Abstract
Pinus thunbergii Parl. is an economically and medicinally important plant, as well as a world-renowned horticultural species of the Pinus genus. Pine wilt disease is a dangerous condition that affects P. thunbergii. However, understanding of the genetics underlying resistance to this disease is poor. Our findings reveal that P. thunbergii’s resistance mechanism is based on differential transcriptome responses generated by the early presence of the pathogen Bursaphelenchus xylophilus, also known as the pine wood nematode. A transcriptome analysis (RNA-seq) was performed to examine gene expression in shoot tissues from resistant and susceptible P. thunbergii trees. RNA samples were collected from the shoots of inoculated pines throughout the infection phases by the virulent Bursaphelenchus xylophilus AMA3 strain. The photosynthesis and plant–pathogen interaction pathways were significantly enriched in the first and third days after infection. Flavonoid biosynthesis was induced in response to late infestation (7 and 14 days post-infestation). Calmodulin, RBOH, HLC protein, RPS, PR1, and genes implicated in phytohormone crosstalk (e.g., SGT1, MYC2, PP2C, and ERF1) showed significant alterations between resistant and susceptible trees. Furthermore, salicylic acid was found to aid pine wood nematodes tolerate adverse conditions and boost reproduction, which may be significant for pine wood nematode colonization within pines. These findings provide new insights into how host defenses overcame pine wood nematode infection in the early stage, which could potentially contribute to the development of novel strategies for the control of pine wilt disease.
1. Introduction
Pinus thunbergii Parl. is a well-known and popular horticultural tree from the genus Pinus. At the same time, it is a valuable economic and therapeutic plant. Wood of the P. thunbergii tree is widely utilized in building, furniture, and paper production, and P. thunbergii seeds can also be turned into oil [1,2]. Moreover, P. thunbergii has traditionally been used for edible and medicinal purposes to treat several disorders, such as neuralgia, acute lung injury, and diabetes [3,4]. As an important commercial pine species, P. thunbergii, which is used for coastal reforestation and landscaping, has been extensively cultivated in China for more than 100 years [5]. However, because the P. thunbergii species is sensitive to pine wood nematode (PWN), its population has declined substantially since the first report of pine wilt disease (PWD) in Nanjing City, China [6,7].
PWD is a severe forest disease affecting the Pinus species, caused by PWN (Bursaphelenchus xylophilus) and resulting in the death of a significant number of pine trees [8,9]. PWN is native to North America, where it very rarely kills native pine trees [10]. East Asia was the primarily affected areas, particularly China, Japan, and Korea, where the pathogen continues to cause irreparable damage to forest ecosystems and severe economic losses [11,12,13]. In Europe, the PWN was first detected in 1999, specifically in southwestern Portugal, and had isolated incursions into Spain. Today, it has spread to more than 30% of Portugal, causing extensive damage to Portuguese forests [14,15]. Moreover, the number of damaged conifer trees is increasing every year. Researchers have implemented a series of strategies to combat PWD, including the removal of dead trees, early molecular detection for conifers, and injection of nematocidal substances [16,17,18]. However, these measures only delayed the spread of PWD and cannot completely control the disease [19]. The breeding program for elite nematode-resistant Pinus species was an efficient and environmentally friendly approach to controlling PWD [20,21,22]. Somatic embryogenesis was the most promising technique for mass propagation of selected elite conifers [23,24]. Many somatic plants have been obtained from Pinus species, such as P. pinea [25], P. thunbergii [21,23,24,26], P. radiata [27], and P. elliottii [28]. However, the resistance of somatic plants to PWN has rarely been described.
RNA-seq is widely used to identify the different expression genes (DEGs) involved in plant and pathogen interactions [29,30]. Over the last decade, it was commonly used to elucidate the resistance mechanisms of pine to PWD. For example, in the study of P. densiflora, the phenylpropanoid biosynthesis, flavonoid biosynthesis, and oxidation–reduction pathways were found to have significant enrichment in response to B. xylophilus infection [31]. Modesto et al. [32] considered cell wall reinforcement and hormone signaling mechanisms essential for the resistance of P. pinaster to B. xylophilus. Wang et al. [33] found the oxidoreductase activity pathway was rapidly activated in response to invasion at 24 h of PWN infestation of P. thunbergii. However, the mechanisms behind the successful defense against PWN in P. thunbergii remain unknown. Previously, we compared changes in DEGs during disease development in resistant and susceptible P. thunbergii (i.e., (ph_1d vs. ph_3d) vs. (kh_1d vs. kh_3d)). The importance of α-linolenic acid metabolism and linoleic acid metabolism in nematode-resistance was found [34]. In this article, we highlighted and analyzed the DEGs in resistant and susceptible P. thunbergii at the same time point (i.e., ph_1d vs. kh_1d) during disease development.
A comparative transcriptome analysis (RNA-seq) of the shoot tissues of resistant and susceptible P. thunbergii following PWN inoculation was carried out to describe the transcriptional differences between the two groups. We used RNA-seq to identify differentially expressed genes (DEGs) between resistant and susceptible P. thunbergii at various time periods after inoculation. The identification of genetic determinants for PWD resistance is crucial for the PWD-resistant clones of P. thunbergii, as well as providing valuable data for resistance gene screening and breeding tactics in other Pinus species.
2. Results
2.1. Resistance Performance of P. thunbergii Somatic Plants
The somatic plants of nematode-resistant P. thunbergii exhibited disease symptoms about two weeks later than the susceptible P. thunbergii seedlings. After 7 days post-infestation (dpi), the tips of the upper branches at the inoculated sites of susceptible P. thunbergii began to droop, and a few needles near the inoculated sites started to lose their luster and even showed a loss of green phenotype, while the resistant P. thunbergii remained healthy (Figure 1A,B). After 14 dpi, the susceptible P. thunbergii showed obvious PWD symptoms, while the nematode-resistant P. thunbergii just began to develop the disease (Figure 1C), exhibiting distinct PWD characteristics only after 28 dpi (Figure 1D). Further statistical research revealed that after 7 dpi, the disease incidence of susceptible P. thunbergii was 66.67%, and the disease index was 0.70, whereas resistant P. thunbergii was 0. At this time point, the resistant P. thunbergii were healthy, which indicated that they suppressed PWN infestation. However, after 14 dpi, the disease incidence and index of the resistant P. thunbergii were up to 62.96% and 0.85, respectively, while for the susceptible P. thunbergii they were 100% and 14.67, respectively (Figure 1E,F). Our results showed that somatic plants were effective in delaying the onset of the PWD. Although the somatic plants finally developed PWD symptoms, it was two weeks later than the susceptible P. thunbergii. This development provided potential data to further elucidate PWD pathogenesis and resistance mechanisms.
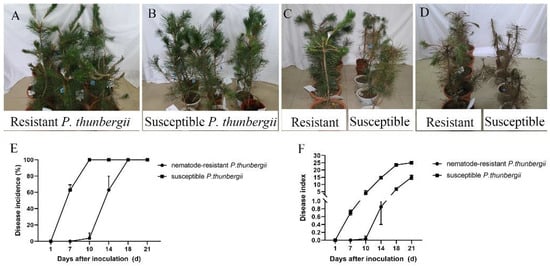
Figure 1.
Performance of resistant and susceptible P. thunbergii after inoculation with pine wood nematode. Data represent mean ± SD. The performance of resistant and susceptible P. thunbergii infected with pine wood nematode for one (A,B), two (C), and four weeks (D). (E,F) indicate disease incidence and disease index of pine trees.
2.2. Transcriptome Assemblies of Resistant and Susceptible P. thunbergii
To understand the molecular basis of P. thunbergii responses to PWN, we analyzed the temporal progression of the pathogenic invasion of the hosts. A comparative transcriptomic analysis (RNA-seq) was conducted in the shoot tissues of resistant and susceptible P. thunbergii following PWN inoculation. The DEGs were selected by comparing infected resistant trees with susceptible ones at fixed times. Seven cDNA libraries created from samples taken at four time points (a total of 239.14 Gb of clean data) were obtained by the transcriptome analysis of 21 samples. The clean data of each sample were >10.33 Gb, and the percentage of the Q30 base was >93.90% (Table S2). Our results revealed that the number of unigenes obtained was 120,249, and the number of transcripts was 192,025, while the average length of N50 unigenes was 1551 bp. BUSCO was employed to evaluate the integrity of the assembly, wherein the scores of unigenes and transcripts were 76.7% and 85%, respectively (Table S3).
2.3. Transcriptomic Responses to PWN Inoculation of P. thunbergii
To explore the global differences in the defense responses of susceptible and resistant P. thunbergii inoculated with PWN, significant DEGs were applied for further analyses at four time points. The result revealed that the maximum DEGs occurred at 7 dpi (total NO. 10,041) when the resistant P. thunbergii trees were healthy and susceptible P. thunbergii began to exhibit disease symptoms. The lowest number of DEGs occurred at 14 dpi (total No. 6316) when both resistant and susceptible P. thunbergii began to show disease symptoms (needles lost their green color). At 3 dpi, the number of up-DEGs was the highest of the four time points, which could be critical for P. thunbergii resistance to PWN inoculation. Furthermore, we discovered that the number of down-DEGs was consistently higher than the number of up-DEGs at each time point, implying that more DEGs were depressed during PWN inoculation in resistant P. thunbergii than in susceptible P. thunbergii, with the highest number of down-DEGs occurring at 7 dpi (No. 7582) (Figure 2A).
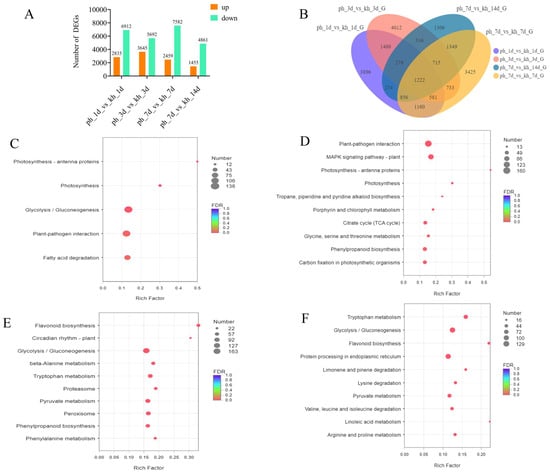
Figure 2.
Comparisons and analysis of DEGs between resistant and susceptible P. thunbergii at four time points. (A) number of DEGs obtained in resistant and susceptible P. thunbergii at 1, 3, 7, and 14 dpi. (B) Venn diagram depicting the number and overlapping relationships of DEGs between different phenotypes at four time points. (C–F) indicate the KEGG enrichment analysis (p < 0.05) for resistant and susceptible P. thunbergii at 1, 3, 7, and 14 dpi, respectively. Resulting p-values were adjusted for control of the false discovery rate (FDR). Circle color denotes the FDR, circle size is proportional to the number of genes involved in the enrichment of the pathway (Count). ph indicates susceptible P. thunbergii; kh indicates nematode-resistant P. thunbergii.
At different time points, there were large numbers of common DEGs. For example, 3561 common DEGs were found between 1 and 3 dpi, while 1222 common DEGs existed in four time points. In addition, several specific DEGs were present at four time points of PWN inoculation. For example, there were 3896, 4012, 3425, and 1306 special DEGs found at 1, 3, 7, and 14 dpi, which indicated that the defense responses of resistant and susceptible P. thunbergii might share some of the same basic defense responses, as well as vary with the disease progression (Figure 2B). To further elucidate the responses of resistant and susceptible P. thunbergii to PWN, we performed a KEGG enrichment analysis of these DEGs at four time points. Approximately 27.77% of the DEGs (numbering 33,367) were annotated by the KEGG database (Table S4). We evaluated the pathway at four time periods with a significance level of p < 0.05. Five significantly enriched pathways linked with the DEGs were discovered at 1 dpi, and pathway enrichment analyses using KEGG terms revealed a considerably larger number of metabolic pathways enriched in subsequent responses at 3, 7, and 14 dpi compared to 1 dpi. This indicated that P. thunbergii activated additional response mechanisms following PWN inoculation at 1 dpi. Photosynthesis antenna proteins and photosynthesis processes ranked among the most significantly enriched at 1 and 3 dpi (Figure 2C,D), while flavonoid biosynthesis ranked among the most significantly enriched at 7 and 14 dpi (Figure 2E,F). In addition, circadian rhythm-plant and linoleic acid metabolism showed higher enrichment levels at 7 and 14 dpi, respectively (Figure 2). Plant immunity pathways, i.e., plant–pathogen interactions and plant MAPK signaling pathways, increased significantly (p < 0.001) at 3 dpi. Phenylpropanoid biosynthesis was stimulated significantly (p < 0.05) at 3 and 7 dpi. Furthermore, at 7 and 14 dpi, the main enriched pathways involved amino acid metabolism (e.g., tryptophan, phenylalanine, and pyruvate metabolism) (Figure 2; Table S5). This could have been related to the creation of downstream secondary metabolites that aid in PWN defense. Thus, PWN infection caused complex and diverse response mechanisms involved in both growth and defense, including photosynthesis, glycolysis/gluconeogenesis, amino acid metabolism, and secondary metabolite biosynthesis.
2.4. Photosynthesis-Related DEGs Responses to PWN Inoculation in P. thunbergii
Photosynthesis and photosynthesis–antenna protein pathways were significantly enriched (p < 0.001) at 1 and 3 dpi, which prompted us to investigate the differences in gene-regulated growth between PWN-resistant and susceptible P. thunbergii. Subsequently, the DEGs associated with photosynthesis were examined. Interestingly, at 1 dpi, the 23 (100%) DEGs for photosynthesis and 12 (100%) DEGs for photosynthesis–antenna protein were upregulated in resistant P. thunbergii. At 3 dpi, all genes expressed upregulation except for one DEG (HLCB1, TRINITY_DN84,366_c0_g1), which was downregulated and involved the photosynthesis–antenna protein pathway in PWN-resistant P. thunbergii (Tables S6 and S7). Nematode-resistant P. thunbergii had considerably more DEGs encoding photosystem I, photosystem II, the cytochrome b6/f complex, photosynthetic electron transport, and F-type ATPase than susceptible P. thunbergii. This finding indicated that the photosynthetic system pathway was considerably impacted and may play a role in P. thunbergii responses to PWN inoculation.
2.5. Gene Regulation Involved in Pattern-Triggered Immunity and Effector-Triggered Immunity in PWN-Induced Immunity of P. thunbergii
We investigated the DEGs connected to P. thunbergii’s defense responses based on the gene expression profile and KEGG enrichment of the organism inoculated with PWN. This study found that after PWN infection, calcium ion-induced reactive oxygen species (ROS); NO signaling pathways; DEGs encoding respiratory burst oxidase homologs (RBOH); and calmodulin (CALM/CML) were significantly altered. Additionally, the two trends were reversed. Nematode-resistant P. thunbergii exhibited higher expression levels in the ROS-regulated branch (three (75%) DEGs for RBOH at 1 dpi and six (85.71%) at 3 dpi) compared to susceptible P. thunbergii. But nematode-resistant P. thunbergii showed lower expression of ten (100%) DEGs of calmodulin (CALM/CML) at 1 dpi and seven (87.50%) at 3 dpi in the NO-regulated branch compared to susceptible P. thunbergii (Figure S1). Calmodulin expression was inhibited in nematode-resistant P. thunbergii; nevertheless, calcium ion control of ROS was elevated, which may have contributed to ROS formation. This finding was supported by the overexpression of oxidative stress-inducible 1 (OXI1), which maintained ROS homeostasis at 3 dpi (Figure 3A). Next, we looked at the hypersensitive response (HR) and defense-related genes involved in pattern-triggered immunity (PTI) and effector-triggered immunity (ETI). For example, SUMM2 (disease resistance protein RPS2) expression was greater in nematode-resistant P. thunbergii at 1 dpi but decreased at 3 dpi, whereas MAP kinase substrate 1 (MKS1) expression was repressed.
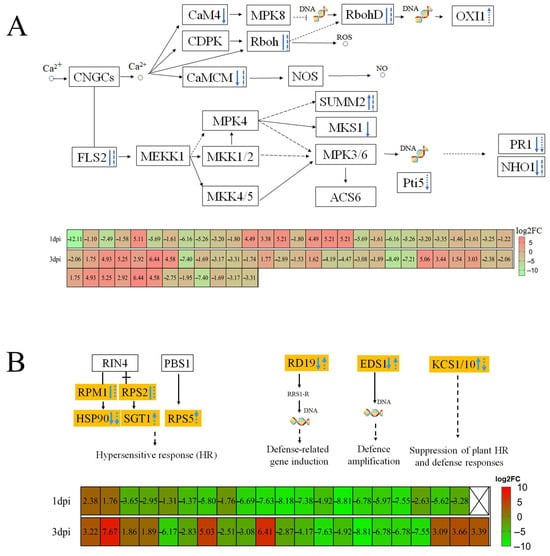
Figure 3.
Transcriptome analysis revealed that a pine wood nematode infestation elicited pattern-triggered immunity and effector-triggered immunity defense responses. (A) Calcium ion-triggered immune response. (B) Resistance gene-induced immune responses. Solid lines represent 1 dpi, dashed lines represent 3 dpi, downward arrows represent lower DEG expression in resistant P. thunbergii than susceptible P. thunbergii and vice versa with the upward arrows, and straight and dashed lines represent both up- and downregulated DEGs. Solid and dashed arrows indicate characterized and predicted pathway steps, respectively.
In early PWN inoculation (at 1 dpi and 3 dpi), the expressions of heat shock protein 90 (HSP90), pathogenesis-related 1(PR1), and NHO1 (glycerol kinase) were suppressed in nematode-resistant P. thunbergii. The defense-related genes RD19 (cathepsin F) and EDS1 (enhanced disease susceptibility 1 protein) were downregulated at 1 dpi and then upregulated at 3 dpi; however, the KCS (3-ketoacyl-CoA synthase) expression showed an opposite trend (Figure 3B). It was found that the HR-gene disease resistance protein RPS5 (RPS5) and SGT1 (suppressor of G2 allele of SKP1), as well as the defense-related genes RD19 and EDS1, were upregulated. Furthermore, the suppression of plant HR and defense response KCS genes was downregulated at 3 dpi, implying that pines’ immunity to PWN was significantly increased at 3 dpi after PWN infection. The defense of P. thunbergii against PWN was complex and required the regulation of multiple genes.
2.6. Signaling Molecule Responses to PWN Infection of P. thunbergii
The ROS signaling system plays a key role in regulating plant stress responses. To further understand the physiological alterations involved in PWN infection, we investigated ROS scavenging in P. thunbergii. From transcriptome sequencing, a total of 13 DEGs related to ROS scavenging were identified at four time points (Table S8). Among these DEGs, the expression levels of superoxide dismutase (SOD) and peroxidase (POD) were higher in nematode-resistant than susceptible P. thunbergii at each time point, although catalase (CAT) expression was the opposite. Six upregulated DEGs encoding for SOD and POD were found only in the nematode-resistant P. thunbergii. Three of the four genes encoding for glutathione reductase were more highly expressed in susceptible P. thunbergii, while one was more highly expressed in nematode-resistant P. thunbergii. Furthermore, one encoding phenylalanine ammonia lyase (PAL) gene exhibited higher expression in susceptible P. thunbergii early in the PWN inoculation (~three times higher than in nematode-resistant P. thunbergii) (Table S8). These results implied that two completely different reactive oxygen species scavenging systems were activated when resistant and susceptible P. thunbergii was threatened by pine wood nematode. In the ROS-regulated branch, three DEGs encoding for OXI1 were upregulated in PWN-resistant P. thunbergii, whereas two DEGs encoding for NDPK2 were suppressed in PWN-resistant P. thunbergii (Figure 4). We speculated that the gene OXI1 may have a negative regulatory effect on NDPK2.

Figure 4.
PWN infestation induced changes in response genes for phytohormones and reactive oxygen species. Solid and dashed arrows indicate characterized and predicted pathway steps, respectively. The red content in the box represented DEGs.
In the ethylene signaling pathway, the expression of Mitogen-Activated Protein Kinase Kinase Kinase 9 (MKK9), ethylene-insensitive 3 (EIN3), and Ethylene Response Factor 1 (ERF1) genes were downregulated, which suggested that the ethylene pathway was suppressed. This resulted in the up-expression of Chitinase B (ChiB) in nematode-resistant P. thunbergii at 3 dpi. Transcription factor MYC2 (a core protein in the JA-induced signaling pathway), was up-expressed in nematode-resistant P. thunbergii. Furthermore, the MKK3-MPK6-MYC2 module positively regulated abscisic acid (ABA) biosynthesis and signaling in Arabidopsis [35]. In this work, the positive regulator of ABA responses (PYL) was up-expressed, while an ABA signaling repressor (protein phosphatase type 2C (PP2C)) was down-expressed at 1 dpi. This suggested that the ABA pathway was activated; however, at 3 dpi, the PYL did not change, and the repressor PP2C was up-expressed, which resulted in MAPKKK (17-18) being repressed (Figure 4; Table S9). MAPKKK17 was induced in response to a mite attack in Arabidopsis [36], while MAPKKK18 (an ABA-activated kinase) was regulated by PP2C, which inhibited the kinase activity of MAPKKK18 [37]. The reduced transcription of MAPKKK18 in Arabidopsis exhibited obviously delayed leaf senescence [38]. Additionally, CAT1 was indirectly regulated by ABA, with the expression of two of the ten genes encoding for CAT1 being significantly upregulated and the other eight genes being downregulated. These results indicated several roles for the ABA pathway during early PWN infection.
We studied the effect of salicylic acid (SA) on PWN survival and proliferation. The result showed that the PWN survival was higher when immersed in an alcohol solution containing 0.1% SA for 1h compared to the alcohol control solution (Figure 5A). Moreover, we found that a greater number of PWN were obtained in dishes sprayed with an alcohol solution containing 0.1% SA than in the control treatment sprayed with 10 mL of alcohol, for 3.5 times (Figure 5B). This indicates that SA could help PWN to resist stressful environments.
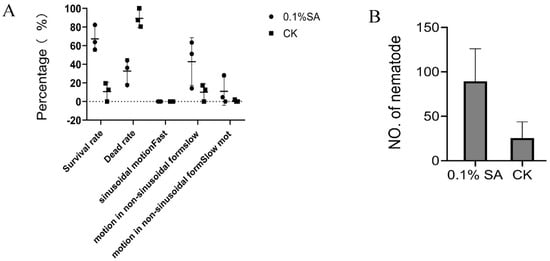
Figure 5.
Effect of salicylic acid (SA) treatment on pine wood nematodes. (A) Pine wood nematode survival after SA treatments. (B) Proliferation of pine wood nematode following SA treatments. An amount of 0.1% SA represents the 0.1% (m/v) SA treatment (ethanol solvent), and CK represents the ethanol solvent control treatment. The data of (B) are expressed as the mean (±SE) of four replicates. Error bars represent the SE.
2.7. Flavonoid Biosynthesis Pathway with DEG-Related Interactions
We compared DEGs along the flavonoid biosynthetic pathway, significantly enriched (p < 0.001) at 7 and 14 dpi (Figure 6). Most enzyme activities were downregulated, except for caffeoyl-CoA O-methyltransferase (CCoAOMT) and flavonoid 3′-monooxygenase (F3′H), which were significantly upregulated (p < 0.05) at 7 dpi and downregulated at 14 dpi (Figures S3 and S4). Phenotypic results showed that nematode-resistant P. thunbergii did not exhibit disease symptoms at 7 dpi (upregulation of CCoAOMT and F3′H) but began showing needle chlorosis only at 14 dpi (CCoAOMT remained unchanged and F3′H was downregulated).
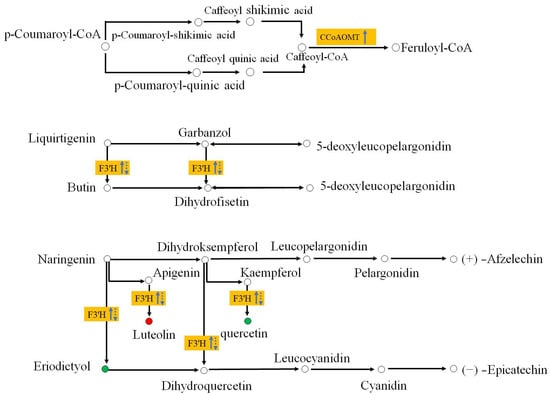
Figure 6.
Flavonoid synthesis differences in PWN-inoculated P. thunbergii. Yellow boxes represent the DEGs between the resistant and susceptible P. thunbergii. Upward-pointing arrows represent up-expression, downward pointing arrows represent down-repression. Solid lines represent the transcription level at 7 dpi; dashed lines represent the transcription level 14 dpi. Red and green circles represent compounds detected in metabolomics, and red represents the abundance of compounds being higher in the resistant pine than the susceptible pine. Green indicates that the abundance of the compound was lower in resistant pine than the susceptible pine (at 7 dpi and 14 dpi).
This result implied that CCoAOMT and F3′H modification might have been correlated with the delayed onset of disease for nematode-resistant P. thunbergii. The metabolomic results revealed that the luteolin metabolite catalyzed by F3′H was more abundant in resistant P. thunbergii, whereas the metabolites of eriodictyol and quercetin were more abundant in susceptible P. thunbergii. This observation raised interesting questions about the abundance of metabolites catalyzed by F3′H. The way in which P. thunbergi resisted late nematode infestation may be significantly impacted by the manner of control of these two metabolites, which was catalyzed by F3′H. Despite the lack of detection of CCoAOMT and Feruloyl-CoA in the metabolomics investigations, CCoAOMT has been linked to a number of leaf diseases (the maize CCoAOMT gene conferred resistance to both gray leaf spot and southern leaf blight, for example) [39]. These results revealed that F3′H and CCoAOMT were important components of the flavonoid metabolic response to protect against PWN. They may be useful for postponing the onset of disease in pine trees since this was especially the case in the early stages of the disease’s development.
2.8. Validation of RNA-seq Expression Data by qRT-PCR
To validate the reliability of the RNA-seq results, 10 unigenes from the highly expressed DEGs were selected for qRT-PCR analysis. Expression of these unigenes differed significantly between susceptible and PWN-resistant P. thunbergii after infection with PWN (Figure 7). The unigenes selected for qRT-PCR analysis were those primarily involved in ROS-responsive genes, disease resistance protein, signal transduction, and integral components of the membrane. The expression pattern of selected unigenes indicated by qRT-PCR agreed well with RNA-seq.
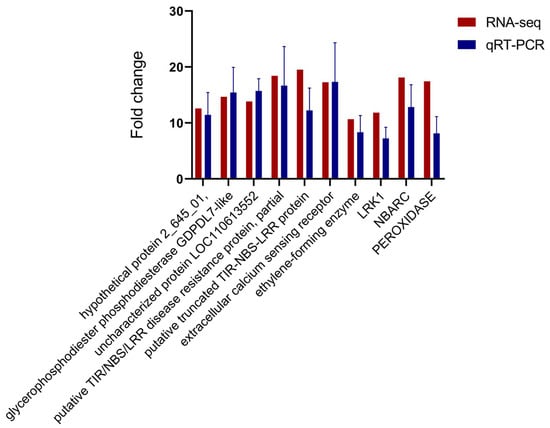
Figure 7.
qRT-PCR validation of unigenes associated with resistance to PWN.
Relative expression levels of qRT-PCR were calculated using Elongation factor 1-alpha as the internal control. The data are expressed as the mean (±SE). Error bars represent the SE.
3. Discussion
One of the most important steps in applying somatic plants for afforestation was determining resistance. Understanding P. thunbergii’s resistance mechanisms to the pine wood nematode was essential for enhancing integrated approaches to managing the worm, such as breeding for resistance or creating novel diagnostic instruments based on targeted resistance markers. According to our findings, susceptible P. thunbergii was more vulnerable to infection (~two weeks) than the somatic plants of resistant P. thunbergii (Figure 1). The transcriptomes of resistant and susceptible P. thunbergii shoot tissues differed remarkably (Figure 2). Compared to earlier studies that used suppression subtractive hybridization, more DEGs were obtained between resistant and susceptible P. thunbergii in this investigation employing next-generation sequencing [40]. The highest level of increased gene expression was seen in the number of DEGs at 3 dpi (NO. 3645), which may represent a crucial threshold for P. thunbergii resistance to PWN. Furthermore, when susceptible P. thunbergii started to wilt, and resistant P. thunbergii was healthy, the quantity of DEGs at 7 dpi (NO. 10041) was highest in the four examined time points. The KEGG enrichment analysis of DEGs revealed that the resistant and susceptible phenotypes differed in important KEGG pathways, indicating that their activated defense mechanisms and genes against PWN infection were qualitatively different.
3.1. Potential Roles of Photosynthesis in Resistance to PWN in Pine
In this investigation, we discovered that the photosynthesis–antenna proteins and photosynthesis pathways were considerably enriched (Figure 2C,D) and that several DEGs of PS I and PS II were upregulated in resistant P. thunbergii (Tables S6 and S7). Glycolysis and TCA cycle pathways were considerably changed (p < 0.001) at 1 and 3 days after infection. Plant photosynthesis produces sugars that travel via glycolysis and the TCA cycle, yielding intermediate metabolites, including pyruvate and acetyl coenzyme A. These compounds are gradually transformed into secondary metabolites, including terpenoids, phenols, and alkaloids, via a succession of oxidative folding and reduction processes. In contrast, pines use secondary metabolites as their principal defense against PWN [41,42]. We hypothesized that the high expression of these photosynthetic genes in resistant trees helped to inhibit PWN infestation and delay the development of PWD in resistant P. thunbergii. For host-pathogen interactions, photosynthetic regulation was developed as an approach to seek to correlate functional manipulation of PS I and PS II with host defense responses derived from primary metabolism [43]. After PWN infestation, photosynthesis-regulated genes responded first, followed by significant modifications in the circadian rhythm-plant pathway (p < 0.001). Our findings suggested that PWN infection disrupted plant growth, leading us to infer that wilting could be linked to altered growth.
The light-harvesting chlorophyll protein complex (LHC) was previously reported to be strongly phosphorylated in resistant wheat plants with stripe rust infection [44], while in this study, the DEGs related to LHC also showed a higher expression level in PWN-resistant P. thunbergii (Tables S6 and S7). Furthermore, the comparative transcriptomic analysis revealed that the majority of photosynthesis-related DEGs (photosystem I, II, cytochrome b6/f complex, photo-synthetic electron transport, and F-type ATPase) were expressed more in resistant P. thunbergii than in susceptible trees, implying an increase in photosynthetic efficacy. In a comparative study between poplar and Lonsdalea populi, higher expression levels of photosynthesis-related genes occurred in more highly resistant poplar, particularly for genes in the LHC [45]. Therefore, more robust photosynthesis might lead to higher levels of resistance in P. thunbergii, which might be an important regulator of PWN resistance. Generally, all these studies demonstrated the potentially critical role of photosynthesis in nematode resistance for P. thunbergii, which provided further new insights into the importance and engagement of photosynthesis-related genes in the regulation of PWN resistance.
3.2. Plant Recognition and Responses to PWN Invasion
In general, plants perceive changes in membrane surface transmembrane potential (Vm), Ca2+ influx, mitogen-activated protein kinase (MAPK) activation, and ROS burst caused by pathogen-associated molecular patterns (PAMP) [46]. These initial signaling events could further activate signal transduction pathways mediated by plant hormones, such as jasmonic acid (JA), salicylic acid (SA), and ethylene (ET) [47]. This eventually led to upregulated transcription levels of defense-related genes and increased levels of defense compounds [48]. The results of this study showed that PWN infection reduced the expression of calmodulin and changed that of RBOH, an important component of the ROS signaling network that affects a number of plant functions. RBOH was engaged in the production of H2O2 during Arabidopsis thaliana hypoxia stress [49], and it was also involved in the formation of aerenchyma in rice roots during oxygen-deficient conditions [50]. In an investigation of Medicago truncatula nodules, innate immunity was activated to mediate rhizobial infection and colonization, and RBOH and calcium-dependent protein kinase (CDPK) were important components of this process. A knock-out of RBOH (MtRbohB or MtRbohD) produced effective nodules [51]. Therefore, we hypothesized that calcium-triggered immunity during the early stage of PWN infection might inhibit infection by regulating ROS burst, stomata, and PWN colonization in host plants.
It has been observed that NHO1 resistance to bacterial and fungal infections is dependent on non-host resistance [52,53]. Interestingly, we found that NHO1 was significantly altered in P. thunbergii against PWN infection, indicating that it was involved in pine PWN resistance. The virulent bacteria appeared to suppress NHO1-mediated resistance by means of an Hrp-dependent virulence mechanism in Arabidopsis thaliana [52], which was consistent with the inhibition of NHO1 at 1 dpi. Furthermore, NHO1 compromised the resistance mediated by RPS and RPM1, and here, transcriptome analysis found that the expressions of RPS and RPM1 were significantly altered, albeit whether they were regulated by NHO1 requires further investigation. OsNHO1 regulates rice resistance to bacterial blight and blast by influencing the wax content and regulating the transcription level of PR genes [54]. Here, we first noticed changes in NHO1 resistance to PWN in P. thunbergii, however, its specific mechanism remains to be further explored.
The immediate increase in heat shock proteins (HSPs) was crucial for the cellular adaptation to environmental changes and for maintaining cellular homeostasis [55]. Prior transcriptome analysis of P. thunbergii found that HSP70 expression was higher in resistant plants than in susceptible ones [40]. In contrast, our study found that HSP90 expression was downregulated at both 1 and 3 dpi in the resistant phenotype. HSP70 and HSP90 may have various defense mechanisms in response to PWN infection. It was previously established that Mi-1-mediated nematode resistance in tomatoes needed the cooperation of HSP90-1 [56]. HSP90, a highly conserved protein in most organisms and involved in numerous biological processes, may be implicated in plants’ fundamental responses to pathogenic organisms [57]. However, it is unclear how HSPs participate in the defense response to PWN infection. In summary, defense regulatory genes such as defense responsive (PR1, NHO1), hypersensitivity responsive (Pti, Rboh), and stress hormone responsive ERF1 were suppressed in resistant P. thunbergii at 1 or 3 dpi, suggesting that resistant P. thunbergii has a protectively modulated defense mechanism during PWD. This was similar to the findings on tomato resistance to bacterial leaf spot (BLS) disease [58]. These findings contributed to a better knowledge of the pine response to PWN and will be essential tools for future PWN engineering.
Phytohormones SA, JA, and ET were signaling chemicals that played important roles in plant development and stress responses [59]. MKS1 was necessary for full SA-dependent resistance, and overexpressing MKS1 in Arabidopsis thaliana activated SA-dependent resistance [60]. EDS1 increased SA production and accumulation while repressing the JA pathway, which was critical in the activation of effector-triggered immunity and plant basal defense [61,62,63], whereas PR1 was a downstream response gene of SA.
In this way, the EDS1, MKS1, and PR1 genes were down-expressed in resistant P. thunbergii, suggesting that the SA pathway was suppressed at 1 dpi. The action of SA on PWN was discovered to boost their survivability during alcohol stress and stimulate proliferation. Simultaneously, resistant P. thunbergii showed increased expression of MYC2 (a key protein in the JA-induced signaling pathway). This demonstrated that suppressing SA and activating the JA pathway during early PWN infection could be beneficial for resistance. In a P. pinaster research, genes encoding JA biosynthesis enzymes (LOX, OPRs) and reacting to JA (ERFs, MYC2) were upregulated in a resistant genotype following inoculation. Additionally, the EDS1 and SA had higher levels in the susceptible genotype, indicating that PWN resistance was associated with the activation of JA and suppression of the SA pathway [32], which aligned with the results of our study. JA-related regulatory gene up-expression also occurred in P. massoniana [64,65], P. densiflora [66], and P. thunbergii [40,67]. These results indicated that the JA pathway might have played a central role in response to PWN in several pine species. The suppression of SA and activation of the JA pathway during early PWN infection might be characteristic of an efficient resistant response in pines; however, further studies are needed.
Ethylene response factors (ERFs) are a large family of transcription factors that play a role in plant responses to biotic and abiotic stressors. Members of ERFs contributed to the integration of the JA and ET pathways. DEGs encoding ERF1 were shown to be suppressed in resistant P. thunbergii at 3 dpi. PYL is a receptor that detects ABA and activates the ABA signaling pathway, which has been implicated in ABA-JA crosstalk during stress responses [68]. PYL was found to mediate the expression of PP2C and MYC2, which were involved in the ABA and JA signaling pathways, respectively [35]. In this work, the positive regulator of ABA response (PYL) was up-expressed and a repressor of ABA signaling, but PP2C was down-expressed at 1 dpi, suggesting activation of the ABA pathway. In Arabidopsis thaliana, the transcription factors MYC and ERF regulated the JA defensive response. ET was necessary to activate the ERF branch, whereas ABA inhibited this defense response and activated the MYC branch [69,70]. In this investigation, P. thunbergii appeared to have a similar regulatory mechanism, namely, the YPL up-expressed and activated the ABA signaling pathway. Furthermore, ERF1 (a critical regulator of the ethylene route) was repressed, whereas MYC2 (a key regulator of the JA pathway) was upregulated during early PWN infection. This seems to indicate a role for the ABA pathway in the early PWN infection of P. thunbergii. The immune regulation involved in hormones such as SA, JA, ET, and ABA appeared to be key to the differences in resistance to PWN in Pinus species. However, the extent to which numerous related genes were involved (e.g., PYL, MKS1, EDS1, MYC2, etc.) in contributing to PWN resistance still requires further validation. In addition, MYC2 was a positive regulator of JAs-mediated flavonoid biosynthesis [71]. The MYC2 was significantly upregulated, which may have led to differences in downstream flavonoid metabolism.
Following nematode recognition, the ROS produced by plants served as signaling molecules to activate defense responses, enhanced plant cell walls via crosslinking, and inhibited nematode migration [72]. However, higher ROS levels were toxic to plant cells, which might lead to their death if not removed in time [65]. In an earlier study of P. thunbergii, DEGs encoding for antioxidant enzymes were highly expressed in the resistance phenotype. For example, POD and CAT were more highly expressed in resistant P. thunbergii [40,68]. Similarly, the ROS-related genes were differentially expressed significantly between resistant and susceptible P. thunbergii when the branches of P. thunbergii were cut and placed in water for incubation and then inoculated with PWN [33]. In the current study, the expression levels of iron superoxide dismutase and peroxidase were higher in the resistant P. thunbergii than in the susceptible P. thunbergii at every time point. The greater expression of SOD was also found in pines with a higher resistance against PWN (e.g., P. densiflora [67], P. pinaster [32,73], P. strobus [74], P. massoniana [65], and P. yunnanensis [75]. Furthermore, POD contributed to cell wall synthesis by catalyzing the oxidative cross-linking of structural cell wall proteins (extensin and HRGPs), which were involved in cell wall-mediated resistance and played an important role in preventing pathogen intrusion [76,77]. As a result, the elevated levels of POD and SOD were advantageous in the protection against PWN.
3.3. Induction of Secondary Metabolic Pathways
Flavonoids are one of the most abundant types of phenolics, which are secondary metabolites produced by plants to protect themselves from diseases. Most enzymes in the flavonoid biosynthesis pathway were down-expressed at 7 and 14 dpi, but two important enzymes were dramatically up-expressed in the resistant genotype (Tables S3 and S4). One of these was CCoAOMT, which catalyzed the methylation of caffeoyl-CoA to produce feruloyl CoA [78]; CCoAOMT catalyzed the transfer of a methyl group from S-adenosylmethionine to a hydroxyl moiety of caffeoyl-CoA as part of the lignin biosynthetic pathway [79]. This enzyme played an important role in the lignin biosynthesis pathway, being involved in the phenylpropanoid pathway and lignin formation [80]. The suppression of PrCCoAOMT expression in P. radiata affected the lignin content and composition, resulting in a decrease in lignin content and an increase in the p-hydroxyphenyl/guaiacyl ratio relative to untransformed controls [81]. Furthermore, the ZmCCoAOMT2 of maize exhibited gene conferring quantitative resistance to both southern leaf blight and gray leaf spot [39]. Lignin is a common polymer found in the cell walls of all vascular plants, hardening and strengthening cell wall structures via covalent crosslinking with cell wall polysaccharides [82]. Based on the positive regulatory link between CCoAOMT and lignin production, we predicted that up-expression of CCoAOMT at 7 dpi could induce PWN resistance, resulting in the cell wall reinforcement to restrict the further invasion by PWN. Another essential enzyme was flavonoid 3′-monooxygenase (F3′H, EC 1.14.14.82), which could catalyze multiple substrates in the flavonoid biosynthesis pathway [83]. Interestingly, our study revealed that the modification of F3’H resulted in the different accumulation of multiple metabolites. Metabolome results showed that eriodictyol and quercetin had lower levels in resistant plants at 7 dpi; however, luteoin was the opposite. Moreover, the concentration of luteoin was significantly higher in resistant P. thunbergii at each time point (Figure S2), which might be developed as a marker metabolite for assessing resistance to PWN in P. thunbergii. Based on the findings of this study, we summarized a proposed P. thunbergii defensive model against PWN infestation (Figure 8). This study provided valuable data on P. thunbergii’s resistance against PWN. These findings will undoubtedly help us better understand the transcriptional defense mechanisms against PWN in P. thunbergii and other Pinus species. However, more extensive functional research of the important genes identified in this study is needed in the future to untangle these complex defense systems.
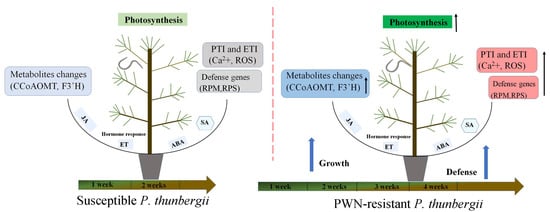
Figure 8.
A proposed model of P. thunbergii’s defense response to Bursaphelenchus xylophilus infection during the onset period. Arrow up represents an upward trend.
Bursaphelenchus xylophilus infection triggered P. thunbergii PTI and ETI, phytohormone production, and induction of metabolites, which integrated multiple regulatory circuits. During the early stages of PWN infection, resistant P. thunbergii expressed more photosynthesis-related genes than susceptible P. thunbergii. Meanwhile, RPS and RPM, calcium-induced immune response genes, and ROS defense genes were all substantially expressed in resistant P. thunbergii. In the late stages of pine wood nematode infestation, secondary metabolism regulation took precedence, and resistant P. thunbergii had increased expression of secondary metabolism-regulating genes (CCoAOMT and F3’H).
4. Materials and Methods
4.1. Plant Materials and Nematode Infections
For this experiment, the PWN-resistant P. thunbergii somatic plants were used as resistant materials. The somatic plants were from the nematode-resistant P. thunbergii family 39 [24]. And somatic plants were the regeneration plants produced by somatic embryogenesis in nematode-resistant P. thunbergii [84]. The disease incidence of PWN-resistant P. thunbergii family 39 was lower by ~30–40% compared to susceptible P. thunbergii following artificial inoculation with the PWN (Wu et al., 2008). The PWN-resistant P. thunbergii family (No. 28–40) was introduced from Japan in 2004 [85,86]. The susceptible P. thunbergii (mortality rates were all 100% after inoculation with PWN in the previous test) from Suqian City, China, was selected as the control [34].
Three-year-old somatic plants and susceptible P. thunbergii were transferred to seedling pots for four months, and then the PWN-resistant and susceptible P. thunbergii with a similar growth status were selected for inoculation with the PWN. The PWN used in this experiment was the highly virulent B. xylophilus strain AMA3 isolate. Nematode infection was conducted as described earlier by our group [34]. The average heights of the resistant and susceptible trees were 1.47 m and 1.51 m, respectively. The branches growing at ~10 cm below the tips of the shoots were cut at an angle (~1 cm long × 1 mm deep) into the xylem. Subsequently, 1000 nematodes (suspended in 1 mL of sterile water) were injected into the longitudinal wounds of the resistant and susceptible P. thunbergii trees. Three branches per tree were inoculated (i.e., 3000 nematodes per pine tree). Samples were collected at 1, 3, 7, and 14 dpi to assess the resistance. Nine pine trees were used as replicates for each treatment. We sampled inoculated trees (both susceptible and resistant trees) at 1, 3, and 7 dpi; resistant trees were also sampled at 14 dpi. The last sampling time was chosen based on previous results, where the needles of resistant and susceptible P. thunbergii turned yellow at 14 and 7 dpi, respectively.
4.2. Assessment of PWN Resistance of Somatic Plants
The PWN resistance of P. thunbergii was divided into six grades: (0) no obvious symptoms; (1) a few needles lost their green color; (2) less than 1/3 needles lost their green color; (3) 1/3 to 1/2 needles lost their green color; (4) more than 1/2 needles lost their green color; and (5) all needles lost their green color). The disease grade and index were calculated based on symptoms over 14 days (onset period). The statistical susceptibility index and susceptibility rate were recorded according to a previously described method.
Disease incidence = Number of diseased pines/Total number of plants investigated × 100%; Disease index = ∑(Number of diseased pines in corresponding grade × Representative value)/Total number of pines investigated × The representative value of the most severe disease grade [45].
4.3. Sample Collection and RNA Extraction
Sampling time points were established, including 1, 3, 7, and 14 (dpi), to assess the resistance of the somatic plants against PWD. At 1, 3, 7, and 14 dpi, the 2 cm long segments of the stems below the inoculation sites were cut off and immediately put into liquid nitrogen. On 14 dpi, only the PWN-resistant P. thunbergii stem tissues were collected, as the susceptible P. thunbergii stems were completely wilted. The last sampling time was carried out according to previous results, where the needles of resistant and susceptible P. thunbergii turned yellow after 14 and 7 dpi, respectively. Three trees representing resistant and susceptible P. thunbergii were selected as biological replicates for each treatment. Next, these samples were stored at −80 °C for further RNA extraction.
The total RNA was extracted from each PWN inoculated tree sample at four time points using TRIzol® Reagent (Plant RNA Purification Reagent for plant tissue) according to the instructions, while genomic DNA was removed using DNase I (TaKara, Nanjing, China). The RNA degradation and contamination were monitored using 1% agarose gels, whereas the integrity and purity of the total RNA quality were determined via a 2100 Bioanalyser (Agilent Technologies, Santa Clara, CA, USA) and quantified using an ND-2000 (NanoDrop Technologies, Wilmington, DE, USA). Only high-quality RNA samples (OD260/280 = 1.8~2.2, OD260/230 ≥ 2.0, RIN ≥ 7.9, 28S:18S ≥ 1.0) were utilized to develop a sequencing library.
4.4. Differential Expression Analysis and Functional Enrichment
To identify the DEGs (differential expression genes) between the resistant and susceptible P. thunbergii samples, the expression levels of each gene were calculated according to the transcripts per million reads (TPM) method. The DEGs with |log2 (foldchange)| ≥ 1 and P-adjust ≤ 0.05 were considered as differentially expressed genes. Furthermore, the databases GO (Gene Ontology, http://www.geneontology.org (accessed on 11 February 2022)) and KEGG (Kyoto Encyclopedia of Genes and Genomes, http://www.genome.jp/kegg/ (accessed on 14 February 2022)) were used to identify functional-enrichment analysis.
4.5. UPLC-MS/MS-Based Non-Targeted Metabolomics Analysis
Samples used for metabolomics analysis were consistent with transcriptomics analysis. The samples were collected at 1, 3, 7, and 14 dpi. Similarly, on 14 dpi, only the PWN-resistant P. thunbergii stem tissues were collected. The 2 cm segments of stems, located below the inoculation sites, were excised and promptly immersed in liquid nitrogen. Metabolite Extraction: 50 mg pine stem segment tissue was accurately weighed, and the metabolites were extracted using a 400 µL methanol/water (4:1, v/v) solution with 0.02 mg/mL L-2-chlorophenylalanin as internal standard. The sample was then re-solubilized with 100 µL solution (acetonitrile: water = 1:1) and extracted by low-temperature ultrasonication for 5 min (5 °C, 40 KHz), followed by centrifugation at 13,000× g and 4 °C for 10 min. The supernatant was transferred to sample vials for LC-MS/MS analysis.
Quality control sample: As a part of the system conditioning and quality control process, a pooled quality control sample (QC) was prepared by mixing equal volumes of all samples. The QC samples were disposed of and tested in the same manner as the analytic samples. This helped to represent the whole sample set, which would be injected at regular intervals (every 5–15 samples) in order to monitor the stability of the analysis.
UHPLC-MS/MS analysis: The LC-MS/MS analysis of the sample was conducted on a Thermo UHPLC-Q Exactive HF-X system equipped with an ACQUITY HSS T3 column (100 mm × 2.1 mm i.d., 1.8 μm; Waters Corporation, Milford, NH, USA) at Majorbio Bio-Pharm Technology Co. Ltd. (Shanghai, China). The mobile phases consisted of 0.1% formic acid in water: acetonitrile (95:5, v/v) (solvent A) and 0.1% formic acid in acetonitrile/isopropanol/water (47.5:47.5, v/v) (solvent B). The flow rate was 0.40 mL/min, and the column temperature was 40 °C. MS conditions: the mass spectrometric data were collected using a Thermo UHPLC-Q Exactive HF-X Mass Spectrometer equipped with an electrospray ionization (ESI) source operating in positive mode and negative mode. The optimal conditions were set as follows: source temperature at 425 °C; sheath gas flow rate at 50 arb; Aux gas flow rate at 13 arb; ion-spray voltage floating (ISVF) at −3500V in negative mode and 3500 V in positive mode, respectively; Normalized collision energy, 20-40-60V rolling for MS/MS. Full MS resolution was 60,000, and MS/MS resolution was 7500. Data acquisition was performed via the Data Dependent Acquisition (DDA) mode. The detection was carried out over a mass range of 70–1050 m/z.
Data analysis: The pretreatment of LC/MS raw data was performed by Progenesis QI version 2.0 (Waters Corporation, Milford, NH, USA) software, and a three-dimensional data matrix in CSV format was exported. The information in this three-dimensional matrix included: sample information, metabolite name, and mass spectral response intensity. Internal standard peaks, as well as any known false positive peaks (including noise, column bleed, and derivatized reagent peaks), were removed from the data matrix, deredundant, and peak pooled. At the same time, the metabolites were identified by searching databases, and the main databases were the HMDB (http://www.hmdb.ca/ (accessed on 10 March 2022)), Metlin (https://metlin.scripps.edu/ (accessed on 11 March 2022)). The data matrix obtained by searching the database was uploaded to the Majorbio cloud platform (https://cloud.majorbio.com (accessed on 11 March 2022)) for data analysis. First, the data matrix was pre-processed, as follows: At least 80% of the metabolic features detected in any set of samples were retained. After filtering, for specific samples with metabolite levels below the lower limit of quantification, the minimum metabolite value was estimated, and each metabolic signature was normalized to the sum. To reduce the errors caused by sample preparation and instrument instability, the response intensities of the sample mass spectrometry peaks were normalized using the sum normalization method to obtain the normalized data matrix. Meanwhile, the variables of QC samples with relative standard deviation (RSD) > 30% were excluded and log10 logarithmicized to obtain the final data matrix for subsequent analysis. Then, the R package “ropls”(Version 1.6.2) was used to perform principal component analysis (PCA) and orthogonal least partial squares discriminant analysis (OPLS-DA), and 7-cycle interactive validation evaluating the stability of the model. The metabolites with VIP > 1, p < 0.05 were determined as significantly different metabolites based on the Variable importance in the projection (VIP) obtained by the OPLS-DA model and the p-value generated by Student’s t test. Differential metabolites among the two groups were mapped into their biochemical pathways via metabolic enrichment and pathway analysis based on the KEGG database (http://www.genome.jp/kegg/ (accessed on 20 March 2022)). These metabolites could be classified according to the pathways they are involved in or the functions they perform. The principle was that the annotation analysis of a single metabolite develops into an annotation analysis of a group of metabolites. Python package “scipy.stats” (https://docs.scipy.org/doc/scipy/ (accessed on 20 March 2022)) was used to perform enrichment analysis to obtain the most relevant biological pathways for experimental treatments.
4.6. Quantitative RT-PCR Analysis
The RNA samples used for the qRT-PCR and transcriptome sequencing were identical. To evaluate the accuracy and reproducibility of the RNA-sequenced (RNA-Seq) expression profiles, quantitative real-time polymerase chain reaction (qRT-PCR) analysis was performed to analyze the expression levels of regulated genes at four distinct time points. The primer pairs (Table S1) for the candidate genes were designed on the NCBI website. Quantitative RT-PCR was run in a 7900 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) with the SYBR Green detection method to verify the transcriptome sequencing results. Quantitative real-time PCR (qRT-PCR) was performed in a 20 μL reaction volume containing 10 μL of SYBR Green Master Mix (Vazyme Biotech, Nanjing, China).
4.7. Nematode Motility and Proliferation in Salicylic Acid Treatment
Salicylic acid (SA) solution: 0.01 g of salicylic acid was dissolved in 10 mL of ethanol solution (5 mL of ethanol and 5 mL of sterile water) at a concentration of 0.1% (w/v) of salicylic acid. SA treatments were in two ways: (1) 100 µL of suspension containing approximately 1000 nematodes was dropped into 10 mL of SA solution and shaken well, left for 30 min, and the movement of nematodes was observed with a light microscope. A 10 mL ethanol solution (5 mL of ethanol and 5 mL of sterile water) was used as a control. (2) 100 µL of suspension containing approximately 1000 nematodes was added onto potato dextrose agar (PDA) covered with B. cinerea and sprayed with 1mL of SA (0.1%) solution. And then, the nematodes were cultured in the dark at 28 °C for 7 days. The nematodes were collected using the Baermann funnel method for approximately 12 h and counted under a light microscope. The same number of nematodes was incubated on PDA plates and sprayed 1 mL of ethanol solution (ethanol/sterile water = 1:1 (v:v)) as a control group.
4.8. Statistical Analysis
Results were expressed as percentages using nonparametric methods. The data about gene expression trends after treatments were analyzed according to the methods of Livak and Schmittgen [87], using a one-way analysis of variance followed by Tukey’s honest significant difference (HSD) post hoc test. The analysis of the Venn diagram and heatmap was performed using R version 3.6.2. In addition, the graphs were created using GraphPad Prism (Patterns & Practices., Redmond, WA, USA) and Adobe Photoshop CS6 (64-bit) software (Adobe, San Jose, CA, USA). Functional analysis was performed using GraphPad Prism (Patterns & Practices, Redmond, WA, USA).
5. Conclusions
Under PWN stress, most of the DEGs were downregulated, which potentially led to the physiologic disorders of P. thunbergii. We systematically analyzed DEGs that were potentially related to PWN resistance. Our results demonstrated that photosynthesis, calcium-triggered immunity, and resistance phytohormone were rapidly induced to respond to the PWN infection (at 1 and 3 dpi). Furthermore, the increased activity of ROS-scavenging enzymes was a beneficial reaction to limit the rapid progression of disease in P. thunbergii. In the early stage of disease development (at 7 dpi), the metabolism of flavonoids might be important in influencing the delayed onset of disease in P. thunbergii. PWN-resistant P. thunbergii exhibited higher antioxidant enzyme activities, photosynthesis capacities, and JA pathway mediation. Overall, we revealed that nematode-resistant and susceptible P. thunbergii had different defense mechanisms in response to PWN. These results provide an important basis for the application of PWN-resistant plants in future artificial afforestation.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ijms25105156/s1.
Author Contributions
J.Y. and X.W. guided the research. T.S. and A.Y. performed the experiments and analyzed the data. T.S. wrote the manuscript. Y.W. (Wang Yahui) and Y.W. (Wang Yang) provided helpful comments on the work and manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Natural Science Foundation of Jiangsu Province of China (BK20230393), the National Key Research and Development Program of China (2021YFD1400900), the Key Laboratory of National Forestry and Grassland Administration on Prevention and Control Technology of Pine Wilt Disease, and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data described in this study can be found in the article and the Supplementary Materials.
Acknowledgments
We thank the Key Laboratory of National Forestry and Grassland Administration on Prevention and Control Technology of Pine Wilt Disease for their support of this project. The authors thank Frank Boehm for revising the article and touching up the language. We thank Weiliang Kong, Lanxiang Lu, and Tongyue Wen for useful discussions; and we thank Lin Rui and Tongyue Wen for assistance in method optimization.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Linton, J.M.; Barne, H.M.; Seale, R.D.; Jone, P.D.; Lowell, E.C.; Hummel, S.S. Suitability of live and fire-killed small-diameter ponderosa and lodgepole pine trees for manufacturing a new structural wood composite. Bioresour. Technol. 2010, 101, 6242–6247. [Google Scholar] [CrossRef] [PubMed]
- Idris, D.; Osman, Ö. Price premiums for certified roundwood: Evidence from auction sales in Turkey. J. For. Res. 2021, 26, 395–399. [Google Scholar]
- Yoon, C.J.; Choi, W.S.; Kang, H.S.; Kim, H.J.; Lee, W.T.; Lee, J.S.; Lee, S.; Son, S.Y.; Lee, C.H.; Sohn, U.D.; et al. Pinus thunbergii Parl. Extracts Reduce Acute Inflammation by Targeting Oxidative Stress. Evid. Based Complement. Altern. Med. 2021, 2021, 7924645. [Google Scholar] [CrossRef] [PubMed]
- Süntar, I.; Demirel, M.A.; Taban, K.; Ceribasi, A.O.; Gok, H.N.; Metkin, G. Assessment of the preventive activity of Pinus brutia Ten. (Pinaceae) against in vivo acute lung injury model. Phytochem. Lett. 2023, 58, 8–18. [Google Scholar] [CrossRef]
- Ding, X.L.; Lin, S.X.; Zhao, R.; Ye, J.R. First report of needle blight on Pinus thunbergii Parl. caused by Fusarium proliferatum in China. Plant Dis. 2022, 106, 2989. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Lin, M.; Li, W.; Fang, Z. The occurrence of a pine wilting disease caused by a nematode found in Nanjing. For. Pest. Dis. 1983, 4, 1–5. [Google Scholar]
- Menendez-Gutierrez, M.; Alonso, M.; Diaz, R. Assessing genetic variation in resistance to pinewood nematode (Bursaphelenchus xylophilus) in Pinus radiata D. Don. Half-Sib. Families. For. 2021, 12, 1474. [Google Scholar]
- Mota, M.M.; Vieira, P. Pine Wilt Disease: A Worldwide Threat to Forest Ecosystems; Springer: Dordrecht, The Netherlands, 2008; ISBN 978-1-4020-8454-6. [Google Scholar]
- Akbulut, S.; Stamps, W.T. Insect vectors of the pinewood nematode: A review of the biology and ecology of Monochamus species. For. Pathol. 2012, 42, 89–99. [Google Scholar] [CrossRef]
- Gruffudd, H.R.; Jenkins, T.A.R.; Evans, H.F. Using an evapo-transpiration model (ETpN) to predict the risk and expression of symptoms of pine wilt disease (PWD) across Europe. Biol. Invasions 2016, 18, 2823–2840. [Google Scholar] [CrossRef]
- Yano, M. Investigation on the cause of pine mortality in Nagasaki. Prefect. Sanrinkoho 1913, 4, 1–14. [Google Scholar]
- Liu, B.; Liu, Q.; Zhou, Z.; Yin, H.; Xie, Y.; Wei, Y. Two terpene synthases in resistant Pinus massoniana contribute to defence against Bursaphelenchus xylophilus. Plant Cell Environ. 2020, 44, 257–274. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Wu, X.Q.; Wen, T.Y.; Feng, Y.Q.; Zhang, Y. Terpene production varies in Pinus thunbergii Parl. with different levels of resistance, with potential effects on pinewood nematode behavior. Forests 2022, 13, 1140. [Google Scholar] [CrossRef]
- Vicente, C.; Espada, M.; Vieira, P.; Mota, M. Erratum to: Pine Wilt Disease: A threat to European forestry. Eur. J. Plant Pathol. 2012, 133, 497. [Google Scholar] [CrossRef]
- De la Fuente, B.; Saura, S.; Beck, P.S. Predicting the spread of an invasive tree pest: The pine wood nematode in Southern Europe. J. Appl. Ecol. 2018, 55, 2374–2385. [Google Scholar] [CrossRef]
- Li, M.; Li, H.; Sheng, R.C.; Sun, H.; Sun, S.H.; Chen, F.M. The first record of Monochamus saltuarius (Coleoptera; Cerambycidae) as vector of Bursaphelenchus xylophilus and its new potential hosts in China. Insects 2020, 11, 636. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, H.; Ding, X.L.; Wang, L.; Wang, X.; Chen, F.M. The detection of pine wilt disease: A literature review. Int. J. Mol. Sci. 2022, 23, 10797. [Google Scholar] [CrossRef] [PubMed]
- Mallez, S.; Castagnone, C.; Lombaert, E.; Castagnone-Sereno, P.; Guillemaud, T. Inference of the worldwide invasion routes of the pinewood nematode Bursaphelenchus xylophilus using approximate Bayesian computation analysis. Peer Community J. 2021, 1, e56. [Google Scholar] [CrossRef]
- De la Fuente, B.; Beck, P. Management measures to control pine wood nematode spread in Europe. J. Appl. Entomol. 2019, 56, 2577–2580. [Google Scholar] [CrossRef]
- Fujimoto, Y.; Toda, T.; Nishimura, K.; Yamate, H.; Fuyuno, S. Breeding project on resistance to the pine-wood nematode. An outline of the research and the achievement of the project for ten years. Bull. For. Tree Breed. Inst. 1989, 7, 1–84. [Google Scholar]
- Maruyama, T.E.; Hosoi, Y. Post-maturation treatment improves and synchronizes somatic embryo germination of three species of Japanese pines. Plant Cell Tiss. Org. 2012, 110, 45–52. [Google Scholar] [CrossRef]
- Hirao, T.; Matsunaga, K.; Hirakawa, H.; Shirasawa, K.; Isoda, K.; Mishima, K.; Tamura, M.; Watanabe, A. Construction of genetic linkage map and identification of a novel major locus for resistance to pine wood nematode in Japanese black pine (Pinus thunbergii). BMC Plant Biol. 2019, 19, 424. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, E.; Hosoi, Y.; Ishii, K. Somatic embryo production and plant regeneration of japanese black pine (Pinus thunbergii Parl). J. For. Res. 2005, 10, 403–407. [Google Scholar] [CrossRef]
- Sun, T.; Wang, Y.; Zhu, L.; Wu, X.Q.; Ye, J.R. Plant regeneration by somatic embryogenesis in Pinus thunbergii resistant to the pine wood nematode. Can. J. For. Res. 2019, 49, 1604–1612. [Google Scholar] [CrossRef]
- Carneros, E.; Celestino, C.; Klimaszewska, K.; Park, Y.S.; Toribio, M.; Bonga, J.M. Plant regeneration in stone pine (Pinus pinea L.) by somatic embryogenesis. Plant Cell Tiss. Org. 2009, 98, 165–178. [Google Scholar] [CrossRef]
- Sun, T.; Wang, Y.; Zhu, L.; Liu, X.W.; Wang, Q.T.; Ye, J.R. Evaluation of somatic embryo production during embryogenic tissue proliferation stage using morphology, maternal genotype, proliferation rate and tissue age of Pinus thunbergii Parl. J. For. Res. 2022, 33, 445–454. [Google Scholar] [CrossRef]
- Montalban, I.A.; De Diego, N.; Moncaleán, P. Enhancing initiation and proliferation in radiata pine (Pinus radiata d. Don) somatic embryogenesis through seed family screening, zygotic embryo staging and media adjustments. Acta Physiol. Plant 2012, 34, 451–460. [Google Scholar] [CrossRef]
- Yang, F.; Xia, X.R.; Ke, X.; Ye, J.R.; Zhu, L. Somatic embryogenesis in slash pine (Pinus elliottii engelm): Improving initiation of embryogenic tissues and maturation of somatic embryos. Plant Cell Tiss. Org. 2020, 143, 159–171. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, Z.; Seidl, M.F.; Majer, A.; Jakse, J.; Javornik, B.; Thomma, B.P.H.J. Broad taxonomic characterization of Verticillium wilt resistance genes reveals ancient origin of the tomato Ve1 immune receptor. Mol. Plant Pathol. 2017, 18, 195–209. [Google Scholar] [CrossRef] [PubMed]
- De la O Leyva-Perez, M.; Jimenez-Ruiz, J.; Cabanas, C.G.L.; Valverde-Corredor, A.; Barroso, J.B.; Francisco, L.; Mercado-Blanco, J. Tolerance of olive (Olea europaea) cv Frantoio to Verticillium dahliae relies on both basal and pathogen-induced differential transcriptomic responses. New Phytol. 2018, 217, 671–686. [Google Scholar] [CrossRef]
- Lee, I.H.; Kim, J.; Woo, K.S.; Jang, K.H.; Kim, Y.H.; Shim, D. De novo assembly and transcriptome analysis of the Pinus densiflora response to pine wilt disease in nature. Plant Biotechnol. Rep. 2018, 12, 229–236. [Google Scholar] [CrossRef]
- Modesto, I.; Sterck, L.; Arbona, V.; Gomez-Cadenas, A.; Carrasquinho, I.; Van de Peer, Y.; Miguel, C.M. Insights into the mechanisms implicated in Pinus pinaster resistance to pinewood nematode. Front. Plant Sci. 2021, 12, 690857. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Wu, X.Q.; Wen, T.Y.; Feng, Y.Q.; Zhang, Y. Transcriptomic analysis reveals differentially expressed genes associated with pine wood nematode resistance in resistant Pinus thunbergii. Tree Physiol. 2023, 43, 995–1008. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Rahman, M.U.; Wu, X.; Ye, J.R. Resistant and Susceptible Pinus thunbergii ParL. Show Highly Divergent Patterns of Differentially Expressed Genes during the Process of Infection by Bursaphelenchus xylophilus. Int. J. Mol. Sci. 2023, 24, 14376. [Google Scholar] [CrossRef] [PubMed]
- Verma, D.; Bhagat, P.K.; Sinha, A.K. MKK3-MPK6-MYC2 module positively regulates ABA biosynthesis and signalling in Arabidopsis. J. Plant Biochem. Biotechnol. 2020, 29, 785–795. [Google Scholar] [CrossRef]
- Romero-Hernandez, G.; Martinez, M. Opposite roles of MAPKKK17 and MAPKKK21 against Tetranychus urticae in Arabidopsis. Front. Plant Sci. 2022, 13, 1038866. [Google Scholar] [CrossRef] [PubMed]
- Tajdel, M.; Mituła, F.; Ludwików, A. Regulation of Arabidopsis MAPKKK18 by ABI1 and SnRK2, components of the ABA signaling pathway. Plant Signal. Behav. 2016, 11, e1139277. [Google Scholar] [CrossRef]
- Zhao, G.; Cheng, Q.; Zhao, Y.; Wu, F.; Mu, B.; Gao, J.; Yang, L.; Yan, J.; Zhang, H.; Cui, X.; et al. The abscisic acid–responsive element binding factors MAPKKK18 module regulates abscisic acid–induced leaf senescence in Arabidopsis. J. Biol. Chem. 2023, 299, 103060. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; He, Y.; Kabahuma, M.; Chaya, T.; Kelly, A.; Borrego, E.; Bian, Y.; Kasmi, F.E.; Yang, L.; Teixeira, P.; et al. A gene encoding maize caffeoyl-CoA O-methyltransferase confers quantitative resistance to multiple pathogens. Nat. Genet. 2017, 49, 1364–1372. [Google Scholar] [CrossRef] [PubMed]
- Hirao, T.; Fukatsu, E.; Watanabe, A. Characterization of resistance to pine wood nematode infection in Pinus thunbergii using suppression subtractive hybridization. BMC Plant Biol. 2012, 12, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, J.; Anjo, S.; Fonseca, L.; Egas, C.; Manadas, B.; Abrantes, I. Bursaphelenchus xylophilus and B. mucronatus secretomes: A comparative proteomic analysis. Sci. Rep. 2016, 6, 39007. [Google Scholar] [PubMed]
- Rodrigues, A.M.; Carrasquinho, I.; António, C. Primary metabolite adjustments associated with pinewood nematode resistance in Pinus pinaster. Front. Plant Sci. 2021, 12, 777681. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, T.B.; Aucique-Perez, C.E.; Einhardt, A.M.; Rodrigues, F.A. Wheat susceptibility to blast is enhanced by a photosynthetic. J. Phytopathol. 2021, 160, 630–639. [Google Scholar] [CrossRef]
- Chen, Y.E.; Cui, J.M.; Su, Y.Q.; Yuan, S.; Yuan, M.; Zhang, H.Y. Influence of stripe rust infection on the photosynthetic characteristics and antioxidant system of susceptible and resistant wheat cultivars at the adult plant stage. Int. J. Mol. Sci. 2015, 6, 779. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, Y.; Yu, R.; Zhang, L.; Yang, Y.; Xiao, D.; Li, A.; Wang, Y. Integrated transcriptomic and metabolomic profiles reveal adaptive responses of three poplar varieties against the bacterial pathogen Lonsdalea populi. Plant Cell Environ. 2023, 46, 306–321. [Google Scholar] [CrossRef] [PubMed]
- Berens, M.L.; Berry, H.M.; Mine, A.; Argueso, C.T.; Tsuda, K. Evolution of hormone signaling networks in plant defense. Annu. Rev. Phytopathol. 2017, 55, 401–425. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Y.; Wang, D.D.; Fang, X.; Chen, X.Y.; Mao, Y.B. Plant specialized metabolism regulated by jasmonate signaling. Plant Cell Physiol. 2020, 60, 2638–2647. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Shu, J.; Xue, H.; Zhang, W.; Zhang, Y.; Liu, Y.; Fang, L.; Wang, Y.; Wang, H. The gut microbiota in camellia weevils arc influenced by plant secondary metabolites and contribute to saponin degradation. Msystems 2020, 5, e00692-19. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.Y.; Huang, Y.C.; Ou, S.L. ERF73/HRE1 is involved in H2O2 production via hypoxia-inducible Rboh gene expression in hypoxia signaling. Protoplasma 2017, 254, 1705–1714. [Google Scholar] [CrossRef]
- Yamauchi, T.; Yoshioka, M.; Fukazawa, A.; Mori, H.; Nishizawa, N.K.; Tsutsumi, N.; Yoshioka, H.; Nakazono, M. An NADPH oxidase RBOH functions in rice roots during lysigenous aerenchyma formation under oxygen-deficient conditions. Plant Cell 2017, 29, 775–790. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Xiao, A.; Dong, R.; Fan, Y.; Zhang, X.; Liu, C.; Wang, C.; Zhu, H.; Duanmu, D.; Cao, Y.; et al. Suppression of innate immunity mediated by the CDPK-Rboh complex is required for rhizobial colonization in Medicago truncatula nodules. New Phytol. 2018, 220, 425–434. [Google Scholar] [CrossRef]
- Lu, M.; Tang, X.; Zhou, J. Arabidopsis NHO1 is required for general resistance against Pseudomonas bacteria. Plant Cell 2001, 13, 437–447. [Google Scholar] [CrossRef][Green Version]
- Kang, L.; Li, J.; Zhao, T.; Xiao, F.; Tang, X.; Thilmony, R.; He, S.; Zhou, J.M. Interplay of the Arabidopsis nonhost resistance gene NHO1 with bacterial virulence. Proc. Natl. Acad. Sci. USA 2003, 100, 3519–3524. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Wang, R.; Khaskhali, S.; Gao, Z.; Guo, W.; Wang, H.; Niu, X.; He, C.; Yu, X.; Chen, Y. A Novel Glycerol Kinase Gene OsNHO1 regulates resistance to bacterial blight and blast diseases in rice. Front. Plant Sci. 2022, 12, 800625. [Google Scholar] [CrossRef]
- Gupta, A.; Bansal, A.; Hashimoto-Torii, K. HSP70 and HSP90 in neurodegenerative diseases. Neurosci. Lett. 2020, 716, 134678. [Google Scholar] [CrossRef]
- Bhattarai, K.K.; Li, Q.; Liu, Y.; Dinesh-Kumar, S.P.; Kaloshian, I. The Mi-1-mediated pest resistance requires Hsp90 and Sgt1. Plant Physiol. 2007, 144, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.S.; Li, Z.Y.; Chen, Y.; Chen, M.; Li, L.C.; Ma, Y.Z. Heat shock protein 90 in plants: Molecular mechanisms and roles in stress responses. Int. J. Mol. Sci. 2012, 13, 15706–15723. [Google Scholar] [CrossRef] [PubMed]
- Ved Prakash, G.; Pandey, S.; Srivastava, S.; Shukla, P.; Kumar, N.; Kumari, M.; Katiyar, R.; Singh, S.; Mishra, A. Chitosan fabricated biogenic silver nanoparticles (Ch@BSNP) protectively modulate the defense mechanism of tomato during bacterial leaf spot (BLS) disease. Plant Physiol. Biochem. 2023, 197, 107637. [Google Scholar]
- Aerts, N.; Mendes, P.M.; Van Wees, S.C.M. Multiple levels of crosstalk in hormone networks regulating plant defense. Plant J. 2021, 105, 489–504. [Google Scholar] [CrossRef]
- Andreasson, E.; Jenkins, T.; Brodersen, P.; Thorgrimsen, S.; Petersen, N.H.T.; Zhu, S.; Qiu, J.L.; Micheelsen, P.; Rocher, A.; Petersen, M.; et al. The MAP kinase substrate MKS1 is a regulator of plant defense responses. EMBO J. 2005, 24, 2579–2589. [Google Scholar] [CrossRef] [PubMed]
- Wiermer, M.; Feys, B.J.; Parker, J.E. Plant immunity: The EDS1 regulatory node. Curr. Opin. Plant Biol. 2005, 1, 383–389. [Google Scholar] [CrossRef]
- Pieterse, C.M.J.; Van der Does, D.; Zamioudis, C.; Leon-Reyes, A.; Wees, S.C.M.V. Hormonal modulation of plant immunity. Annu. Rev. Cell Dev. Biol. 2011, 28, 489–521. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, X. Salicylic acid: Biosynthesis, perception, and contributions to plant immunity. Curr. Opin. Plant Biol. 2019, 50, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wei, Y.; Xu, L.; Hao, Y.; Chen, X.; Zhou, Z. Transcriptomic profiling reveals differentially expressed genes associated with pine wood nematode resistance in masson pine (Pinus massoniana Lamb.). Sci. Rep. 2017, 7, 4693. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Liang, G.; Huang, A.; Zhang, F.; Guo, W. Comparative study on the MRNA Expression of Pinus massoniana infected by Bursaphelenchus xylophilus. J. For. Res. 2020, 2020, 75–86. [Google Scholar] [CrossRef]
- Shin, H.; Lee, H.; Woo, K.S.; Noh, E.W.; Koo, Y.B.; Lee, K.J. Identification of genes upregulated by pinewood nematode inoculation in Japanese red pine. Tree Physiol. 2009, 29, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Nose, M.; Shiraishi, S. Comparison of the gene expression profiles of resistant and non-resistant Japanese black pine inoculated with pine wood nematode using a modified Long SAGE technology. For. Pathol. 2011, 41, 143–145. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Z.; Gao, J.; Wang, P.; Hu, T.; Wang, Z.; Hou, Y.; Wan, Y.; Liu, W.; Xie, S.; et al. Arabidopsis Duodecuple Mutant of PYL ABA Receptors Reveals PYL Repression of ABA-Independent SnRK2 Activity. Cell Rep. 2018, 23, 3340–3351. [Google Scholar] [CrossRef] [PubMed]
- Ambawat, S.; Sharma, P.; Yadav, N.R.; Yadav, R.C. MYB transcription factor genes as regulators for plant responses: An overview. Physiol. Mol. Biol. Plants 2013, 19, 307–321. [Google Scholar] [CrossRef]
- Vos, I.A.; Verhage, A.; Watt, L.; Vlaardingerbroek, I.; Wees, S.C.V. Abscisic acid is essential for rewiring of jasmonic acid-dependent defenses during herbivory. BioRxiv 2019. [Google Scholar] [CrossRef]
- Chini, A.; Boter, M.; Solano, R. Plant oxylipins: COI1/JAZs/MYC2 as the core jasmonic acid-signalling module. FEBS J. 2009, 276, 4682–4692. [Google Scholar] [CrossRef] [PubMed]
- Couto, D.; Zipfel, C. Regulation of pattern recognition receptor signaling in plants. Nat. Rev. Immunol. 2016, 16, 537–552. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, D.; Trindade, C.; Usie, A.; Meireles, B.; Barbosa, P.; Fortes, A.; Resquita, C.; Costa, R.; Ramos, A. Expression profiling in Pinus pinaster in response to infection with the pine wood nematode Bursaphelenchus xylophilus. Forests 2017, 8, 279. [Google Scholar] [CrossRef]
- Hwang, H.S.; Han, J.Y.; Choi, Y.E. Enhanced accumulation of pinosylvin stilbenes and related gene expression in Pinus strobus after infection of pine wood nematode. Tree Physiol. 2021, 41, 1972–1987. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, D.; Trindade, C.; Usie, A.; Meireles, B.; Fortes, A.M.; Guimaraes, J.B.; Simoes, F.; Costa, R.L.; Ramos, A.M. Comparative transcriptomic response of two Pinus species to infection with the pine wood nematode Bursaphelenchus xylophilus. Forests 2020, 11, 204. [Google Scholar] [CrossRef]
- Hückelhoven, R. Cell wall-associated mechanisms of disease resistance and susceptibility. Annu. Rev. Phytopathol. 2007, 45, 101–127. [Google Scholar] [CrossRef] [PubMed]
- Deepak, S.; Shailasree, S.; Kini, R.K.; Muck, A.; Mithofer, A.; Shetty, S.H. Hydroxyproline-rich glycoproteins and plant defense. J. Phytopathol. 2010, 158, 585–593. [Google Scholar]
- Shaipulah, N.F.M.; Muhlemann, J.K.; Woodworth, B.D.; Moerkercke, A.; Verdonk, J.C.; Ramirez, A.A.; Haring, M.A.; Dudareva, N.; Schuurink, R.C. CCoAOMT down-regulation activates anthocyanin biosynthesis in Petunia. Plant Physiol. 2016, 170, 717–731. [Google Scholar] [CrossRef] [PubMed]
- Boerjan, W.; Ralph, J.; Baucher, M. Lignin biosynthesis. Annu. Rev. Plant Biol. 2003, 54, 519–546. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.X.; Ni, R.; Gao, S. Molecular cloning and characterization of two distinct caffeoyl CoA O-methyltransferases (CCoAOMTs) from the liverwort Marchantia paleacea. Plant Sci. 2022, 314, 111102. [Google Scholar] [CrossRef]
- Wagner, A.; Tobimatsu, Y.; Phillips, L.; Flint, H.; Torr, R.; Donaldson, L.; Pears, L.; Ralph, J. CCoAOMT suppression modifies lignin composition in Pinus radiata. Plant J. 2011, 67, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Sattler, S.E.; Funnell-Harris, D.L. Modifying lignin to improve bioenergy feedstocks: Strengthening the barrier against pathogens. Front. Plant Sci. 2013, 4, 70. [Google Scholar] [CrossRef] [PubMed]
- Kumari, G.; Nigam, V.K.; Pandey, D.M. The molecular docking and molecular dynamics study of flavonol synthase and flavonoid 3′-monooxygenase enzymes involved for the enrichment of kaempferol. J. Biomol. Struct. Dyn. 2023, 41, 2478–2491. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Wang, Y.; Wu, X.; Ye, J.R.; Cheng, F. Promoting the application of Pinus thunbergii Parl. to enhance the growth and survival rates of post-germination somatic plantlets. BMC Plant Biol. 2023, 23, 195. [Google Scholar] [CrossRef] [PubMed]
- Toda, T. Studies on the breeding for resistance to the pine wilt disease in Pinus densiflora and P. thunbergii. Bull. For. Tree Breed. Cent. 2004, 20, 83–217. [Google Scholar]
- Wu, X.; Zhang, Y.; Chen, W.; Liu, J.; Ye, J.R. Resistance and histopathological observation of wilt-resistant Pinus thunbergii families from Japan to Bursaphelenchus xylophilus. Acta Phytopathol. Sin. 2008, 38, 44–50. (In Chinese) [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).