed]
- Bang, O.Y.; Kim, E.H. Mesenchymal Stem Cell-Derived Extracellular Vesicle Therapy for Stroke: Challenges and Progress. Front. Neurol. 2019, 10, 211. [Google Scholar] [CrossRef]
- Bian, X.; Ma, K.; Zhang, C.; Fu, X. Therapeutic angiogenesis using stem cell-derived extracellular vesicles: An emerging approach for treatment of ischemic diseases. Stem Cell Res. Ther. 2019, 10, 158. [Google Scholar] [CrossRef]
- Ma, K.; Zhu, B.; Wang, Z.; Cai, P.; He, M.; Ye, D.; Yan, G.; Zheng, L.; Yang, L.; Zhao, J. Articular chondrocyte-derived extracellular vesicles promote cartilage differentiation of human umbilical cord mesenchymal stem cells by activation of autophagy. J. Nanobiotechnol. 2020, 18, 163. [Google Scholar] [CrossRef] [PubMed]
- Natasha, G.; Gundogan, B.; Tan, A.; Farhatnia, Y.; Wu, W.; Rajadas, J.; Seifalian, A.M. Exosomes as immunotheranostic nanoparticles. Clin. Ther. 2014, 36, 820–829. [Google Scholar] [CrossRef]
- Shi, M.-M.; Yang, Q.-Y.; Monsel, A.; Yan, J.Y.; Dai, C.X.; Zhao, J.Y.; Shi, G.C.; Zhou, M.; Zhu, X.M.; Li, S.K.; et al. Preclinical efficacy and clinical safety of clinical-grade nebulized allogenic adipose mesenchymal stromal cells-derived extracellular vesicles. J. Extracell. Vesicles 2021, 10, e12134. [Google Scholar] [CrossRef]
- Xie, X.; Song, Q.; Dai, C.; Cui, S.; Tang, R.; Li, S.; Chang, J.; Li, P.; Wang, J.; Li, J.; et al. Clinical safety and efficacy of allogenic human adipose mesenchymal stromal cells-derived exosomes in patients with mild to moderate Alzheimer’s disease: A phase I/II clinical trial. Gen. Psychiatry 2023, 36, e101143. [Google Scholar] [CrossRef]
- Attaluri, S.; Jaimes Gonzalez, J.; Kirmani, M.; Vogel, A.D.; Upadhya, R.; Kodali, M.; Madhu, L.N.; Rao, S.; Shuai, B.; Babu, R.S.; et al. Intranasally administered extracellular vesicles from human induced pluripotent stem cell-derived neural stem cells quickly incorporate into neurons and microglia in 5xFAD mice. Front. Aging Neurosci. 2023, 15, 1200445. [Google Scholar] [CrossRef] [PubMed]
- Kodali, M.; Castro, O.W.; Kim, D.-K.; Thomas, A.; Shuai, B.; Attaluri, S.; Upadhya, R.; Gitai, D.; Madhu, L.N.; Prockop, D.J.; et al. Intranasally Administered Human MSC-Derived Extracellular Vesicles Pervasively Incorporate into Neurons and Microglia in both Intact and Status Epilepticus Injured Forebrain. Int. J. Mol. Sci. 2019, 21, 181. [Google Scholar] [CrossRef] [PubMed]
- Doeppner, T.R.; Herz, J.; Görgens, A.; Schlechter, J.; Ludwig, A.K.; Radtke, S.; de Miroschedji, K.; Horn, P.A.; Giebel, B.; Hermann, D.M. Extracellular vesicles improve post-stroke neuroregeneration and prevent postischemic immunosuppression. Stem Cells Transl. Med. 2015, 4, 1131–1143. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bi, T.; Yang, S. Exosomal microRNA-150-5p from bone marrow mesenchymal stromal cells mitigates cerebral ischemia/reperfusion injury via targeting toll-like receptor 5. Bioengineered 2022, 13, 3030–3043. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Pan, J.; Li, Y.; Jiang, Y.; Zheng, H.; Shi, R.; Zhang, Q.; Liu, C.; Tian, H.; Zhang, Z.; et al. Extracellular vesicles from adipose-derived stem cells promote microglia M2 polarization and neurological recovery in a mouse model of transient middle cerebral artery occlusion. Stem Cell Res. Ther. 2022, 13, 21. [Google Scholar] [CrossRef]
- Zhang, Y.; Chopp, M.; Meng, Y.; Katakowski, M.; Xin, H.; Mahmood, A.; Xiong, Y. Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. J. Neurosurg. 2015, 122, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Phelps, J.; Sanati Nezhad, A.; Ungrin, M.; Duncan, N.A.; Sen, A. Bioprocessing of mesenchymal stem cells and their derivatives: Toward cell-free therapeutics. Stem Cells Int. 2018, 2018, 9415367. [Google Scholar] [CrossRef] [PubMed]
- Phelps, J.; Hart, D.A.; Mitha, A.P.; Duncan, N.A.; Sen, A. Physiological oxygen conditions enhance the angiogenic properties of extracellular vesicles from human mesenchymal stem cells. Stem Cell Res. Ther. 2023, 14, 218. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Kallos, M.S.; Hunter, C.; Sen, A. Improved expansion of human bone marrow-derived mesenchymal stem cells in microcarrier-based suspension culture. J. Tissue Eng. Regen. Med. 2012, 8, 210–225. [Google Scholar] [CrossRef]
- Jorgenson, K.D.; Hart, D.A.; Krawetz, R.; Sen, A. Production of adult human synovial fluid-derived mesenchymal stem cells in stirred-suspension culture. Stem Cells Int. 2018, 2018, 8431053. [Google Scholar] [CrossRef]
- Allen, L.M.; Matyas, J.; Ungrin, M.; Sen, A. Serum-free culture of human mesenchymal stem cell aggregates in suspension bioreactors for tissue engineering applications. Stem Cells Int. 2019, 2019, 4607461. [Google Scholar] [CrossRef] [PubMed]
- Gobin, J.; Muradia, G.; Mehic, J.; Westwood, C.; Couvrette, L.; Stalker, A.; Bigelow, S.; Luebbert, C.C.; Bissonnette, F.S.-D.; Johnston, M.J.W.; et al. Hollow-fiber bioreactor production of extracellular vesicles from human bone marrow mesenchymal stromal cells yields nanovesicles that mirrors the immuno-modulatory antigenic signature of the producer cell. Stem Cell Res. Ther. 2021, 12, 127. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Wu, X. Exosomes produced from 3D cultures of umbilical cord mesenchymal stem cells in a hollow-fiber bioreactor show improved osteochondral regeneration activity. Cell Biol. Toxicol. 2020, 36, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Wang, B.; Tang, T.; Lv, L.; Ding, Z.; Li, Z.; Hu, R.; Wei, Q.; Shen, A.; Fu, Y.; et al. Three-dimensional culture of MSCs produces exosomes with improved yield and enhanced therapeutic efficacy for cisplatin-induced acute kidney injury. Stem Cell Res. Ther. 2020, 11, 206. [Google Scholar] [CrossRef] [PubMed]
- De Almeida Fuzeta, M.; Bernardes, N.; Oliveira, F.D.; Costa, A.C.; Fernandes-Platzgummer, A.; Farinha, J.P.; Rodrigue, C.A.V.; Jung, S.; Tseng, R.-J.; Milligan, W.; et al. Scalable Production of Human Mesenchymal Stromal Cell-Derived Extracellular Vesicles Under Serum-/Xeno-Free Conditions in a Microcarrier-Based Bioreactor Culture System. Front. Cell Dev. Biol. 2020, 8, 553444. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.H.G.; Costa, M.S.; Painho, B.; Sousa, C.D.; Carrondo, I.; Oltra, E.; Pelacho, B.; Prosper, F.; Isidro, I.A.; Alves, P.; et al. Enhanced bioprocess control to advance the manufacture of mesenchymal stromal cell-derived extracellular vesicles in stirred-tank bioreactors. Biotechnol. Bioeng. 2023, 120, 2725–2741. [Google Scholar] [CrossRef] [PubMed]
- Phelps, J.; Leonard, C.; Shah, S.; Krawetz, R.; A Hart, D.; A Duncan, N.; Sen, A. Production of mesenchymal progenitor cell-derived extracellular vesicles in suspension bioreactors for use in articular cartilage repair. Stem Cells Transl. Med. 2022, 11, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Kronstadt, S.M.; Patel, D.B.; Born, L.J.; Levy, D.; Lerman, M.J.; Mahadik, B.; McLoughlin, S.T.; Fasuyi, A.; Fowlkes, L.; Van Heyningen, L.H.; et al. Mesenchymal Stem Cell Culture within Perfusion Bioreactors Incorporating 3D-Printed Scaffolds Enables Improved Extracellular Vesicle Yield with Preserved Bioactivity. Adv. Healthc. Mater. 2023, 12, 2300584. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Bae, Y.; Kwon, Y.; Kim, S.; Park, J. Extracellular vesicles generated using bioreactors and their therapeutic effect on the acute kidney injury model. Adv. Healthc. Mater. 2021, 11, e2101606. [Google Scholar] [CrossRef]
- Guo, S.; Debbi, L.; Zohar, B.; Samuel, R.; Arzi, R.S.; Fried, A.I.; Carmon, T.; Shevach, D.; Redenski, I.; Schlachet, I.; et al. Stimulating Extracellular Vesicles Production from Engineered Tissues by Mechanical Forces. Nano Lett. 2021, 21, 2497–2504. [Google Scholar] [CrossRef]
- Hessvik, N.P.; Llorente, A. Current knowledge on exosome biogenesis and release. Cell Mol. Life Sci. 2018, 75, 193–208. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Sen, A.; Rosenberg, L.; Behie, L.A. Human mesenchymal stem cell culture: Rapid and efficient isolation and expansion in a defined serum-free medium. J. Tissue Eng. Regen. Med. 2012, 6, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Sen, A.; Behie, L.A. The development of a medium for the in vitro expansion of mammalian neural stem cells. Can. J. Chem. Eng. 1999, 77, 963–972. [Google Scholar] [CrossRef]
- Luo, W.; Xiong, W.; Zhou, J.; Fang, Z.; Chen, W.; Fan, Y.; Li, F. Laminar shear stress delivers cell cycle arrest and anti-apoptosis to mesenchymal stem cells. Acta Biochim. Biophys. Sin. 2011, 43, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Tu, M.-L.; Wang, H.-Q.; Sun, X.-D.; Chen, L.-J.; Peng, X.-C.; Yuan, Y.-H.; Li, R.-M.; Ruan, X.-Z.; Li, D.-S.; Xu, Y.-J.; et al. Pim-1 is up-regulated by shear stress and is involved in shear stress-induced proliferation of rat mesenchymal stem cells. Life Sci. 2011, 88, 233–238. [Google Scholar] [CrossRef]
- Riddle, R.C.; Taylor, A.F.; Genetos, D.C.; Donahue, H.J. MAP kinase and calcium signaling mediate fluid flow-induced human mesenchymal stem cell proliferation. Am. J. Physiol. Physiol. 2006, 290, C776–C784. [Google Scholar] [CrossRef] [PubMed]
- Sonam, S.; Sathe, S.R.; Yim, E.K.F.; Sheetz, M.P.; Lim, C.T. Cell contractility arising from topography and shear flow determines human mesenchymal stem cell fate. Sci. Rep. 2016, 6, 20415. [Google Scholar] [CrossRef] [PubMed]
- Tsai, A.-C.; Jeske, R.; Chen, X.; Yuan, X.; Li, Y. Influence of Microenvironment on Mesenchymal Stem Cell Therapeutic Potency: From Planar Culture to Microcarriers. Front. Bioeng. Biotechnol. 2020, 8, 640. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.K.-L.; Chen, X.; Lim, Y.M.; Reuveny, S.; Oh, S.K.W. Inhibition of ROCK-myosin II signaling pathway enables culturing of human pluripotent stem cells on microcarriers without extracellular matrix coating. Tissue Eng. Part. C Methods 2014, 20, 227–238. [Google Scholar] [CrossRef]
- Naqvi, S.M.; McNamara, L.M. Stem Cell Mechanobiology and the Role of Biomaterials in Governing Mechanotransduction and Matrix Production for Tissue Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 597661. [Google Scholar] [CrossRef]
- McBeath, R.; Pirone, D.M.; Nelson, C.M.; Bhadriraju, K.; Chen, C.S. Cell Shape, Cytoskeletal Tension, and RhoA Regulate Stem Cell Lineage Commitment. Dev. Cell 2004, 6, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Fagiani, E.; Christofori, G. Angiopoietins in angiogenesis. Cancer Lett. 2013, 328, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Chen-Konak, L.; Guetta-Shubin, Y.; Yahav, H.; Shay-Salit, A.; Zilberman, M.; Binah, O.; Resnick, N. Transcriptional and post-translation regulation of the Tiel receptor by fluid shear stress changes in vascular endothelial cells. FASEB J. 2003, 17, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Pankajakshan, D.; Agrawal, D.K. Mesenchymal Stem Cell Paracrine Factors in Vascular Repair and Regeneration. J. Biomed. Technol. Res. 2014, 1. [Google Scholar] [CrossRef] [PubMed]
- Becquart, P.; Cruel, M.; Hoc, T.; Sudre, L.; Pernelle, K.; Bizios, R.; Logeart-Avramoglou, D.; Petite, H.; Bensidhoum, M. Human mesenchymal stem cell responses to hydrostatic pressure and shear stress. Eur. Cell Mater. 2016, 31, 160–173. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Gu, Y.; Li, C.; Wang, C.-R.; Feng, Z.-G.; Qiu, R.-X.; Chen, B.; Li, J.-X.; Zhang, S.-W.; Wang, Z.-G.; et al. Response of mesenchymal stem cells to shear stress in tissue-engineered vascular grafts. Acta Pharmacol. Sin. 2009, 30, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhu, S.; Yuan, C.; Wang, L.; Xu, J.; Liu, Z. Shear stress promotes differentiation of stem cells from human exfoliated deciduous teeth into endothelial cells via the downstream pathway of VEGF-Notch signaling. Int. J. Mol. Med. 2018, 42, 1827–1836. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Riha, G.M.; Yan, S.; Li, M.; Chai, H.; Yang, H.; Yao, Q.; Chen, C. Shear Stress Induces Endothelial Differentiation From a Murine Embryonic Mesenchymal Progenitor Cell Line. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1817–1823. [Google Scholar] [CrossRef] [PubMed]
- Mojsilovic-Petrovic, J.; Callaghan, D.; Cui, H.; Dean, C.; Stanimirovic, D.B.; Zhang, W. Hypoxia-inducible factor-1 (HIF-1) is involved in the regulation of hypoxia-stimulated expression of monocyte chemoattractant protein-1 (MCP-1/CCL2) and MCP-5 (Ccl12) in astrocytes. J. Neuroinflamm. 2007, 4, 12. [Google Scholar] [CrossRef]
- Tucker, D.; Still, K.; Blom, A.; Kafienah, W. Over-Confluence of expanded bone marrow mesenchymal stem cells ameliorates their chondrogenic capacity in 3D cartilage tissue engineering. bioRxiv 2020. [Google Scholar] [CrossRef]
- Kim, D.S.; Lee, M.W.; Lee, T.; Sung, K.W.; Koo, H.H.; Yoo, K.H. Cell culture density affects the stemness gene expression of adipose tissue-derived mesenchymal stem cells. Biomed. Rep. 2017, 6, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Basmaeil, Y.S.; Algudiri, D.; Alenzi, R.; Al Subayyil, A.; Alaiya, A.; Khatlani, T. HMOX1 is partly responsible for phenotypic and functional abnormalities in mesenchymal stem cells/stromal cells from placenta of preeclampsia (PE) patients. Stem Cell Res. Ther. 2020, 11, 30. [Google Scholar] [CrossRef] [PubMed]
- Dunn, L.L.; Kong, S.M.Y.; Tumanov, S.; Chen, W.; Cantley, J.; Ayer, A.; Maghzal, G.J.; Midwinter, R.G.; Chan, K.H.; Ng, M.K.C.; et al. Hmox1 (Heme Oxygenase-1) Protects Against Ischemia-Mediated Injury via Stabilization of HIF-1α (Hypoxia-Inducible Factor-1α). Arterioscler. Thromb. Vasc. Biol. 2021, 41, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Dulak, J.; Łoboda, A.; Zagórska, A.; Józkowicz, A. Complex role of heme oxygenase-1 in angiogenesis. Antioxid. Redox Signal 2004, 6, 858–866. [Google Scholar] [PubMed]
- Zhang, Y.; Ravikumar, M.; Ling, L.; Nurcombe, V.; Cool, S.M. Age-Related Changes in the Inflammatory Status of Human Mesenchymal Stem Cells: Implications for Cell Therapy. Stem Cell Rep. 2021, 16, 694–707. [Google Scholar] [CrossRef]
- Suvakov, S.; Cubro, H.; White, W.M.; Tobah, Y.S.B.; Weissgerber, T.L.; Jordan, K.L.; Zhu, X.Y.; Woollard, J.R.; Chebib, F.T.; Milic, N.M.; et al. Targeting senescence improves angiogenic potential of adipose-derived mesenchymal stem cells in patients with preeclampsia. Biol. Sex Differ. 2019, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Wang, H.; Tran, K.; Civini, S.; Jin, P.; Castiello, L.; Feng, J.; Kuznetsov, S.A.; Robey, P.G.; Sabatino, M.; et al. Human bone marrow stromal cell confluence: Effects on cell characteristics and methods of assessment. Cytotherapy 2015, 17, 897–911. [Google Scholar] [CrossRef]
- Ma, H.; Calderon, T.M.; Fallon, J.T.; Berman, J.W. Hepatocyte growth factor is a survival factor for endothelial cells and is expressed in human atherosclerotic plaques. Atherosclerosis 2002, 164, 79–87. [Google Scholar] [CrossRef]
- Hu, B.; Cheng, S.-Y. Angiopoietin-2: Development of inhibitors for cancer therapy. Curr. Oncol. Rep. 2009, 11, 111–116. [Google Scholar] [CrossRef]
- Jin, H.; Park, S.; Oh, W.; Yang, Y.S.; Kim, S.W.; Choi, S.J. Down-regulation of CD105 is associated with multi-lineage differentiation in human umbilical cord blood-derived mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2009, 381, 676–681. [Google Scholar] [CrossRef]
- Dry, H.; Jorgenson, K.; Ando, W.; Hart, D.A.; Frank, C.B.; Sen, A. Effect of calcium on the proliferation kinetics of synovium-derived mesenchymal stromal cells. Cytotherapy 2013, 15, 805–819. [Google Scholar] [CrossRef] [PubMed]
- Savina, A.; Furlán, M.; Vidal, M.; Colombo, M.I. Exosome release is regulated by a calcium-dependent mechanism in K562 cells. J. Biol. Chem. 2003, 278, 20083–20090. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.; Azimi, I.; Monteith, G.; Bebawy, M. Ca(2+) mediates extracellular vesicle biogenesis through alternate pathways in malignancy. J. Extracell. Vesicles 2020, 9, 1734326. [Google Scholar] [CrossRef] [PubMed]
- Krämer-Albers, E.-M.; Bretz, N.; Tenzer, S.; Tenzer, S.; Winterstein, C.; Möbius, W.; Berger, H.; Nave, K.-A.; Schild, H.; Trotter, J. Oligodendrocytes secrete exosomes containing major myelin and stress-protective proteins: Trophic support for axons? Proteom. Clin. Appl. 2007, 1, 1446–1461. [Google Scholar] [CrossRef] [PubMed]
- Corrigan, M.A.; Johnson, G.P.; Stavenschi, E.; Riffault, M.; Labour, M.N.; Hoey, D.A. TRPV4-mediates oscillatory fluid shear mechanotransduction in mesenchymal stem cells in part via the primary cilium. Sci. Rep. 2018, 8, 3824. [Google Scholar] [CrossRef]
- Gardiner, C.; Di Vizio, D.; Sahoo, S.; Théry, C.; Witwer, K.W.; Wauben, M.; Hill, A.F. Techniques used for the isolation and characterization of extracellular vesicles: Results of a worldwide survey. J. Extracell. Vesicles 2016, 5, 32945. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Liu, H.; Tian, Y.; Xue, C.; Niu, Q.; Chen, C.; Yan, X. Analysis of extracellular vesicle DNA at the single-vesicle level by nano-flow cytometry. J. Extracell. Vesicles 2022, 11, e12206. [Google Scholar] [CrossRef]
- Tóth, E.Á.; Turiák, L.; Visnovitz, T.; Cserép, C.; Mázló, A.; Sódar, B.W.; Försönits, A.I.; Petővári, G.; Sebestyén, A.; Komlósi, Z.; et al. Formation of a protein corona on the surface of extracellular vesicles in blood plasma. J. Extracell. Vesicles 2021, 10, e12140. [Google Scholar] [CrossRef]
- Wolf, M.; Poupardin, R.W.; Ebner-Peking, P.; Andrade, A.C.; Blöchl, C.; Obermayer, A.; Gomes, F.G.; Vari, B.; Maeding, N.; Eminger, E.; et al. A functional corona around extracellular vesicles enhances angiogenesis, skin regeneration and immunomodulation. J. Extracell. Vesicles 2022, 11, e12207. [Google Scholar] [CrossRef]
- Weksler, B.; Romero, I.A.; Couraud, P.-O. The hCMEC/D3 cell line as a model of the human blood brain barrier. Fluids Barriers CNS 2013, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Daniels, B.P.; Cruz-Orengo, L.; Pasieka, T.J.; Couraud, P.-O.; Romero, I.A.; Weksler, B.; Cooper, J.A.; Doering, T.L.; Klein, R.S. Immortalized human cerebral microvascular endothelial cells maintain the properties of primary cells in an in vitro model of immune migration across the blood brain barrier. J. Neurosci. Methods 2013, 212, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Soler-Botija, C.; Monguió-Tortajada, M.; Munizaga-Larroudé, M.; Galvez-Monton, C.; Bayes-Genis, A.; Roura, S. Mechanisms governing the therapeutic effect of mesenchymal stromal cell-derived extracellular vesicles: A scoping review of preclinical evidence. Biomed. Pharmacother. 2022, 147, 112683. [Google Scholar] [CrossRef] [PubMed]
- Jung, S. Serum-Free Conditions for Rapid Isolation and Long-Term Expansion of Highly Homogenous Human Mesenchymal Stem Cells. Ph.D.Thesis, University of Calgary, Calgary, AB, Canada, 2009. [Google Scholar]
- Phelps, J.; Hassanpour-Tamrin, S.; Duncan, N.A.; Sen, A. Considerations for the Collection of Mesenchymal Stem Cell Derived Extracellular Vesicles from Serum Free Medium; University of Calgary: Calgary, AB, Canada, 2024; to be submitted. [Google Scholar]
- Piovesan, A.; Pelleri, M.C.; Antonaros, F.; Strippoli, P.; Caracausi, M.; Vitale, L. On the length, weight and GC content of the human genome. BMC Res. Notes 2019, 12, 106. [Google Scholar] [CrossRef]
- Carpentier, G.; Berndt, S.; Ferratge, S.; Rasband, W.; Cuendet, M.; Uzan, G.; Albanese, P. Angiogenesis Analyzer for ImageJ—A comparative morphometric analysis of “Endothelial Tube Formation Assay” and “Fibrin Bead Assay”. Sci. Rep. 2020, 10, 11568. [Google Scholar] [CrossRef]


| Gene | Primer Sequence (5′-3′) | Origin |
|---|---|---|
| 18S | F: TGG TCG CTC GCT CCT CTC C R: CGC CTG CTG CCT TCC TTG G | NR_003286 |
| ANG1 | F: CCT GAT CTT ACA CGG TGC R: GCT TTC ATA ATC GCT TCT | NM_001314051 |
| BCL2 | F: GAT GAC TGA GTA CCT GAA CC R: AGT TCC ACA AAG GCA TCC | EU287875 |
| FGF2 | F: CGC GGT TGC AAC GGG AT R: GGG TTC ACG GAT GGT TGT CT | NM_27968 |
| HIF1 | F: CCA GTT ACG TTC CTT CGA TCA GT R: TTT GAG GAC TTG CGC TTT CA | NM_001243084 |
| HMOX1 | F: ATG ACA CCA AGG ACC AGA GC R: GTG TAA GGA CCC ATC GGA GA | NM_002133 |
| IL6 | F: TCA ATA TTA GAG TCT CAA CCC CCA R: TTC TCT TTC GTT CCC GGT GG | NM_000600 |
| MCP1 | F: GCA ATC AAT GCC CCA GTC AC R: TCT TTG GGA CAC TTG CTG CT | S71513 |
| SDF1 | F: GGA CTT TCC GCT AGA CCC AC R: GCC CGA TCC CAG ATC AAT GT | NM_199168 |
| TGFB1 | F: GGG GAA ATT GAG GGC TTT CG R: CCA GGA CCT TGC TGT ACT GC | NM_000660 |
| VEGF | F: ACG GTC CCT CTT GGA ATT GG R: GGC CGC GGT GTG TCT A | M32977 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phelps, J.; Hart, D.A.; Mitha, A.P.; Duncan, N.A.; Sen, A. Extracellular Vesicles Generated by Mesenchymal Stem Cells in Stirred Suspension Bioreactors Promote Angiogenesis in Human-Brain-Derived Endothelial Cells. Int. J. Mol. Sci. 2024, 25, 5219. https://doi.org/10.3390/ijms25105219
Phelps J, Hart DA, Mitha AP, Duncan NA, Sen A. Extracellular Vesicles Generated by Mesenchymal Stem Cells in Stirred Suspension Bioreactors Promote Angiogenesis in Human-Brain-Derived Endothelial Cells. International Journal of Molecular Sciences. 2024; 25(10):5219. https://doi.org/10.3390/ijms25105219
Chicago/Turabian StylePhelps, Jolene, David A. Hart, Alim P. Mitha, Neil A. Duncan, and Arindom Sen. 2024. "Extracellular Vesicles Generated by Mesenchymal Stem Cells in Stirred Suspension Bioreactors Promote Angiogenesis in Human-Brain-Derived Endothelial Cells" International Journal of Molecular Sciences 25, no. 10: 5219. https://doi.org/10.3390/ijms25105219






