Protein Citrullination by Peptidyl Arginine Deiminase/Arginine Deiminase Homologs in Members of the Human Microbiota and Its Recognition by Anti-Citrullinated Protein Antibodies
Abstract
1. Introduction
1.1. Influence of the Microbiome on Autoimmunity
1.2. Homologs of PADs/ADs in the Human Microbiome and Their Implications in RA
2. Results
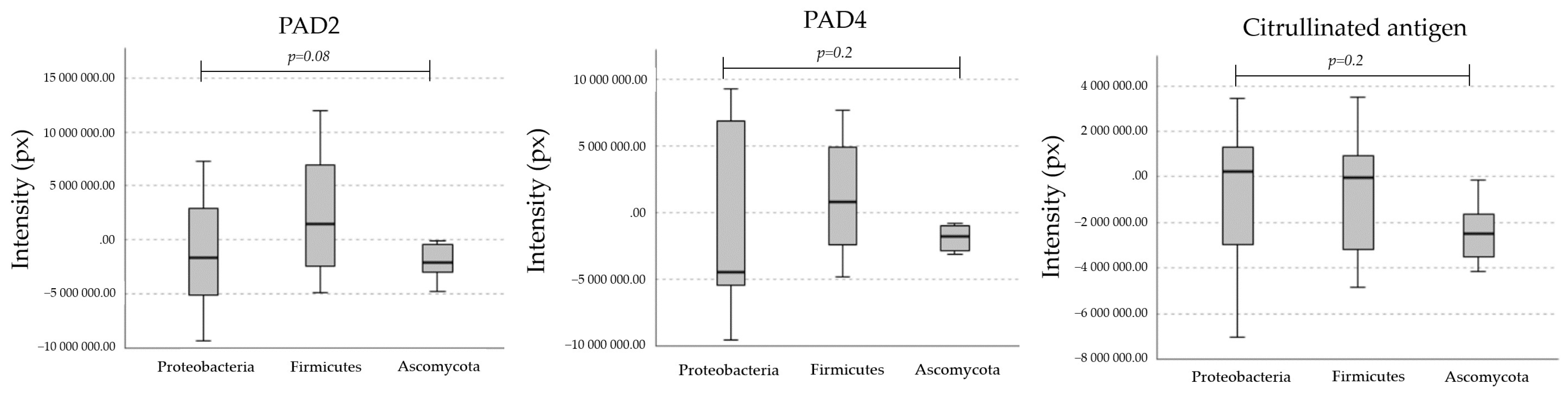
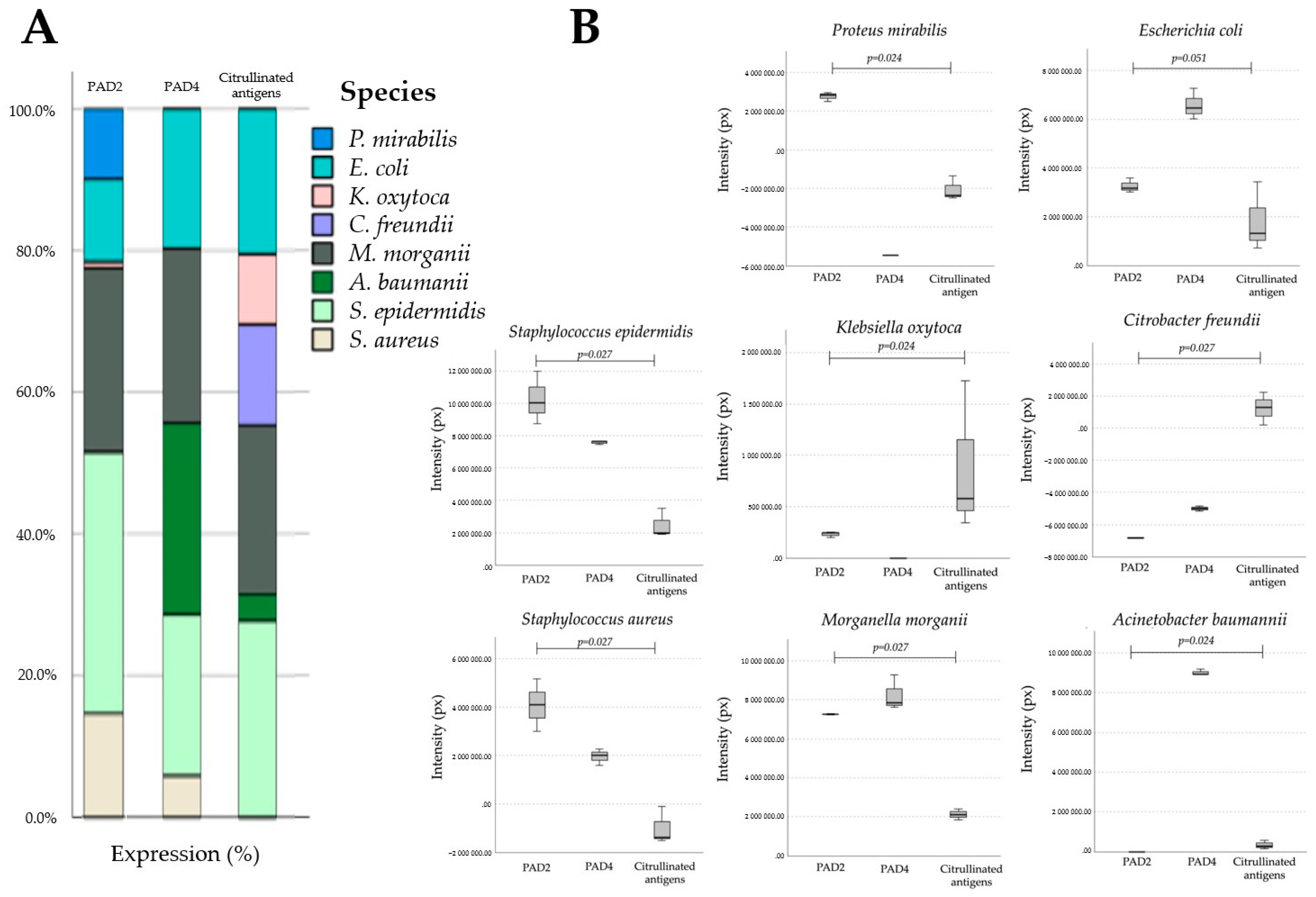
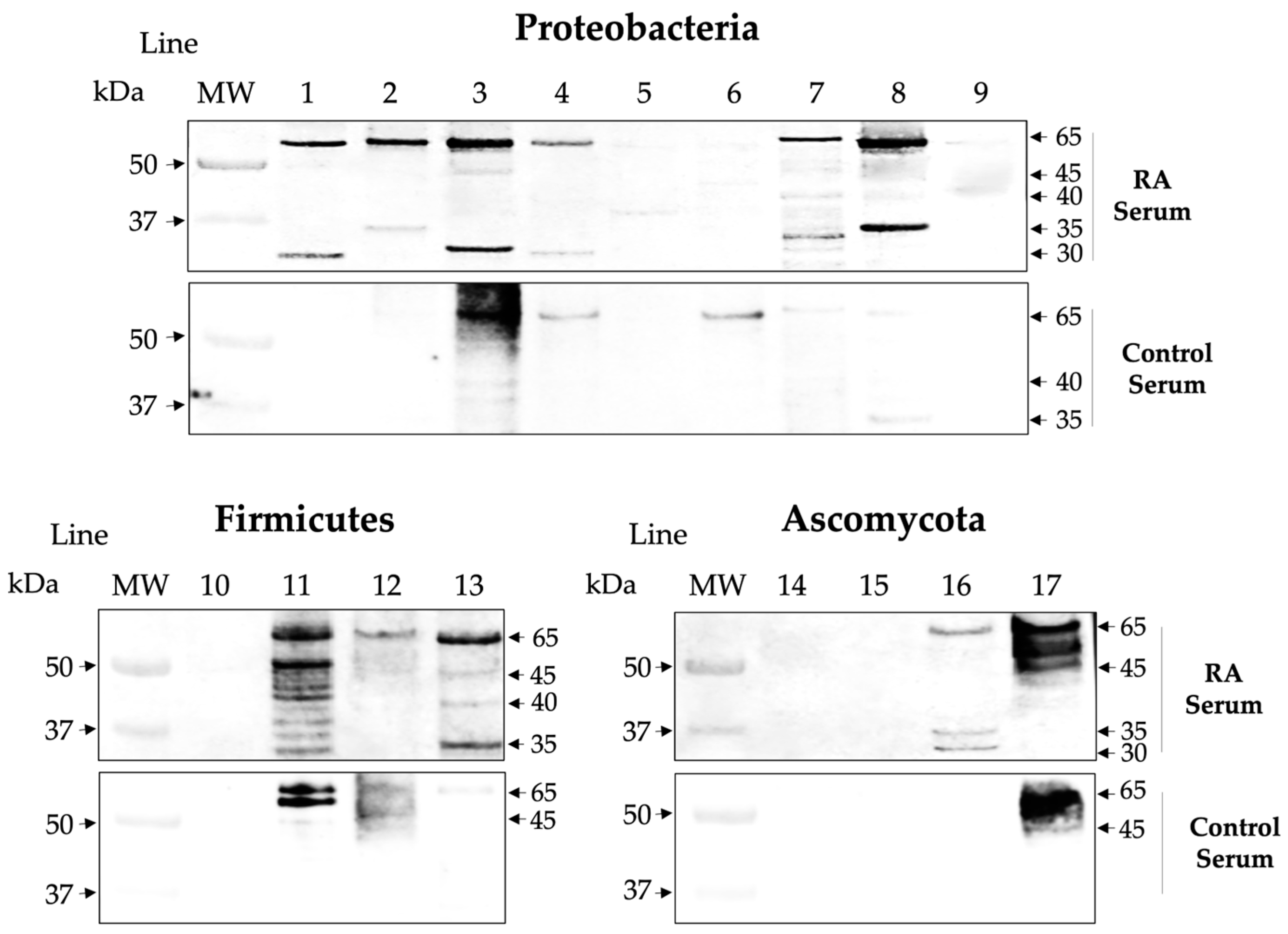
2.1. Homologs of PADs/ADs Are Present in Microbial Extracts
2.2. Microbial Proteins Are Endogenously Citrullinated
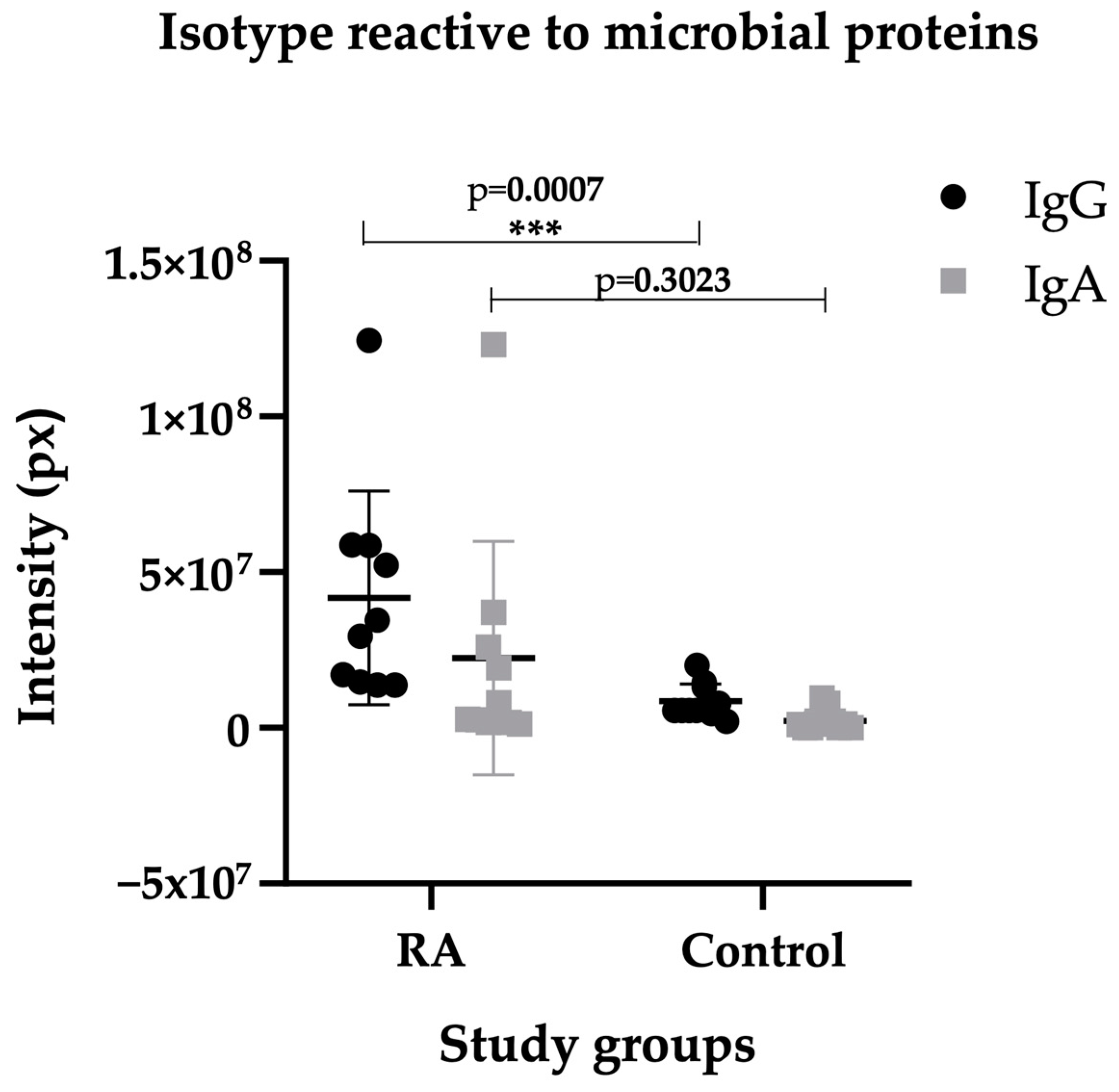
2.3. Isotype IgG of RA Sera Recognizes Antigens of the Microbiota
2.4. Affinity ACPAs Recognize Citrullinated Antigens of the Microbiota
2.5. Citrullination Activity in Microbial Cultures
3. Discussion
4. Materials and Methods
4.1. Type of Study
4.2. Population and Sample
4.3. Biological Samples
4.4. Determination of Anti-CCP Antibodies by ELISA
4.5. Affinity ACPAs Obtained by Elution
4.6. Microbial Cultures Using Selective and Differential Mediums
4.7. Microbial Antigenic Extraction and Quantification
4.8. SDS-PAGE and Western Blot Analyses
4.9. Citrullination Activity in Microbial Cultures
4.10. Statistic Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lederberg, J.; McCray, A.T. ‘Ome sweet’Omics–A genealogical treasury of words. Scientist 2001, 15, 7. [Google Scholar]
- Dekaboruah, E.; Vasant-Suryavanshi, M.; Chettri, D.; Kumar-Verma, A. Human microbiome: An academic update on human body site specific surveillance and its possible role. Arch. Microbiol. 2020, 202, 2147–2167. [Google Scholar] [CrossRef]
- Sender, R.; Fuchs, S.; Milo, R. Are we really vastly outnumbered? Revisiting the ratio of bacterial to host cells in humans. Cell 2016, 164, 337–340. [Google Scholar] [CrossRef]
- Kalbermatter, C.; Fernandez-Trigo, N.; Christensen, S.C. Maternal microbiota, early life colonization and breast milk drive immune development in the newborn. Front. Immunol. 2021, 12, 683022. [Google Scholar] [CrossRef]
- Rooney, C.M.; Mankia, K.; Emery, P. The role of the microbiome in driving RA-related autoimmunity. Front. Cell Dev. Biol. 2020, 8, 538130. [Google Scholar] [CrossRef]
- Del Cheiro, F.; Vernocchi, P.; Petrucca, A.; Paci, P.; Fuentes, S.; Patrico, G.; Capuani, G.; Masotti, A.; Reddel, S.; Russo, A.; et al. Phylogenetic and metabolic tracking of gut microbiota during perinatal development. PLoS ONE 2015, 10, e0137347. [Google Scholar] [CrossRef] [PubMed]
- Schanche, M.; Avershina, E.; Dotterud, C.; Øien, T.; Storrø, O.; Johnsen, R.; Rudi, K. High-resolution analyses of overlap in the microbiota between mothers and their children. Curr. Microbiol. 2015, 71, 283–290. [Google Scholar] [CrossRef] [PubMed]
- The Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef]
- Hooks, K.B.; O’Malley, M.A. Dysbiosis and its discontents. mBio 2017, 8, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.E.; Kahana, D.D.; Ghuman, S.; Wilson, H.P.; Wilson, J.; Kim, S.C.J.; Lagishetty, V.; Jacobs, J.P.; Sinha-Hikim, A.P.; Friedman, T.C. Unhealthy lifestyle and gut dysbiosis: A better understanding of the effects of poor diet and nicotine on the intestinal microbiome. Front. Endocrinol. 2021, 12, 649. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Abele, G.; Miggiano, D.; Gasbarrini, A.; Mele, M.C. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Sasso, J.M.; Ammar, R.M.; Tenchov, R.; Lemmel, S.; Kelber, O.; Grieswelle, M.; Zhou, Q.A. Gut Microbiome–Brain Alliance: A Landscape View into Mental and Gastrointestinal Health and Disorders. ACS Chem. Neurosci. 2023, 14, 1717–1763. [Google Scholar] [CrossRef] [PubMed]
- Nenciarini, S.; Renzi, S.; di Paola, M.; Meriggi, N.; Cavalieri, D. Ascomycetes yeasts: The hidden part of human microbiome. WIREs Mech. Dis. 2024, 7, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Davidson, A.; Diamond, B. Autoimmune diseases. N. Engl. J. Med. 2001, 345, 340–350. [Google Scholar] [CrossRef]
- Alzabin, S.; Venables, P.J. Etiology of autoimmune disease: Past, present and future. Expert. Rev. Clin. Immunol. 2012, 8, 111–113. [Google Scholar] [CrossRef]
- Belkaid, Y.; Hand, T.W. Role of the microbiota in immunity and inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef]
- Sai, A.; Shetty, G.B.; Shetty, P.; Nanjeshgowda, H.L. Influence of gut microbiota on autoimmunity: A narrative review. Brain Behav. Immun. Integr. 2024, 5, 100046. [Google Scholar] [CrossRef]
- di Paola, M.; Rizzetto, L.; Stefanini, I.; Vitali, F.; Massi-Benedetti, C.; Tocci, N.; Romani, L.; Ramazzotti, M.; Lionetti, P.; De Filippo, C.; et al. Comparative immunophenotyping of Saccharomyces cerevisiae and Candidas spp. strains from Crohn’s disease patients and their interactions with the gut microbiome. J. Transl. Autoimmun. 2020, 3, 100036. [Google Scholar] [CrossRef] [PubMed]
- Mann, E.R.; Li, X. Intestinal antigen-presenting cells in mucosal immune homeostasis: Crosstalk between dendritic cells, macrophages and B-cells. World J. Gastroenterol. 2014, 20, 9653–9664. [Google Scholar] [CrossRef] [PubMed]
- Gury-BenAri, M.; Thaiss, C.A.; Serafini, N.; Winter, D.R.; Giladi, A.; Lara-Astiaso, D.; Levy, M.; Salame, T.M.; Weiner, A.; David, E.; et al. The spectrum and regulatory landscape of intestinal innate lymphoid cells are shaped by the microbiome. Cell 2016, 166, 1231–1246.e13. [Google Scholar] [CrossRef]
- Zhang, D.; Frenette, P.S. Cross talk between neutrophils and the microbiota. Blood 2019, 133, 2168–2177. [Google Scholar] [CrossRef]
- Abraham, S.N.; St. John, A.L. Mast cell-orchestrated immunity to pathogens. Nat. Rev. Immunol. 2010, 10, 440–452. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Bao, X.; Zheng, M. CD8+ T-cell immunity orchestrated by iNKT cells. Front. Immunol. 2023, 13, 1109347. [Google Scholar] [CrossRef]
- Bachem, A.; Makhlouf, C.; Binger, K.J.; de Souza, D.P.; Tull, D.; Hochheiser, K.; Whitney, P.G.; Fernandez-Ruiz, D.; Dähling, S.; Kastenmüller, W.; et al. Microbiota-derived short-chain fatty acids promote the memory potential of antigen-activated CD8+ T cells. Immunity 2019, 51, 285–297.e5. [Google Scholar] [CrossRef]
- Zhao, Z.; Ren, J.; Dai, C.; Kannapell, C.C.; Wang, H.; Gaskin, F.; Fu, S.M. Nature of T cell epitopes in lupus antigens and HLA-DR determines autoantibody initiation and diversification. Ann. Rheum. Dis. 2019, 78, 380–390. [Google Scholar] [CrossRef]
- Dessen, A.; Lawrence, C.M.; Cupo, S.; Zaller, D.M.; Wiley, D.C. X-ray crystal structure of HLA-DR4 (DRA*0101, DRB1*0401) complexed with a peptide from human collagen II. Immunity 1997, 7, 473–481. [Google Scholar] [CrossRef]
- van Drongelen, V.; Ali, W.H.; Holoshitz, J. Uncovering a shared epitope-activated protein citrullination pathway. J. Immunol. 2020, 205, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Vossenaar, E.R.; Zendman, A.J.; van Venrooij, W.J.; Pruijn, G.J. PAD, a growing family of citrullinating enzymes: Genes, features and involvement in disease. Bioessays 2003, 25, 1106–1118. [Google Scholar] [CrossRef]
- Foulquier, C.; Sebbag, M.; Clavel, C.; Chapuy-Regaud, S.; Al Badine, R.; Mechin, M.C. Peptidyl arginine deiminase type 2 (PAD-2) and PAD-4 but not PAD-1, PAD-3, and PAD-6 are expressed in rheumatoid arthritis synovium in close association with tissue inflammation. Arthritis Rheum. 2007, 56, 3541–3553. [Google Scholar] [CrossRef] [PubMed]
- Khandpur, R.; Carmona-Rivera, C.; Vivekanandan-Giri, A.; Gizinski, A.; Yalavarthi, S.; Knight, J.S. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci. Transl. Med. 2013, 5, 178ra40. [Google Scholar] [CrossRef]
- Rebernick, R.; Fahmy, L.; Glover, C.; Bawadekar, M.; Shim, D.; Holmes, C.L. DNA area and NETosis analysis (DANA): A high-throughput method to quantify neutrophil extracellular traps in fluorescent microscope images. Biol. Proced. Online 2018, 20, 7. [Google Scholar] [CrossRef] [PubMed]
- Shelef, M.A.; Sokolove, J.; Lahey, L.J.; Wagner, C.A.; Sackmann, E.K.; Warner, T.F. Peptidylarginine deiminase 4 contributes to tumor necrosis factor a-induced inflammatory arthritis. Arthritis Rheum. 2014, 66, 1482–1491. [Google Scholar] [CrossRef] [PubMed]
- Holmes, C.L.; Shim, D.; Kernien, J.; Johnson, C.J.; Nett, J.E.; Shelef, M.A. Insight into Neutrophil Extracellular Traps through Systematic Evaluation of Citrullination and Peptidylarginine Deiminases. J. Immunol. Res. 2019, 2019, 2160192. [Google Scholar] [CrossRef]
- Bashar, S.J.; Holmes, C.L.; Shelef, M.A. Macrophage extracellular traps require peptidylarginine deiminase 2 and 4 and are a source of citrullinated antigens bound by rheumatoid arthritis autoantibodies. Front. Immunol. 2024, 15, 1167362. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Li, S.; Chen, B.; Zhu, Q.; Xu, L.; Li, F. Serum Anti-Citrullinated Protein Antibodies and Rheumatoid Factor Increase the Risk of Rheumatoid Arthritis–Related Interstitial Lung Disease: A Meta-Analysis. Clin. Rheumatol. 2021, 40, 4533–4543. [Google Scholar] [CrossRef]
- Willemze, A.; van der Woude, D.; Ghidey, W.; Levarht, E.N.; Stoeken-Rijsbergen, G.; Verduyn, W.; de Vries, R.R.; Houwing-Duistermaat, J.J.; Huizinga, T.W.; Trouw, L.A.; et al. The interaction between HLA shared epitope alleles and smoking and its contribution to autoimmunity against several citrullinated antigens. Arthritis Rhem. 2011, 63, 1823–1832. [Google Scholar] [CrossRef]
- Yu, K.; Proost, P. Insights into peptidylarginine deiminase expression and citrullination pathways. Trends Cell Biol. 2022, 32, 746–761. [Google Scholar] [CrossRef] [PubMed]
- Lappin, D.F.; Apatzidou, D.; Quirke, A.M.; Oliver-Bell, J.; Butcher, J.P.; Kinane, D.F.; Riggio, M.P.; Venables, P.; McInnes, I.B.; Culshaw, S. Influence of periodontal disease, Porphyromonas gingivalis and cigarette smoking on systemic anti- citrullinated peptide antibody titres. J. Clin. Periodontol. 2013, 40, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Brandl, C.; Bucci, L.; Schett, G.; Zaiss, M.M. Crossing the barriers: Revisiting the gut feeling in rheumatoid arthritis. Eur. J. Immunol. 2021, 51, 798–810. [Google Scholar] [CrossRef]
- Oliveira, S.R.; de Arruda, J.A.A.; Corrêa, J.D.; Carvalho, V.F.; Medeiros, J.D.; Schneider, A.H.; Machado, C.C.; Duffles, L.F.; Fernandes, G.R.; Calderaro, D.C.; et al. Methotrexate and Non-Surgical Periodontal Treatment Change the Oral–Gut Microbiota in Rheumatoid Arthritis: A Prospective Cohort Study. Microorganisms 2024, 12, 68. [Google Scholar] [CrossRef]
- Maeda, Y.; Takeda, K. Host-microbiota interactions in rheumatoid arthritis. Exp. Mol. Med. 2019, 51, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Wei, Y.; Zhu, Y.; Xie, Z.; Hai, Q.; Li, Z.; Qin, D. Gut microbiota and rheumatoid arthritis: From pathogenesis to novel therapeutic opportunities. Front. Immunol. 2022, 13, 1007165. [Google Scholar] [CrossRef] [PubMed]
- Picchianti-Diamanti, A.; Rosado, M.; D’Amelio, R. Infectious agents and inflammation: The role of microbiota in autoimmune arthritis. Front. Microbiol. 2018, 8, 2696. [Google Scholar] [CrossRef]
- Hill, J.A.; Southwood, S.; Sette, A.; Jevnikar, A.M.; Bell, D.A.; Cairns Cutting, E. The conversion of arginine to citrulline allows for a high-affinity peptide interaction with the rheumatoid arthritis-associated HLA-DRB1*0401 MHC class II molecule. J. Immunol. 2003, 171, 538–541. [Google Scholar] [CrossRef]
- Engström, M.; Eriksson, K.; Lee, L.; Hermansson, M.; Johansson, A.; Nicholas, A.P.; Gerasimcik, N.; Lundberg, K.; Klareskog, K.; Catrina, A.I.; et al. Increase citrullination and expression of peptidylarginine deiminases independently of P. gingivalis and A. actinomycetemcomitans in gingival tissue of patients with periodontitis. J. Transl. Med. 2018, 16, 214. [Google Scholar] [CrossRef]
- Kosgodage, U.S.; Matewele, P.; Mastroianni, G.; Kraev, I.; Brotherton, D.; Awamaria, B.; Nicholas, A.P.; Lange, S.; Inal, J.M. Peptidylarginine deiminase inhibitors reduce bacterial membrane vesicle release and sensitize bacteria to antibiotic treatment. Front. Cell Infect. Microbiol. 2019, 9, 227. [Google Scholar] [CrossRef] [PubMed]
- Weawsiangsang, S.; Rattanachak, N.; Jongjitvimol, T.; Jaifoo, T.; Charoensit, P.; Viyoch, J.; Jongjitwimol, J. Hydroquinine inhibits the growth of multidrug-resistant Pseudomonas aeruginosa via the suppression of the arginine deiminase pathway genes. Int. J. Mol. Sci. 2023, 24, 13914. [Google Scholar] [CrossRef]
- Cummings, T.F.M.; Gori, K.; Sanchez-Pulido, L.; Gavriilidis, G.; Moi, D.; Wilson, A.R.; Murchison, E.; Dessimoz, C.; Ponting, C.P.; Christophorou, M.A. Citrullination was introduced into animals by horizontal gene transfer from cyanobacteria. Mol. Biol. Evol. 2021, 39, msab317. [Google Scholar] [CrossRef]
- Maresz, K.J.; Hellvard, A.; Sroka, A.; Adamowicz, K.; Bielecka, E.; Koziel, J.; Gawron, K.; Mizgalska, D.; Marcinska, K.A.; Benedyk, M.; et al. Porphyromonas gingivalis facilitates the development and progression of destructive arthritis through its unique bacterial peptidylarginine deiminase (PAD). PLoS Pathog. 2013, 9, e1003627. [Google Scholar] [CrossRef]
- Jung, H.; Jung, S.M.; Rim, Y.A.; Park, N.; Nam, Y.; Lee, J.; Park, S.-H.; Ju, J.H. Arthritic role of Porphyromonas gingivalis in collagen-induced arthritis mice. PLoS ONE 2017, 12, e0188698. [Google Scholar] [CrossRef]
- Lac, P.; Saunders, S.; Tutunea-Fatan, E.; Barra, L.; Bell, D.A.; Cairns, E. Immune responses to peptides containing homocitrulline or citrulline in the DR4-transgenic mouse model of rheumatoid arthritis. J. Autoimmun. 2018, 89, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Engdahl, C.; Bang, H.; Dietel, K.; Lang, S.C.; Harre, U.; Schett, G. Periarticular bone loss in arthritis is induced by autoantibodies against citrullinated vimentin. J. Bone Min. Res. 2017, 32, 1681–1691. [Google Scholar] [CrossRef] [PubMed]
- Dusad, A.; Duryee, M.J.; Shaw, A.T.; Klassen, L.W.; Anderson, D.R.; Wang, D.; Ren, K.; Gravallese, E.M.; O’Dell, J.R.; Mikuls, T.R. Induction of bone loss in DBA/1J mice immunized with citrullinated autologous mouse type II collagen in the absence of adjuvant. Immunol. Res. 2014, 58, 51–60. [Google Scholar] [CrossRef]
- Arnoux, F.; Mariot, C.; Peen, E.; Lambert, N.C.; Balandraud, N.; Roudier, J.; Auger, I. Peptidyl arginine deiminase immunization induces anticitrullinated protein antibodies in mice with particular MHC types. Proc. Natl. Acad. Sci. USA 2017, 114, E10169–E10177. [Google Scholar] [CrossRef]
- Castillo, D.M.; Lafaurie, G.I.; Romero-Sánchez, C.; Delgadillo, N.A.; Castillo, Y.; Bautista-Molano, W.; Pacheco-Tena, C.; Bello-Gualtero, J.M.; Chalem-Choueka, P.; Castellanos, J.E. The Interaction Effect of Anti-RgpA and Anti-PPAD Antibody Titers: An Indicator for Rheumatoid Arthritis Diagnosis. J. Clin. Med. 2023, 12, 3027. [Google Scholar] [CrossRef] [PubMed]
- Stobernack, T.; Glasner, C.; Junker, S.; Gabarrini, G.; De Smit, M.; Van Winkelhoff, A.J.; De Jong, A.; Otto, A.; Becher, D.; Van Dijl, J.M. Extracellular Proteome and Citrullinome of the Oral Pathogen Porphyromonas gingivalis. J. Proteome Res. 2016, 15, 4532–4543. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wright, K.; Davis, J.M.; Jeraldo, P.; Marietta, E.V.; Murray, J.; Nelson, H.; Matteson, E.L.; Taneja, V. An expansion of rare lineage intestinal microbes characterizes rheumatoid arthritis. Genome Med. 2016, 8, 14. [Google Scholar] [CrossRef]
- Toyofuku, M.; Nomura, N.; Eberl, L. Types and origins of bacterial membrane vesicles. Nat. Rev. Microbiol. 2019, 17, 13–24. [Google Scholar] [CrossRef]
- Doward, D.W.; Garon, C.F. DNA is packaged within membrane-derived vesicles of Gram-negative but not Gram-positive bacteria. Appl. Environ. Microbiol. 1990, 56, 1960–1962. [Google Scholar] [CrossRef]
- Blenkinsopp, H.C.; Seidler, K.; Barrow, M. Microbial imbalance and intestinal permeability in the pathogenesis of rheumatoid arthritis: A mechanism review with a focus on bacterial translocation, citrullination, and probiotic intervention. J. Am. Nutr. Assoc. 2024, 43, 59–76. [Google Scholar] [CrossRef]
- Jeong, S.H.; Nam, Y.; Jung, H.; Kim, J.; Rim, Y.A.; Park, N.; Lee, K.; Choi, S.; Jang, Y.; Kim, Y. Interrupting oral infection of Porphyro-monas gingivalis with anti-fima antibody attenuates bacterial dissemination to the arthritic joint and improves experimental arthritis. Exp. Mol. Med. 2018, 50, e460-11. [Google Scholar] [CrossRef]
- Van Der Heijden, I.M.; Wilbrink, B.; Tchetverikov, I.; Schrijver, I.A.; Schouls, L.M.; Hazenberg, M.P.; Breedveld, F.C.; Tak, P.P. Presence of bacterial DNA and bacterial peptidoglycans in joints of patients with rheumatoid arthritis and other arthritides. Arthritis Rheum. 2000, 43, 593–598. [Google Scholar] [CrossRef]
- Bennike, T.B.; Ellingsen, T.; Glerup, H.; Bonderup, O.K.; Carlsen, T.G.; Meyer, M.K.; Bøgsted, M.; Christiansen, G.; Birkelund, S.; Andersen, V. Proteome analysis of rheumatoid arthritis gut mucosa. J. Proteome Res. 2017, 16, 346–354. [Google Scholar] [CrossRef]
- Laemmli, U.K.; Favre, M. Maturation of the head of bacteriophage T4 I. DNA packaging events. J. Mol. Biol. 1973, 80, 575–599. [Google Scholar] [CrossRef] [PubMed]
- Towbin, H.T.; Staehlin, T.; Gordon, J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocelulose sheets: Procedure and some applications. Proc. Natl. Acad. Sci. USA 1979, 76, 4350–4354. [Google Scholar] [CrossRef] [PubMed]
- Papapetropoulos, A.; Foresti, R.; Ferdinandy, P. Pharmacology of the ‘gasotransmitters’ NO, CO and H2S: Translational opportunities. Br. J. Pharmacol. 2015, 172, 1395–1396. [Google Scholar] [CrossRef] [PubMed]


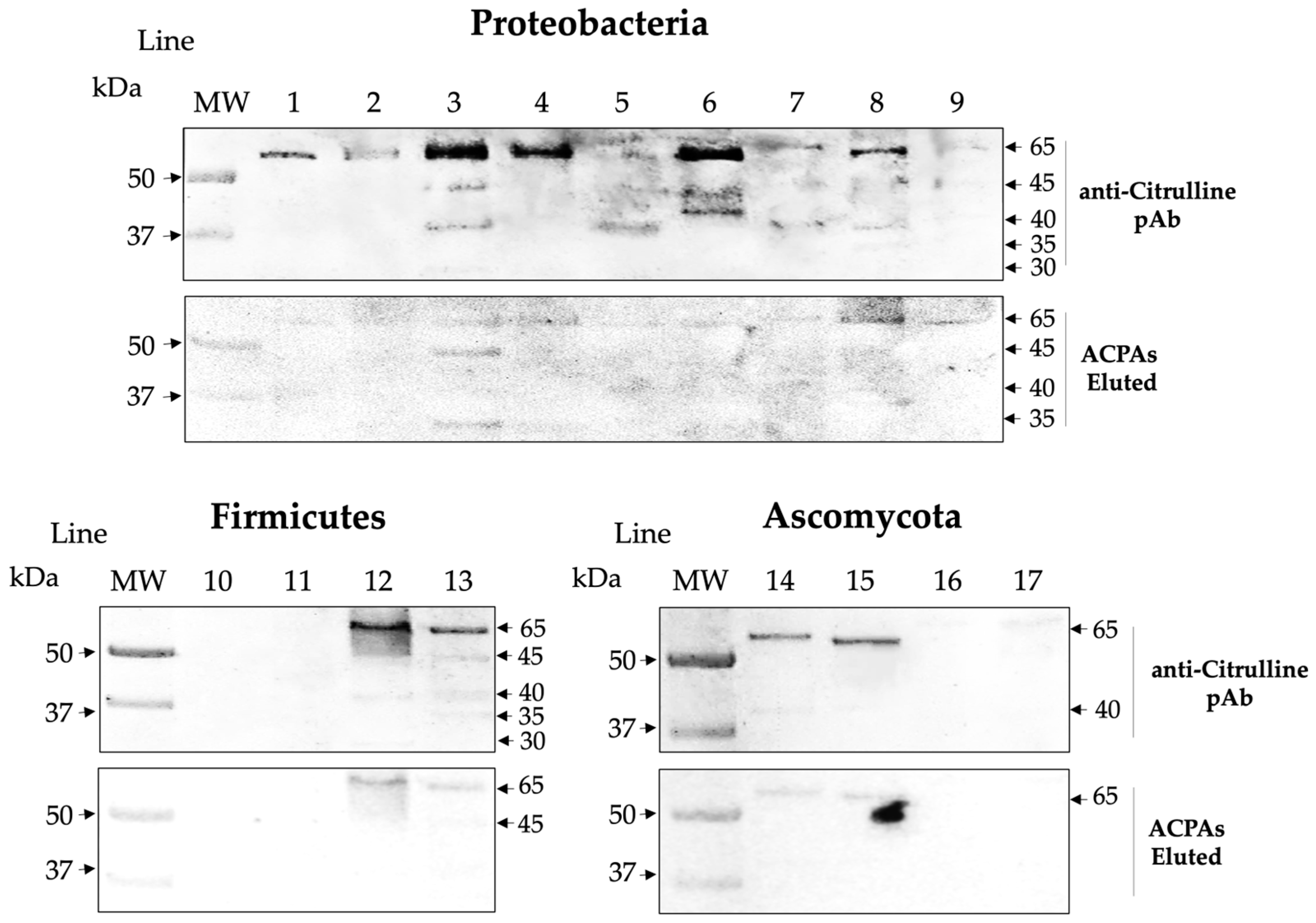
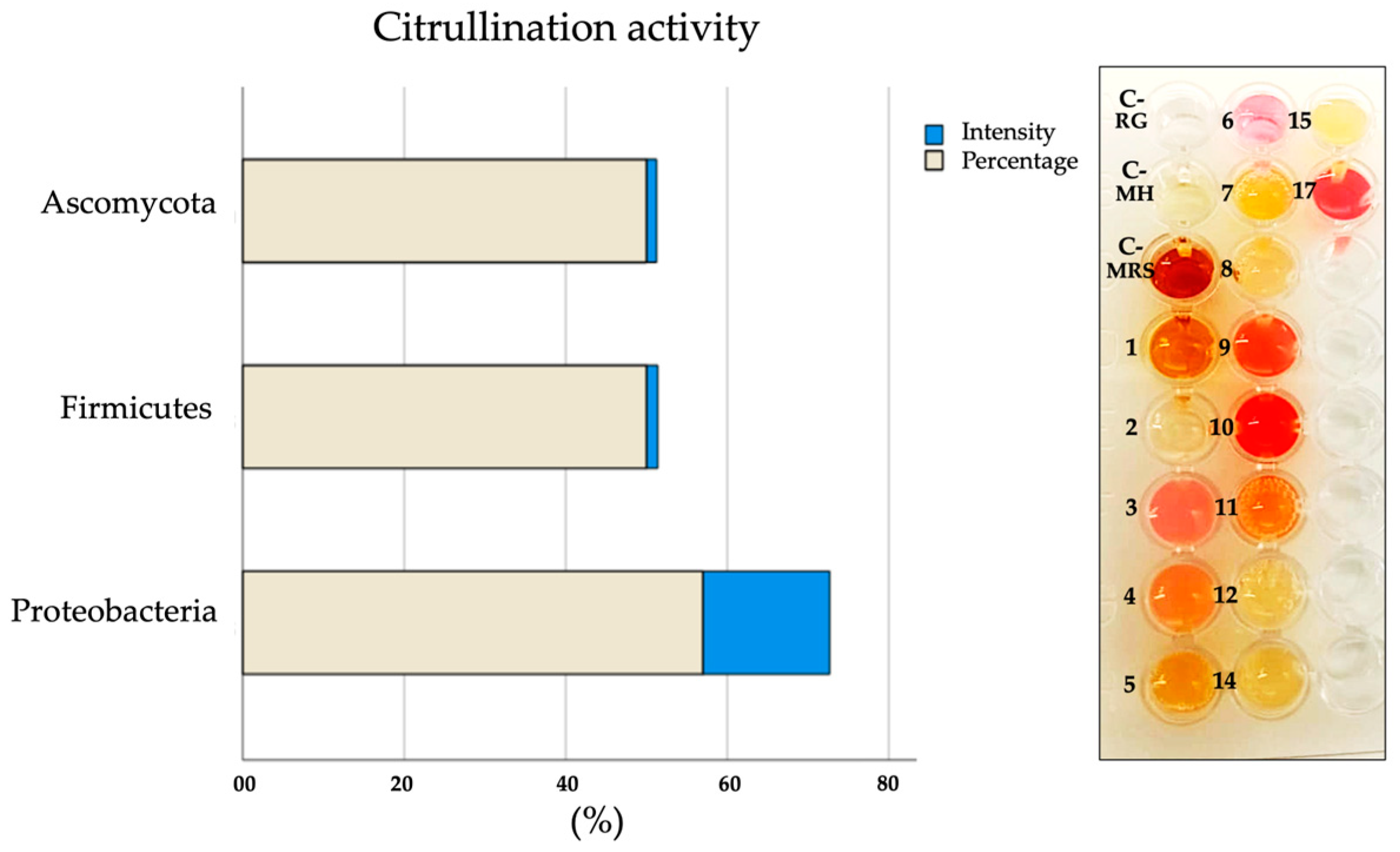
| Phyla | Firmicutes | Ascomycota | Proteobacteria | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 |
| Citrullination activity | − | − | + | + | + | + | − | − | + | + | + | − | ND | − | − | ND | + |
| Expression of citrullinated antigens | − | − | + | + | + | + | − | − | + | + | + | + | − | + | + | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Pérez, M.-E.; Nieto-Torres, E.; Bollain-y-Goytia, J.-J.; Delgadillo-Ruíz, L. Protein Citrullination by Peptidyl Arginine Deiminase/Arginine Deiminase Homologs in Members of the Human Microbiota and Its Recognition by Anti-Citrullinated Protein Antibodies. Int. J. Mol. Sci. 2024, 25, 5192. https://doi.org/10.3390/ijms25105192
Pérez-Pérez M-E, Nieto-Torres E, Bollain-y-Goytia J-J, Delgadillo-Ruíz L. Protein Citrullination by Peptidyl Arginine Deiminase/Arginine Deiminase Homologs in Members of the Human Microbiota and Its Recognition by Anti-Citrullinated Protein Antibodies. International Journal of Molecular Sciences. 2024; 25(10):5192. https://doi.org/10.3390/ijms25105192
Chicago/Turabian StylePérez-Pérez, María-Elena, Enrique Nieto-Torres, Juan-José Bollain-y-Goytia, and Lucía Delgadillo-Ruíz. 2024. "Protein Citrullination by Peptidyl Arginine Deiminase/Arginine Deiminase Homologs in Members of the Human Microbiota and Its Recognition by Anti-Citrullinated Protein Antibodies" International Journal of Molecular Sciences 25, no. 10: 5192. https://doi.org/10.3390/ijms25105192
APA StylePérez-Pérez, M.-E., Nieto-Torres, E., Bollain-y-Goytia, J.-J., & Delgadillo-Ruíz, L. (2024). Protein Citrullination by Peptidyl Arginine Deiminase/Arginine Deiminase Homologs in Members of the Human Microbiota and Its Recognition by Anti-Citrullinated Protein Antibodies. International Journal of Molecular Sciences, 25(10), 5192. https://doi.org/10.3390/ijms25105192






