NR4A2 as a Novel Target Gene for Developmental and Epileptic Encephalopathy: A Systematic Review of Related Disorders and Therapeutic Strategies
Abstract
:1. Introduction
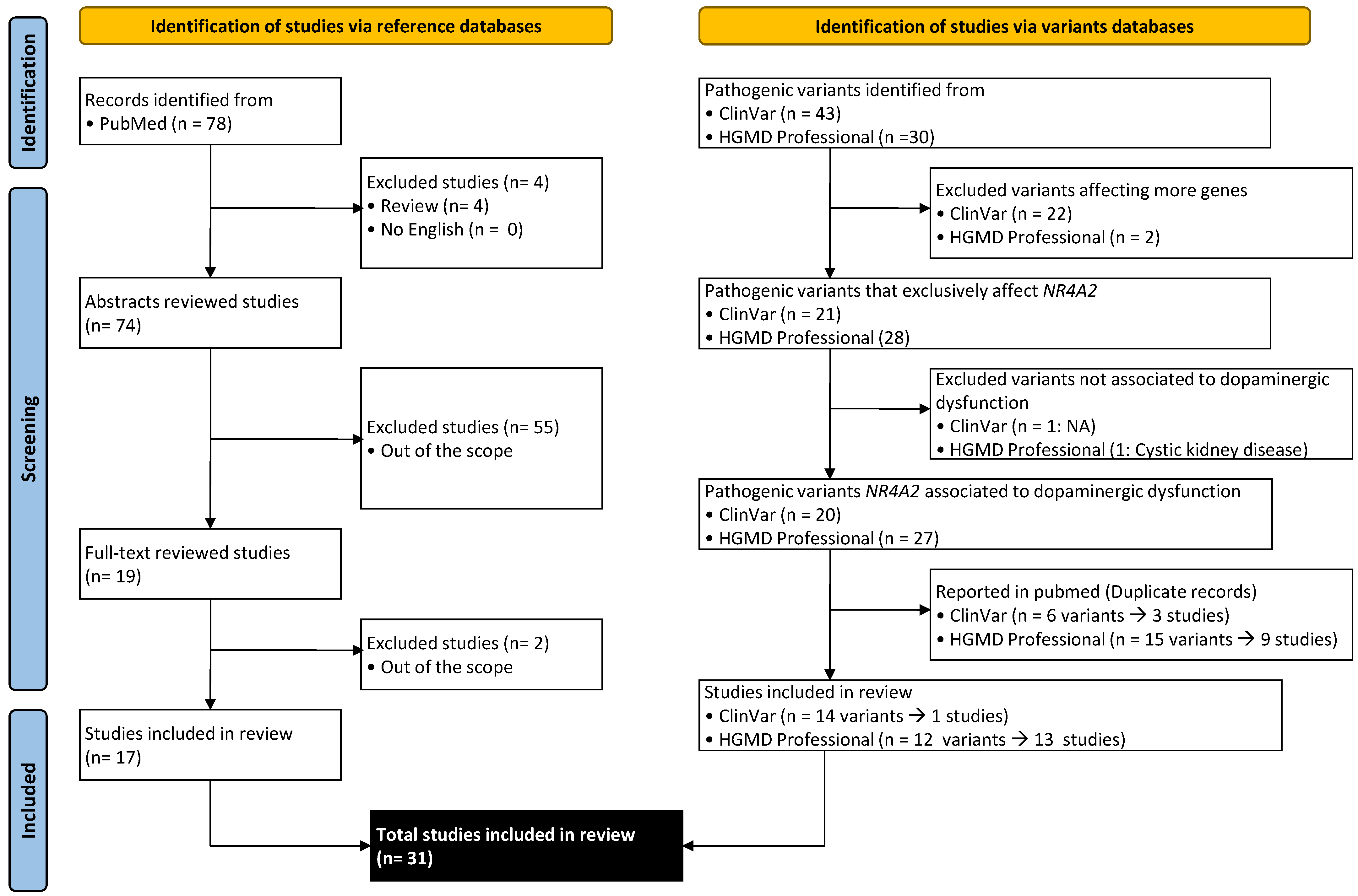
2. Methods
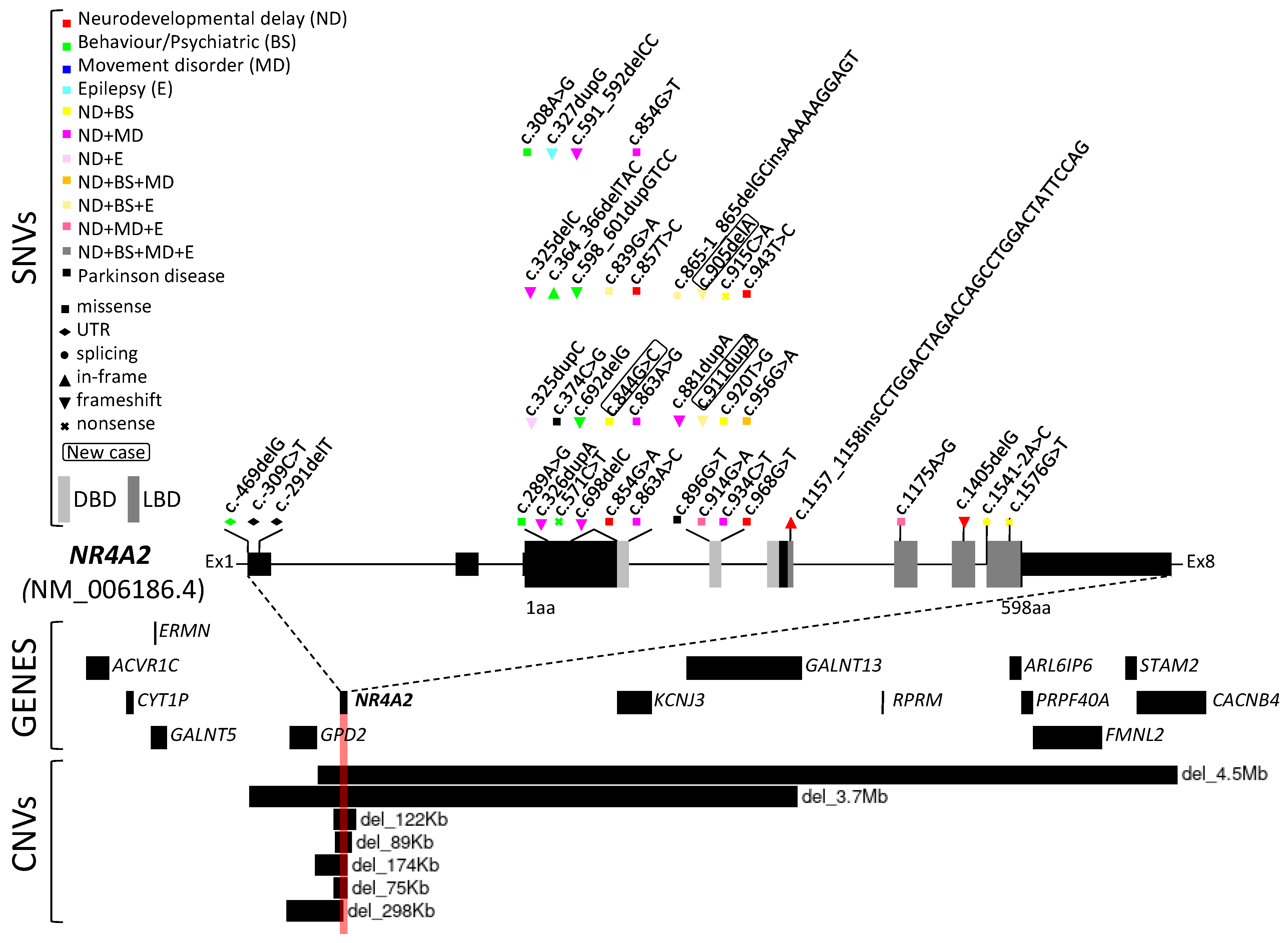
3. Results
3.1. Case Reports—Clinical Findings
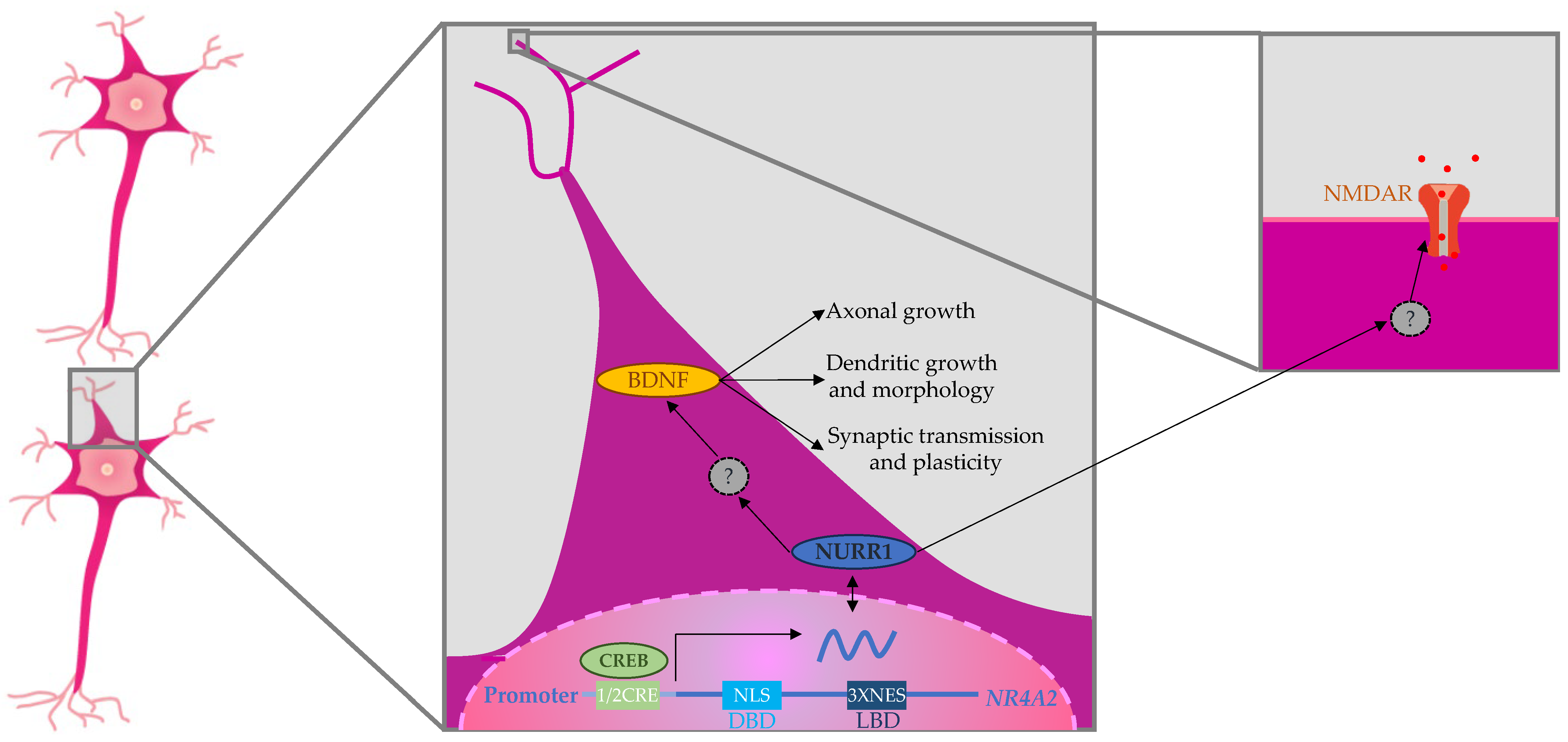
3.2. NR4A2 and Neurodegenerative Disease
3.3. Treatments
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, Z.; Benoit, G.; Liu, J.; Prasad, S.; Aarnisalo, P.; Liu, X.; Xu, H.; Walker, N.P.C.; Perlmann, T. Structure and Function of Nurr1 Identifies a Class of Ligand-Independent Nuclear Receptors. Nature 2003, 423, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Flaig, R.; Greschik, H.; Peluso-Iltis, C.; Moras, D. Structural Basis for the Cell-Specific Activities of the NGFI-B and the Nurr1 Ligand-Binding Domain. J. Biol. Chem. 2005, 280, 19250–19258. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.-H.; Han, B.-S.; Moon, J.; Kim, D.-J.; Shin, J.; Rajan, S.; Nguyen, Q.T.; Sohn, M.; Kim, W.-G.; Han, M.; et al. Nuclear Receptor Nurr1 Agonists Enhance Its Dual Functions and Improve Behavioral Deficits in an Animal Model of Parkinson’s Disease. Proc. Natl. Acad. Sci. USA 2015, 112, 8756–8761. [Google Scholar] [CrossRef]
- de Vera, I.M.S.; Munoz-Tello, P.; Zheng, J.; Dharmarajan, V.; Marciano, D.P.; Matta-Camacho, E.; Giri, P.K.; Shang, J.; Hughes, T.S.; Rance, M.; et al. Defining a Canonical Ligand-Binding Pocket in the Orphan Nuclear Receptor Nurr1. Structure 2019, 27, 66–77.e5. [Google Scholar] [CrossRef] [PubMed]
- Robinson-Rechavi, M.; Escriva Garcia, H.; Laudet, V. The Nuclear Receptor Superfamily. J. Cell Sci. 2003, 116, 585–586. [Google Scholar] [CrossRef] [PubMed]
- Brelivet, Y.; Kammerer, S.; Rochel, N.; Poch, O.; Moras, D. Signature of the Oligomeric Behaviour of Nuclear Receptors at the Sequence and Structural Level. EMBO Rep. 2004, 5, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Martinat, C.; Bacci, J.-J.; Leete, T.; Kim, J.; Vanti, W.B.; Newman, A.H.; Cha, J.H.; Gether, U.; Wang, H.; Abeliovich, A. Cooperative Transcription Activation by Nurr1 and Pitx3 Induces Embryonic Stem Cell Maturation to the Midbrain Dopamine Neuron Phenotype. Proc. Natl. Acad. Sci. USA 2006, 103, 2874–2879. [Google Scholar] [CrossRef] [PubMed]
- Kadkhodaei, B.; Ito, T.; Joodmardi, E.; Mattsson, B.; Rouillard, C.; Carta, M.; Muramatsu, S.; Sumi-Ichinose, C.; Nomura, T.; Metzger, D.; et al. Nurr1 Is Required for Maintenance of Maturing and Adult Midbrain Dopamine Neurons. J. Neurosci. Off. J. Soc. Neurosci. 2009, 29, 15923–15932. [Google Scholar] [CrossRef] [PubMed]
- Messmer, K.; Remington, M.P.; Skidmore, F.; Fishman, P.S. Induction of Tyrosine Hydroxylase Expression by the Transcription Factor Pitx3. Int. J. Dev. Neurosci. Off. J. Int. Soc. Dev. Neurosci. 2007, 25, 29–37. [Google Scholar] [CrossRef]
- Saijo, K.; Winner, B.; Carson, C.T.; Collier, J.G.; Boyer, L.; Rosenfeld, M.G.; Gage, F.H.; Glass, C.K. A Nurr1/CoREST Pathway in Microglia and Astrocytes Protects Dopaminergic Neurons from Inflammation-Induced Death. Cell 2009, 137, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Buervenich, S.; Carmine, A.; Arvidsson, M.; Xiang, F.; Zhang, Z.; Sydow, O.; Jönsson, E.G.; Sedvall, G.C.; Leonard, S.; Ross, R.G.; et al. NURR1 Mutations in Cases of Schizophrenia and Manic-Depressive Disorder. Am. J. Med. Genet. 2000, 96, 808–813. [Google Scholar] [CrossRef]
- Le, W.-D.; Xu, P.; Jankovic, J.; Jiang, H.; Appel, S.H.; Smith, R.G.; Vassilatis, D.K. Mutations in NR4A2 Associated with Familial Parkinson Disease. Nat. Genet. 2003, 33, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.P.; McEvoy, A.; Conneely, O.M.; Bresnihan, B.; FitzGerald, O. Involvement of the Nuclear Orphan Receptor NURR1 in the Regulation of Corticotropin-Releasing Hormone Expression and Actions in Human Inflammatory Arthritis. Arthritis Rheum. 2001, 44, 782–793. [Google Scholar] [CrossRef] [PubMed]
- O’Kane, M.; Markham, T.; McEvoy, A.N.; Fearon, U.; Veale, D.J.; FitzGerald, O.; Kirby, B.; Murphy, E.P. Increased Expression of the Orphan Nuclear Receptor NURR1 in Psoriasis and Modulation Following TNF-Alpha Inhibition. J. Invest. Dermatol. 2008, 128, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Achiron, A.; Grotto, I.; Balicer, R.; Magalashvili, D.; Feldman, A.; Gurevich, M. Microarray Analysis Identifies Altered Regulation of Nuclear Receptor Family Members in the Pre-Disease State of Multiple Sclerosis. Neurobiol. Dis. 2010, 38, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Ramos, L.L.P.; Monteiro, F.P.; Sampaio, L.P.B.; Costa, L.A.; Ribeiro, M.D.O.; Freitas, E.L.; Kitajima, J.P.; Kok, F. Heterozygous Loss of Function of NR4A2 Is Associated with Intellectual Deficiency, Rolandic Epilepsy, and Language Impairment. Clin. Case Rep. 2019, 7, 1582–1584. [Google Scholar] [CrossRef] [PubMed]
- Wirth, T.; Mariani, L.L.; Bergant, G.; Baulac, M.; Habert, M.-O.; Drouot, N.; Ollivier, E.; Hodžić, A.; Rudolf, G.; Nitschke, P.; et al. Loss-of-Function Mutations in NR4A2 Cause Dopa-Responsive Dystonia Parkinsonism. Mov. Disord. 2020, 35, 880–885. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Gupta, A.; Zech, M.; Sigafoos, A.N.; Clark, K.J.; Dincer, Y.; Wagner, M.; Humberson, J.B.; Green, S.; van Gassen, K.; et al. De Novo Variants of NR4A2 Are Associated with Neurodevelopmental Disorder and Epilepsy. Genet. Med. Off. J. Am. Coll. Med. Genet. 2020, 22, 1413–1417. [Google Scholar] [CrossRef] [PubMed]
- Jesús, S.; Hinarejos, I.; Carrillo, F.; Martínez-Rubio, D.; Macías-García, D.; Sánchez-Monteagudo, A.; Adarmes, A.; Lupo, V.; Pérez-Dueñas, B.; Mir, P.; et al. NR4A2 Mutations Can Cause Intellectual Disability and Language Impairment with Persistent Dystonia-Parkinsonism. Neurol. Genet. 2021, 7, e543. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Lévy, J.; Grotto, S.; Mignot, C.; Maruani, A.; Delahaye-Duriez, A.; Benzacken, B.; Keren, B.; Haye, D.; Xavier, J.; Heulin, M.; et al. NR4A2 Haploinsufficiency Is Associated with Intellectual Disability and Autism Spectrum Disorder. Clin. Genet. 2018, 94, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Duyzend, M.H.; Coe, B.P.; Baker, C.; Hoekzema, K.; Gerdts, J.; Turner, T.N.; Zody, M.C.; Beighley, J.S.; Murali, S.C.; et al. Genome Sequencing Identifies Multiple Deleterious Variants in Autism Patients with More Severe Phenotypes. Genet. Med. Off. J. Am. Coll. Med. Genet. 2019, 21, 1611–1620. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Xu, W.; Xiao, M.; Lu, Y.; Lan, X.; Tang, X.; Xu, N.; Yu, G.; Zhang, H.; Wu, S. Two Novel Heterozygous Truncating Variants in NR4A2 Identified in Patients with Neurodevelopmental Disorder and Brief Literature Review. Front. Neurosci. 2022, 16, 956429. [Google Scholar] [CrossRef] [PubMed]
- Krgovic, D.; Gorenjak, M.; Rihar, N.; Opalic, I.; Stangler Herodez, S.; Gregoric Kumperscak, H.; Dovc, P.; Kokalj Vokac, N. Impaired Neurodevelopmental Genes in Slovenian Autistic Children Elucidate the Comorbidity of Autism with Other Developmental Disorders. Front. Mol. Neurosci. 2022, 15, 912671. [Google Scholar] [CrossRef] [PubMed]
- Winter, B.; Krämer, J.; Meinhardt, T.; Berner, D.; Alt, K.; Wenzel, M.; Winkelmann, J.; Zech, M. NR4A2 and Dystonia with Dopa Responsiveness. Mov. Disord. 2021, 36, 2203–2204. [Google Scholar] [CrossRef] [PubMed]
- Reuter, M.S.; Krumbiegel, M.; Schlüter, G.; Ekici, A.B.; Reis, A.; Zweier, C. Haploinsufficiency of NR4A2 Is Associated with a Neurodevelopmental Phenotype with Prominent Language Impairment. Am. J. Med. Genet. A 2017, 173, 2231–2234. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Feliciano, P.; Shu, C.; Wang, T.; Astrovskaya, I.; Hall, J.B.; Obiajulu, J.U.; Wright, J.R.; Murali, S.C.; Xu, S.X.; et al. Integrating de Novo and Inherited Variants in 42,607 Autism Cases Identifies Mutations in New Moderate-Risk Genes. Nat. Genet. 2022, 54, 1305–1319. [Google Scholar] [CrossRef] [PubMed]
- Kaplanis, J.; Samocha, K.E.; Wiel, L.; Zhang, Z.; Arvai, K.J.; Eberhardt, R.Y.; Gallone, G.; Lelieveld, S.H.; Martin, H.C.; McRae, J.F.; et al. Evidence for 28 Genetic Disorders Discovered by Combining Healthcare and Research Data. Nature 2020, 586, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Feliciano, P.; Zhou, X.; Astrovskaya, I.; Turner, T.N.; Wang, T.; Brueggeman, L.; Barnard, R.; Hsieh, A.; Snyder, L.G.; Muzny, D.M.; et al. Exome Sequencing of 457 Autism Families Recruited Online Provides Evidence for Autism Risk Genes. NPJ Genom. Med. 2019, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Vissers, L.E.L.M.; van Nimwegen, K.J.M.; Schieving, J.H.; Kamsteeg, E.-J.; Kleefstra, T.; Yntema, H.G.; Pfundt, R.; van der Wilt, G.J.; Krabbenborg, L.; Brunner, H.G.; et al. A Clinical Utility Study of Exome Sequencing versus Conventional Genetic Testing in Pediatric Neurology. Genet. Med. Off. J. Am. Coll. Med. Genet. 2017, 19, 1055–1063. [Google Scholar] [CrossRef]
- Sleiman, P.M.A.; Healy, D.G.; Muqit, M.M.K.; Yang, Y.X.; Van Der Brug, M.; Holton, J.L.; Revesz, T.; Quinn, N.P.; Bhatia, K.; Diss, J.K.J.; et al. Characterisation of a Novel NR4A2 Mutation in Parkinson’s Disease Brain. Neurosci. Lett. 2009, 457, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Grimes, D.A.; Han, F.; Panisset, M.; Racacho, L.; Xiao, F.; Zou, R.; Westaff, K.; Bulman, D.E. Translated Mutation in the Nurr1 Gene as a Cause for Parkinson’s Disease. Mov. Disord. 2006, 21, 906–909. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Tsai, M.T.; Shaw, C.K.; Chen, C.H. Mutation Analysis of the Human NR4A2 Gene, an Essential Gene for Midbrain Dopaminergic Neurogenesis, in Schizophrenic Patients. Am. J. Med. Genet. 2001, 105, 753–757. [Google Scholar] [CrossRef] [PubMed]
- Rossen, J.L.; Bohnsack, B.L.; Zhang, K.X.; Ing, A.; Drackley, A.; Castelluccio, V.; Ralay-Ranaivo, H. Evaluation of Genetic Testing in a Cohort of Diverse Pediatric Patients in the United States with Congenital Cataracts. Genes 2023, 14, 608. [Google Scholar] [CrossRef] [PubMed]
- Dzinovic, I.; Boesch, S.; Skorvanek, M.; Necpal, J.; Svantnerova, J.; Pavelekova, P.; Havrankova, P.; Tsoma, E.; Indelicato, E.; Runkel, E.; et al. Genetic Overlap between Dystonia and Other Neurologic Disorders: A Study of 1,100 Exomes. Park. Relat. Disord. 2022, 102, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Klee, E.W.; Cousin, M.A.; e Vairo, F.P.; Morales-Rosado, J.A.; Macke, E.L.; Jenkinson, W.G.; Ferrer, A.; Schultz-Rogers, L.E.; Olson, R.J.; Oliver, G.R.; et al. Impact of Integrated Translational Research on Clinical Exome Sequencing. Genet. Med. Off. J. Am. Coll. Med. Genet. 2021, 23, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Zech, M.; Jech, R.; Boesch, S.; Škorvánek, M.; Weber, S.; Wagner, M.; Zhao, C.; Jochim, A.; Necpál, J.; Dincer, Y.; et al. Monogenic Variants in Dystonia: An Exome-Wide Sequencing Study. Lancet. Neurol. 2020, 19, 908–918. [Google Scholar] [CrossRef]
- Barge-Schaapveld, D.Q.C.M.; Ofman, R.; Knegt, A.C.; Alders, M.; Höhne, W.; Kemp, S.; Hennekam, R.C.M. Intellectual Disability and Hemizygous GPD2 Mutation. Am. J. Med. Genet. A 2013, 161A, 1044–1050. [Google Scholar] [CrossRef] [PubMed]
- Shimojima, K.; Okamoto, N.; Yamamoto, T. Possible Genes Responsible for Developmental Delay Observed in Patients with Rare 2q23q24 Microdeletion Syndrome: Literature Review and Description of an Additional Patient. Congenit. Anom. Kyoto 2017, 57, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Gabaldon-Albero, A.; Baviera, R.; Hernandez-Muela, S.; Garces, M.; Villanueva, V.; Martínez, F. Translational Research Group in Genetics, Instituto de Investigación Sanitaria La Fe, Valencia, Spain. 2024; manuscript in preparation. [Google Scholar]
- Jankovic, J.; Chen, S.; Le, W.D. The Role of Nurr1 in the Development of Dopaminergic Neurons and Parkinson’s Disease. Prog. Neurobiol. 2005, 77, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Moon, M.; Jeong, I.; Kim, C.-H.; Kim, J.; Lee, P.K.J.; Mook-Jung, I.; Leblanc, P.; Kim, K.-S. Correlation between Orphan Nuclear Receptor Nurr1 Expression and Amyloid Deposition in 5XFAD Mice, an Animal Model of Alzheimer’s Disease. J. Neurochem. 2015, 132, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Kadkhodaei, B.; Alvarsson, A.; Schintu, N.; Ramsköld, D.; Volakakis, N.; Joodmardi, E.; Yoshitake, T.; Kehr, J.; Decressac, M.; Björklund, A.; et al. Transcription Factor Nurr1 Maintains Fiber Integrity and Nuclear-Encoded Mitochondrial Gene Expression in Dopamine Neurons. Proc. Natl. Acad. Sci. USA 2013, 110, 2360–2365. [Google Scholar] [CrossRef] [PubMed]
- Al-Nusaif, M.; Lin, Y.; Li, T.; Cheng, C.; Le, W. Advances in NURR1-Regulated Neuroinflammation Associated with Parkinson’s Disease. Int. J. Mol. Sci. 2022, 23, 16184. [Google Scholar] [CrossRef] [PubMed]
- Wellenbrock, C.; Hedrich, K.; Schäfer, N.; Kasten, M.; Jacobs, H.; Schwinger, E.; Hagenah, J.; Pramstaller, P.P.; Vieregge, P.; Klein, C. NR4A2 Mutations Are Rare among European Patients with Familial Parkinson’s Disease. Ann. Neurol. 2003, 54, 415. [Google Scholar] [CrossRef]
- Zimprich, A.; Asmus, F.; Leitner, P.; Castro, M.; Bereznai, B.; Homann, N.; Ott, E.; Rutgers, A.W.F.; Wieditz, G.; Trenkwalder, C.; et al. Point Mutations in Exon 1 of the NR4A2 Gene Are Not a Major Cause of Familial Parkinson’s Disease. Neurogenetics 2003, 4, 219–220. [Google Scholar]
- Hering, R.; Petrovic, S.; Mietz, E.-M.; Holzmann, C.; Berg, D.; Bauer, P.; Woitalla, D.; Müller, T.; Berger, K.; Krüger, R.; et al. Extended Mutation Analysis and Association Studies of Nurr1 (NR4A2) in Parkinson Disease. Neurology 2004, 62, 1231–1232. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, P.; Lohmann, E.; Pollak, P.; Durif, F.; Tranchant, C.; Agid, Y.; Dürr, A.; Brice, A. Absence of NR4A2 Exon 1 Mutations in 108 Families with Autosomal Dominant Parkinson Disease. Neurology 2004, 62, 2133–2134. [Google Scholar] [CrossRef]
- Liu, H.; Liu, H.; Li, T.; Cui, J.; Fu, Y.; Ren, J.; Sun, X.; Jiang, P.; Yu, S.; Li, C. NR4A2 Genetic Variation and Parkinson’s Disease: Evidence from a Systematic Review and Meta-Analysis. Neurosci. Lett. 2017, 650, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Hawk, J.D.; Bookout, A.L.; Poplawski, S.G.; Bridi, M.; Rao, A.J.; Sulewski, M.E.; Kroener, B.T.; Manglesdorf, D.J.; Abel, T. NR4A Nuclear Receptors Support Memory Enhancement by Histone Deacetylase Inhibitors. J. Clin. Invest. 2012, 122, 3593–3602. [Google Scholar] [CrossRef]
- Català-Solsona, J.; Miñano-Molina, A.J.; Rodríguez-Álvarez, J. Nr4a2 Transcription Factor in Hippocampal Synaptic Plasticity, Memory and Cognitive Dysfunction: A Perspective Review. Front. Mol. Neurosci. 2021, 14, 786226. [Google Scholar] [CrossRef]
- Terzioglu-Usak, S.; Negis, Y.; Karabulut, D.S.; Zaim, M.; Isik, S. Cellular Model of Alzheimer’s Disease: Aβ1-42 Peptide Induces Amyloid Deposition and a Decrease in Topo Isomerase IIβ and Nurr1 Expression. Curr. Alzheimer Res. 2017, 14, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Parra-Damas, A.; Valero, J.; Chen, M.; España, J.; Martín, E.; Ferrer, I.; Rodríguez-Alvarez, J.; Saura, C.A. Crtc1 Activates a Transcriptional Program Deregulated at Early Alzheimer’s Disease-Related Stages. J. Neurosci. Off. J. Soc. Neurosci. 2014, 34, 5776–5787. [Google Scholar] [CrossRef] [PubMed]
- Moon, M.; Jung, E.S.; Jeon, S.G.; Cha, M.-Y.; Jang, Y.; Kim, W.; Lopes, C.; Mook-Jung, I.; Kim, K.-S. Nurr1 (NR4A2) Regulates Alzheimer’s Disease-Related Pathogenesis and Cognitive Function in the 5XFAD Mouse Model. Aging Cell 2019, 18, e12866. [Google Scholar] [CrossRef] [PubMed]
- Stiller, T.; Merk, D. Exploring Fatty Acid Mimetics as NR4A Ligands. J. Med. Chem. 2023, 66, 15362–15369. [Google Scholar] [CrossRef] [PubMed]
- Qiao, S.; Tao, S.; Rojo de la Vega, M.; Park, S.L.; Vonderfecht, A.A.; Jacobs, S.L.; Zhang, D.D.; Wondrak, G.T. The Antimalarial Amodiaquine Causes Autophagic-Lysosomal and Proliferative Blockade Sensitizing Human Melanoma Cells to Starvation- and Chemotherapy-Induced Cell Death. Autophagy 2013, 9, 2087–2102. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-I.; Jeon, S.G.; Kim, K.A.; Kim, Y.J.; Song, E.J.; Choi, J.; Ahn, K.J.; Kim, C.-J.; Chung, H.Y.; Moon, M.; et al. The Pharmacological Stimulation of Nurr1 Improves Cognitive Functions via Enhancement of Adult Hippocampal Neurogenesis. Stem Cell Res. 2016, 17, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Willems, S.; Marschner, J.A.; Kilu, W.; Faudone, G.; Busch, R.; Duensing-Kropp, S.; Heering, J.; Merk, D. Nurr1 Modulation Mediates Neuroprotective Effects of Statins. Adv. Sci. Weinh. Baden-Wurtt. Ger. 2022, 9, e2104640. [Google Scholar] [CrossRef] [PubMed]
- Vietor, J.; Gege, C.; Stiller, T.; Busch, R.; Schallmayer, E.; Kohlhof, H.; Höfner, G.; Pabel, J.; Marschner, J.A.; Merk, D. Development of a Potent Nurr1 Agonist Tool for In Vivo Applications. J. Med. Chem. 2023, 66, 6391–6402. [Google Scholar] [CrossRef] [PubMed]
- García-Yagüe, J.; Cuadrado, A. Mechanisms of NURR1 Regulation: Consequences for Its Biological Activity and Involvement in Pathology. Int. J. Mol. Sci. 2023, 24, 12280. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Shang, J.; Kojetin, D.J. Molecular Basis of Ligand-Dependent Nurr1-RXRα Activation. eLife 2023, 12, e85039. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.O.; Battagello, D.S.; Cardoso, A.R.; Hauser, D.N.; Bittencourt, J.C.; Correa, R.G. Dopamine: Functions, Signaling, and Association with Neurological Diseases. Cell Mol. Neurobiol. 2019, 39, 31–59. [Google Scholar] [CrossRef] [PubMed]
- Fuertinger, S.; Zinn, J.C.; Sharan, A.D.; Hamzei-Sichani, F.; Simonyan, K. Dopamine drives left-hemispheric lateralization of neural networks during human speech. J. Comp. Neurol. 2018, 526, 920–931. [Google Scholar] [CrossRef] [PubMed]
- García-Yagüe, J.; Rada, P.; Rojo, A.I.; Lastres-Becker, I.; Cuadrado, A.; Tong, X.; Zhang, D.; Buelow, K.; Guha, A.; Arthurs, B.; et al. Nuclear import and export signals control the subcellular localization of Nurr1 protein in response to oxidative stress. J. Biol. Chem. 2013, 288, 5506–5517. [Google Scholar] [CrossRef] [PubMed]
- Volakakis, N.; Kadkhodaei, B.; Joodmardi, E.; Wallis, K.; Panman, L.; Silvaggi, J.; Spiegelman, B.M.; Perlmann, T. NR4A orphan nuclear receptors as mediators of CREB-dependent neuroprotection. Proc. Natl. Acad. Sci. USA 2010, 107, 12317–12322. [Google Scholar] [CrossRef] [PubMed]
- Barneda-Zahonero, B.; Servitja, J.-M.; Badiola, N.; Miñano-Molina, A.J.; Fadó, R.; Saura, C.A.; Rodríguez-Alvarez, J. Nurr1 protein is required for N-methyl-D-aspartic acid (NMDA) receptor-mediated neuronal survival. J. Biol. Chem. 2012, 287, 11351–11362. [Google Scholar] [CrossRef] [PubMed]
- AlRuwaili R, Al-Kuraishy HM, Al-Gareeb AI, Ali NH, Alexiou A, Papadakis M, Saad HM, Batiha GE. The Possible Role of Brain-derived Neurotrophic Factor in Epilepsy. Neurochem. Res. 2024, 49, 533–547. [Google Scholar] [CrossRef] [PubMed]
| Reference | Patient 1 [30] | Patient 2 [16] | Patient 3 [17] | Patient 4 [17] | Patient 5 [18] | Patient 6 [18] | Patient 7 [18] | Patient 8 [18] | Patient 9 [18] |
| Age (years)/Sex | 15/F | 9/M | 32/M | 57/F | 15/F | 12/M | 9/F | 3/F | 5/M |
| Motor milestones | Delayed | Normal | Normal | Clumsiness | Delayed | Normal | NA | Delayed | Delayed |
| Hypotonia | NA | No | No | NA | NA | Yes | NA | Yes | Yes |
| ID severity | NA | Mild | Mild | Mild | Severe | Mild | Mild-moderate | Severe | Mild |
| Language | NA | Delayed | Delayed | Normal | NA | LD | NA | NA | LD |
| Psychiatric and behavioral | NA | No | No | No | ASD | Hyperactivity Anxiety | NA | No | Attachment disorder, ADHD |
| Epilepsy/Age at onset | No | Yes/5 years | Yes/26 years | No | Yes/6.5 years | Yes/10 years | No | Yes/5 months | No |
| Seizure type/Epilepsy classification | - | Rolandic epilepsy | Generalized tonic-clonic seizures | - | Focal motor | Rolandic epilepsy | - | Infantile spasms | - |
| Refractory epilepsy | No | No | NA | - | No | No | - | No | - |
| Movement disorder/Age at onset | - | No | Dystonia- Parkinsonism/ 29 years | Dystonia- Parkinsonism/ 30 years | No | No | Dystonia Choreo-athetosis Ataxic gait/NA | Dystonia/ NA | No |
| Dopa responsive | NA | - | Yes | Yes | - | - | NA | NA | - |
| Other signs | - | - | - | - | - | RWLD | - | Microcephaly CVI | Hyposensitivity CVI |
| MRI | NA | Normal | NA | Thinning of substantia nigra | Normal | Normal | Gliosis of thalamus and basal ganglia. | Cerebellar atrophy | NA |
| Variant (NM_006186.3) | c.920T > G | c.326dupA | c.326dupA | c.881dupA | c.839G > A | c.865-1_865delGCinsAAA | c.914G > A | c.1175A > G | c.1576G > T |
| Type | Missense | Frameshift | Frameshift | Frameshift | Missense | Splicing | Missense | Missense | Nonsense |
| de novo | Yes | Yes | Yes | NA | Yes | Yes | Yes | Yes | NA |
| Reference | Patient 10 [18] | Patient 11 [18] | Patient 12 [18] | Patient 13 [19] | Patient 14 [25] | Patient 15 [23] | Patient 16 [23] | Patient 17 | Patient 18 |
| Age (years)/Sex | 2/M | 4/F | 19/F | 30/M | 2.5/M | 11/M | 12/M | 11/F | 25/F |
| Motor milestones | Delayed | Delayed | Delayed | Clumsiness | Delayed | Normal | Delayed | Clumsiness | Normal |
| Hypotonia | Yes | Yes | Yes | No | Yes | Yes | Yes | No | No |
| ID severity | Severe | Moderate | Severe | Mild | Mild-moderate | Mild | Mild | Mild | Moderate |
| Language | Normal | LD | LD | LD | Delay | LD | Delay | LD | LD |
| Psychiatric and behavioral problems | No | No | No | ADHD Trichotilomania | No | ADHD | ADHD | ADHD | Delusions, hallucinations, and mood swings |
| Epilepsy/Age at onset | Yes/6 months | No | No | No | NA | No | No | No | Yes/23 years |
| Seizure type/Epilepsy classification | Infantile spasms Lennox–Gastaut Syndrome | - | - | - | - | - | - | - | Focal (motor) |
| Refractory epilepsy | Yes | - | - | - | - | - | - | - | Yes |
| Movement disorder/Age at onset | No | No | No | Motor tics, cervical dystonia, dystonia-parkinsonism/16 years | Multifocal dystonia/22 months | No | No | No | Oculogyric crisis/ 24 years |
| Dopa responsive | - | - | - | NA | Yes | - | - | - | NA |
| Other signs/symptoms | Sensory and sleep disorder | - | - | - | - | RWLD | RWLD | RWLD | RWLD |
| MRI | Pontine hypoplasia Ventriculomegaly | Normal | Normal | Normal | Normal | Normal | Normal | Normal | Ventriculomegaly Widened subarachnoid space |
| Variant (NM_006186.3) | c.325dupC | c.857T > C | c.968G > T | c.956G > A | c.863A > G | c.1541-2A > C | c.915C > A | c.844G > C | c.905delA |
| Type | Frameshift | Missense | Missense | Missense | Missense | Splicing | Nonsense | Missense | Frameshift |
| de novo | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Reference | Patient 19 | Patient 20 [38] | Patient 21 [26] | Patient 22 [21] | Patient 23 [21] | Patient 24 [21] | Patient 25 [39] | Patient 26 [18] | |
| Age (years)/Sex | 32/F | 25/F | 8/F | 17/M | 8/F | 9/M | 6/F | 43/M | |
| Motor milestones | Clumsiness | Normal | Delayed | Normal | Normal | Normal | Delayed | Delayed | |
| Hypotonia | No | ||||||||
| ID severity | Mild | Moderate | Mild | Mild-moderate | NA | NA | Severe | Severe | |
| Language | LD | Delay | LD | Delay | LD | LD | LD | NA | |
| Psychiatric and behavioral problems | Behavioral disorder | NA | No | ASD | Restlessness | ASD | NA | ADHD | |
| ADHD | Behavioral disorder | Behavioral disorder | |||||||
| Epilepsy/Age at onset | Yes/26 years | NA | NA | No | No | No | NA | Yes/13 years | |
| Seizure type/Epilepsy classification | Focal (motor) | - | - | - | - | - | - | Lennox–Gastaut Syndrome | |
| Refractory epilepsy | Yes | - | - | - | - | - | - | Yes | |
| Movement disorder/Age at onset | No | No | No | No | No | No | NA | Ataxia/adulthood | |
| Dopa responsive | - | - | - | - | - | - | - | NA | |
| Other signs/symptoms | RWLD | - | - | RWLD | - | - | - | - | |
| MRI | Bilateral operculo-insular polymicrogyria foci | Normal | NA | NA | NA | NA | Normal | Widened subarachnoid space | |
| Variant (NM_006186.3) | c.911dupA | 2q24.1(157161283-157459740)×1 | 2q24.1(157120975–157210126)×1 | 2q24.1(157094848_157216692)×1 | 2q24.1(157141281_157216692)×1 | 2q24.1(157141280_157315748)×1 | 2q23.3q24.1(152796289–157299545)×1 | 2q23.3q24.1(154790212_158488241)×1 | |
| Type/size | Frameshift | 298 kb | 89 kb | 122 kb | 75 kb | 174 kb | 4.5 Mb | >3.6 Mb | |
| de novo | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gabaldon-Albero, A.; Mayo, S.; Martinez, F. NR4A2 as a Novel Target Gene for Developmental and Epileptic Encephalopathy: A Systematic Review of Related Disorders and Therapeutic Strategies. Int. J. Mol. Sci. 2024, 25, 5198. https://doi.org/10.3390/ijms25105198
Gabaldon-Albero A, Mayo S, Martinez F. NR4A2 as a Novel Target Gene for Developmental and Epileptic Encephalopathy: A Systematic Review of Related Disorders and Therapeutic Strategies. International Journal of Molecular Sciences. 2024; 25(10):5198. https://doi.org/10.3390/ijms25105198
Chicago/Turabian StyleGabaldon-Albero, Alba, Sonia Mayo, and Francisco Martinez. 2024. "NR4A2 as a Novel Target Gene for Developmental and Epileptic Encephalopathy: A Systematic Review of Related Disorders and Therapeutic Strategies" International Journal of Molecular Sciences 25, no. 10: 5198. https://doi.org/10.3390/ijms25105198





