Molecular Docking Approach for Biological Interaction of Green Synthesized Nanoparticles
Abstract
1. Introduction
2. Methodology
3. Concept of Nanotechnology
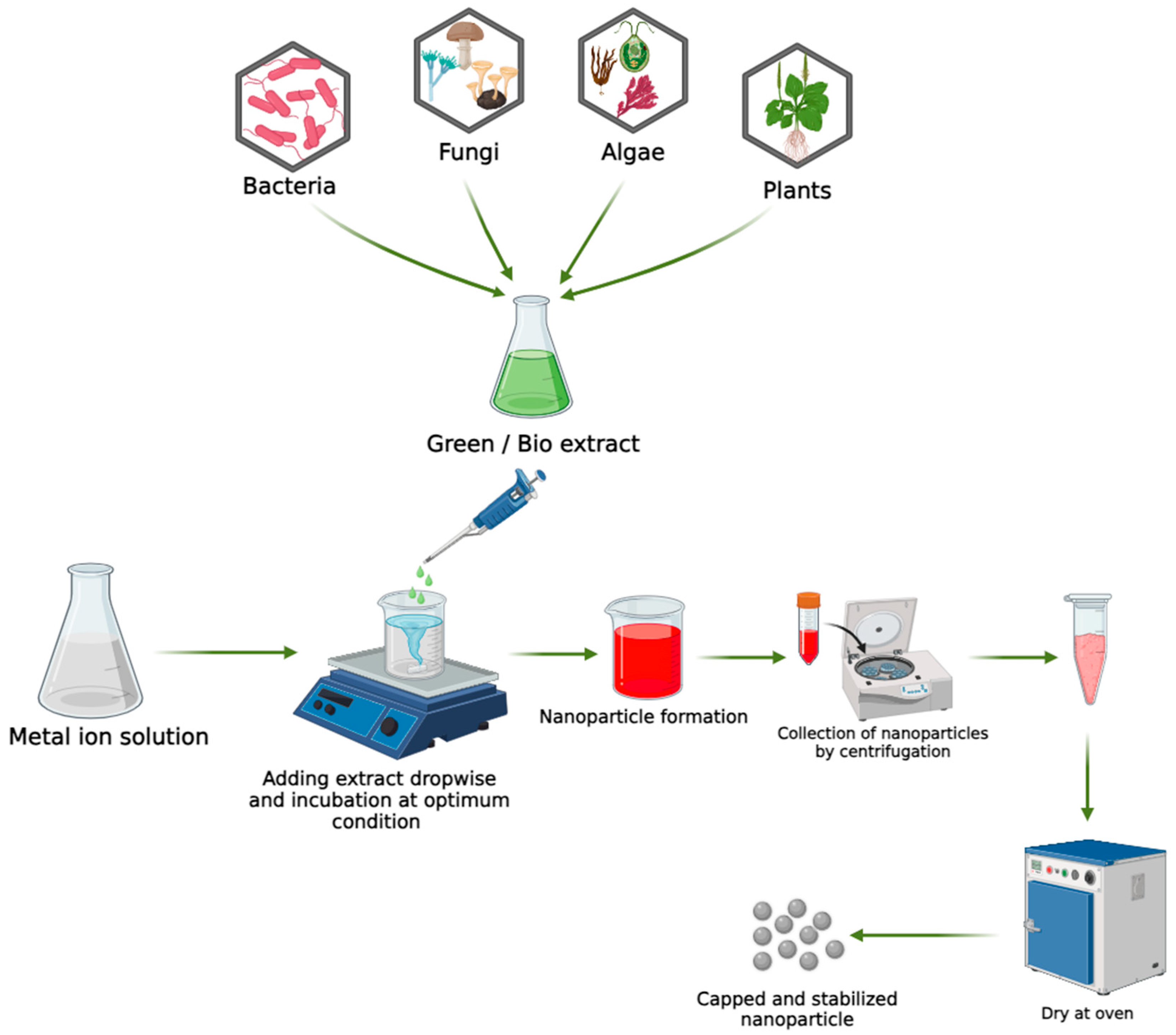
4. Green/Biological Approaches for the Synthesis of Metal Nanoparticles
4.1. Biological Synthesis Using Microorganisms
4.2. Biological Synthesis Using Fungi
4.3. Biosynthesis of NPs Using Algae
4.4. Biological Synthesis Using Plant Extracts
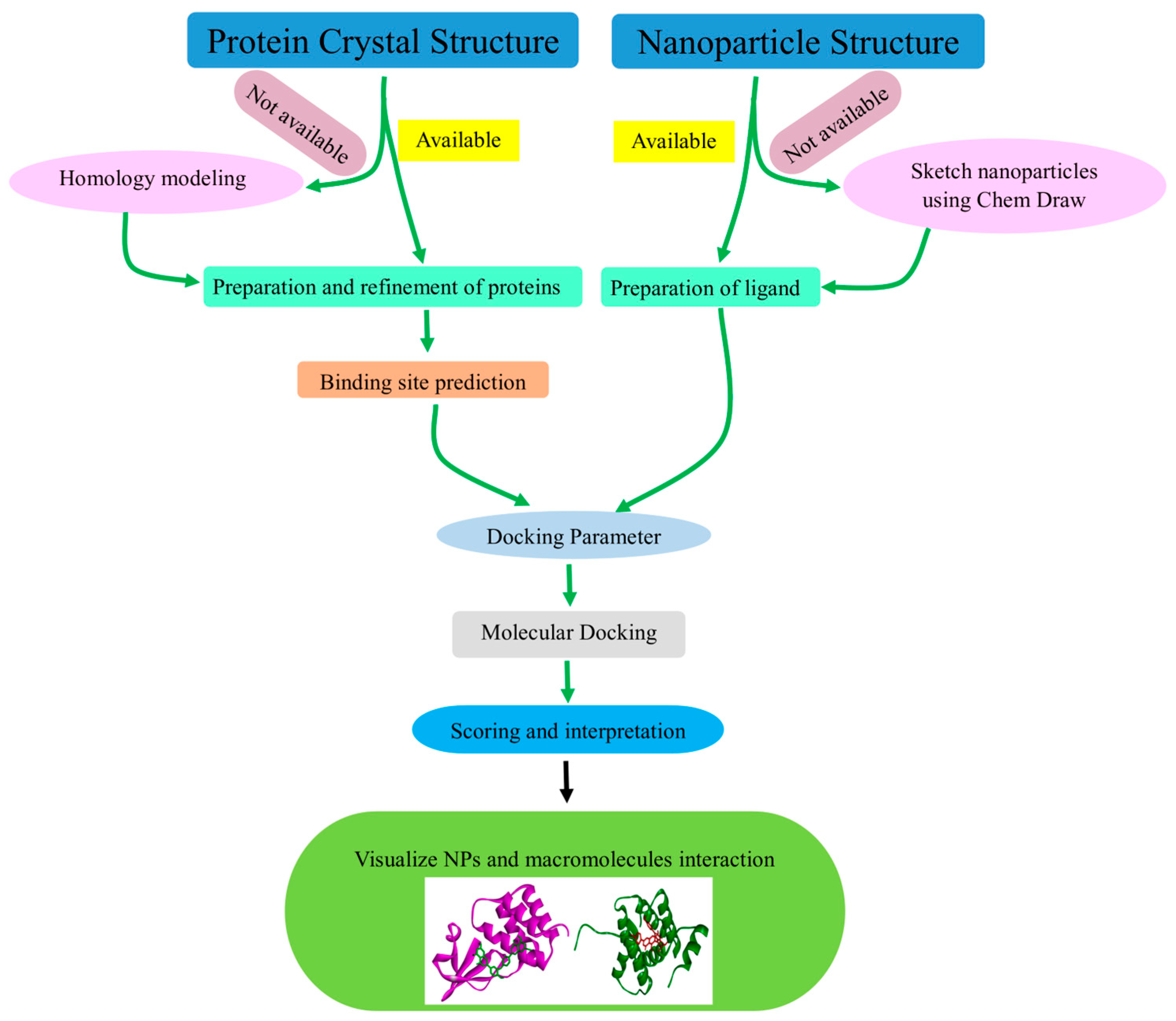
5. Software Utilized in NPs Molecular Docking
6. Analysis of Biomacromolecule–NP Interactions Using Molecular Docking
6.1. Zinc Oxide Nanoparticles
6.2. Copper Oxide Nanoparticles
6.3. Silver Nanoparticles
6.4. Gold Nanoparticles
6.5. Iron Oxide Nanoparticles
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gaur, M.; Misra, C.; Yadav, A.B.; Swaroop, S.; Maolmhuaidh, F.; Bechelany, M.; Barhoum, A. Biomedical Applications of Carbon Nanomaterials: Fullerenes, Quantum Dots, Nanotubes, Nanofibers, and Graphene. Materials 2021, 14, 5978. [Google Scholar] [CrossRef] [PubMed]
- Barhoum, A.; Pal, K.; Rahier, H.; Uludag, H.; Kim, I.S.; Bechelany, M. Nanofibers as new-generation materials: From spinning and nano-spinning fabrication techniques to emerging applications. Appl. Mater. Today 2019, 17, 1–35. [Google Scholar] [CrossRef]
- Rassaei, L.; Marken, F.; Sillanpää, M.; Amiri, M.; Cirtiu, C.M.; Sillanpää, M. Nanoparticles in electrochemical sensors for environmental monitoring. TrAC Trends Anal. Chem. 2011, 30, 1704–1715. [Google Scholar] [CrossRef]
- Islam, F.; Shohag, S.; Uddin, M.J.; Islam, M.R.; Nafady, M.H.; Akter, A.; Mitra, S.; Roy, A.; Emran, T.B.; Cavalu, S. Exploring the journey of zinc oxide nanoparticles (ZnO-NPs) toward biomedical applications. Materials 2022, 15, 2160. [Google Scholar] [CrossRef]
- Pagadala, N.S.; Syed, K.; Tuszynski, J. Software for Molecular Docking: A Review. Biophys. Rev. 2017, 9, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Cho, D.; Ko, K.C.; Lamiel-Garcia, O.S.; Bromley, T.; Lee, J.Y.; Illas, F. Effect of Size and Structure on the Ground-State and Excited-State Electronic Structure of TiO2 Nanoparticles. J. Chem. Theory Comput. 2016, 12, 3751–3763. [Google Scholar] [CrossRef] [PubMed]
- Carnal, F.; Clavier, A.; Stoll, S. Polypeptide Nanoparticle Interactions and Corona Formation Investigated by Monte Carlo Simulations. Polymers 2016, 8, 203. [Google Scholar] [CrossRef] [PubMed]
- Zhdanov, V.P.; Cho, N.J. Kinetics of the Formation of a Protein Corona around Nanoparticles. Math. Biosci. 2016, 282, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Volgin, I.V.; Larin, S.V.; Lyulin, A.V.; Lyulin, S.V. Coarse-Grained Molecular-Dynamics Simulations of Nanoparticle Diffusion in Polymer Nanocomposites. Polymer 2018, 145, 80–87. [Google Scholar] [CrossRef]
- Kokarneswaran, M.; Selvaraj, P.; Ashokan, T.; Perumal, S.; Sellappan, P.; Murugan, K.D.; Ramalingam, S.; Mohan, N.; Chandrasekaran, V. Discovery of carbon nanotubes in sixth century BC potteries from Keeladi, India. Sci. Rep. 2020, 10, 19786. [Google Scholar] [CrossRef]
- Chen, J.; Wei, S.; Xie, H. A brief introduction of carbon nanotubes: History, synthesis, and properties. J. Phys. Conf. Ser. 2021, 1948, 012184. [Google Scholar] [CrossRef]
- Kumari, S.C.; Dhand, V.; Padma, P.N. Green synthesis of metallic nanoparticles: A review. Nanomaterials 2021, 259–281. [Google Scholar] [CrossRef]
- Gupta, D.; Thakur, A.; Gupta, T.K. Green and sustainable synthesis of nanomaterials: Recent advancements and limitations. Environ. Res. 2023, 231, 116316. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, P.; Kaur, M.; Rathee, J.; Sharma, A. Unveiling Biological Synthesis of Metal Nanoparticles, their Characterization and Scopes: A Review. Int. J. Multidiscip. Res. 2023, 5, 1–24. [Google Scholar]
- Sukul, P.K.; Kar, C. Green Conversion Methods to Prepare Nanoparticle. Bioinspired and Green Synthesis of Nanostructures: A Sustainable Approach. In Bioinspired and Green Synthesis of Nanostructures: A Sustainable Approach; Wiley Online Library: New York, NY, USA, 2023; pp. 115–139. [Google Scholar]
- De, A.; Roopa, R.A.; Manasa, H.S.; Guin, M. Green Chemistry Principles and Spectroscopic Methods Applied to Nanomaterials. Nanobiomater. Perspect. Med. Appl. Diagn. Treat. Dis. 2023, 145, 54–91. [Google Scholar]
- Azizi, S.; Ahmad, M.B.; Namvar, F.; Mohamad, R. Green biosynthesis and characterization of zinc oxide nanoparticles using brown marine macroalga Sargassum muticum aqueous extract. Mater. Lett. 2014, 116, 275–277. [Google Scholar] [CrossRef]
- Banerjee, S.; Islam, J.; Mondal, S.; Saha, A.; Saha, B.; Sen, A. Proactive attenuation of arsenic-stress by nano-priming: Zinc Oxide Nanoparticles in Vigna mungo (L.) Hepper trigger antioxidant defense response and reduce root-shoot arsenic translocation. J. Hazard. Mater. 2023, 446, 130735. [Google Scholar] [CrossRef]
- Banerjee, S.; Mondal, S.; Islam, J.; Sarkar, R.; Saha, B.; Sen, A. Rhizospheric nano-remediation salvages arsenic genotoxicity: Zinc-oxide nanoparticles articulate better oxidative stress management, reduce arsenic uptake, and increase yield in Pisum sativum (L.). Sci. Total Environ. 2024, 913, 169493. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, Y.; Du, L.; Yao, H.; Wang, J.; Luo, G. Precipitation preparation of high surface area and porous nanosized ZnO by continuous gas-based impinging streams in unconfined space. Indus. Eng. Chem. Res. 2016, 55, 11943–11949. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Husen, A. Green synthesis, characterization and uses of palladium/platinum nanoparticles. Nanoscale Res. Lett. 2016, 11, 1–13. [Google Scholar] [CrossRef]
- Stepanov, A.L.; Nuzhdin, V.I.; Valeev, V.F.; Kreibig, U. Optical properties of metal nanoparticles. In Proceedings of the ICONO 2010: International Conference on Coherent and Nonlinear Optics, Kazan, Russia, 23–27 August 2010; SPIE: Bellingham, WA, USA, 2011; pp. 543–552. [Google Scholar]
- Basu, S.; Maji, P.; Ganguly, J. Rapid green synthesis of silver nanoparticles by aqueous extract of seeds of Nyctanthes arbor-tristis. Appl. Nanosci. 2015, 6, 1–5. [Google Scholar] [CrossRef]
- Abboud, Y.; Saffaj, T.; Chagraoui, A.; El Bouari, A.; Brouzi, K.; Tanane, O.; Ihssane, B. Biosynthesis, characterization and antimicrobial activity of copper oxide nanoparticles (CONPs) produced using brown alga extract (Bifurcaria bifurcata). Appl. Nanosci. 2014, 4, 571–576. [Google Scholar] [CrossRef]
- Naika, H.R.; Lingaraju, K.; Manjunath, K.; Kumar, D.; Nagaraju, G.; Suresh, D.; Nagabhushana, H. Green synthesis of CuO nanoparticles using Gloriosa superba L. extract and their antibacterial activity. J. Taibah Univ. Sci. 2018, 9, 7–12. [Google Scholar] [CrossRef]
- Taghavi Fardood, S.; Ramazani, A. Green synthesis and characterization of copper oxide nanoparticles using coffee powder extract. J. Nanostruct. 2016, 6, 167–171. [Google Scholar]
- Issaabadi, Z.; Nasrollahzadeh, M.; Sajadi, S.M. Green synthesis of the copper nanoparticles supported on bentonite and investigation of its catalytic activity. J. Clean. Prod. 2015, 142, 3584–3591. [Google Scholar] [CrossRef]
- Nagajyothi, P.C.; Muthuraman, P.; Sreekanth, T.V.M.; Kim, D.H.; Shim, J. Green synthesis: In-vitro anticancer activity of copper oxide nanoparticles against human cervical carcinoma cells. Arab. J. Chem. 2017, 10, 215–225. [Google Scholar] [CrossRef]
- Ndolomingo, M.J.; Meijboom, R. Determination of the surface area and sizes of supported copper nanoparticles through organothiol adsorption—Chemisorption. Appl. Surf. Sci. 2016, 390, 224–235. [Google Scholar] [CrossRef]
- Hortin, J.; Anderson, A.; Britt, D.; Jacobson, A.; Mclean, J. Copper oxide nanoparticle dissolution at alkaline pH is controlled by dissolved organic matter: Influence of soil-derived organic matter, wheat, bacteria, and nanoparticle coating. Environ. Sci. 2020, 7, 2618–2631. [Google Scholar] [CrossRef]
- Babu, M.G.; Gunasekaran, P. Production and structural characterization of crystalline silver nanoparticles from Bacillus cereus isolate. Colloids Surf. B Biointerfaces 2009, 74, 191–195. [Google Scholar] [CrossRef]
- Jo, J.H.; Singh, P.; Kim, Y.J.; Wang, C.; Mathiyalagan, R.; Jin, C.G.; Yang, D.C. Pseudomonas deceptionensis DC5-mediated synthesis of extracellular silver nanoparticles. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1576–1581. [Google Scholar] [CrossRef]
- Devi, L.S.; Joshi, S.R. Ultrastructures of silver nanoparticles biosynthesized using endophytic fungi. J. Microsc. Ultrastruct. 2015, 3, 29–37. [Google Scholar] [PubMed]
- Zhao, X.; Zhou, L.; RiazRajoka, M.S.; Yan, L.; Jiang, C.; Shao, D.; Jin, M. Fungal silver nanoparticles: Synthesis, application and challenges. Crit. Rev. Biotechnol. 2018, 38, 817–835. [Google Scholar] [CrossRef] [PubMed]
- Raja, S.; Ramesh, V.; Thivaharan, V. Green biosynthesis of silver nanoparticles using Calliandra haematocephala leaf extract, their antibacterial activity and hydrogen peroxide sensing capability. Arab. J. Chem. 2017, 10, 253–261. [Google Scholar] [CrossRef]
- Ibrahim, H.M.M. Green synthesis and characterization of silver nanoparticles using banana peel extract and their antimicrobial activity against representative microorganisms. J. Radiat. Res. Appl. Sci. 2015, 8, 265–275. [Google Scholar] [CrossRef]
- Kasithevar, M.; Saravanan, M.; Prakash, P.; Kumar, H.; Ovais, M.; Barabadi, H.; Shinwari, Z.K. Green synthesis of silver nanoparticles using Alysicarpus monilifer leaf extract and its antibacterial activity against MRSA and CoNS isolates in HIV patients. J. Interdiscip. Nanomed. 2017, 2, 131–141. [Google Scholar] [CrossRef]
- Jana, N.R.; Earhart, C.; Ying, J.Y. Synthesis of water-soluble and functionalized nanoparticles by silica coating. Chem. Mater. 2007, 19, 5074–5082. [Google Scholar] [CrossRef]
- Annamalai, J.; Nallamuthu, T. Characterization of biosynthesized gold nanoparticles from aqueous extract of Chlorella vulgaris and their anti-pathogenic properties. Appl. Nanosci. 2015, 5, 603–607. [Google Scholar] [CrossRef]
- Sharma, B.; Purkayastha, D.D.; Hazra, S.; Thajamanbi, M.; Bhattacharjee, C.R.; Ghosh, N.N.; Rout, J. Biosynthesis of fluorescent gold nanoparticles using an edible freshwater red alga, Lemanea fluviatilis (L.) C. Ag. and antioxidant activity of biomatrix loaded nanoparticles. Bioprocess Biosyst. Eng. 2014, 37, 2559–2565. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.; Bhuyan, B.; Purkayastha, D.D.; Dey, M.; Dhar, S.S. Green synthesis of gold nanoparticles using Pogestemon benghalensis (B) O. Ktz. leaf extract and studies of their photocatalytic activity in degradation of methylene blue. Mater. Lett. 2015, 148, 37–40. [Google Scholar] [CrossRef]
- Jafarizad, A.; Safaee, K.; Gharibian, S.; Omidi, Y.; Ekinci, D. Biosynthesis and in-vitro study of gold nanoparticles using mentha and pelargonium extracts. Proc. Mater. Sci. 2015, 11, 224–230. [Google Scholar] [CrossRef]
- Kumar, V.; Bano, D.; Mohan, S.; Singh, D.K.; Hasan, S.H. Sunlight-induced green synthesis of silver nanoparticles using aqueous leaf extract of Polyalthia longifolia and its antioxidant activity. Mater. Lett. 2016, 181, 371–377. [Google Scholar] [CrossRef]
- Swain, S.; Barik, S.K.; Behera, T.; Nayak, S.K.; Sahoo, S.K.; Mishra, S.S.; Swain, P. Green synthesis of gold nanoparticles using root and leaf extracts of Vetiveria zizanioides and Cannabis sativa and its antifungal activities. Bio Nano Sci. 2016, 6, 205–213. [Google Scholar] [CrossRef]
- Majumdar, R.; Bag, B.G.; Ghosh, P. Mimusops elengi bark extract mediated green synthesis of gold nanoparticles and study of its catalytic activity. Appl. Nanosci. 2015, 6, 521–528. [Google Scholar] [CrossRef]
- Ahmad, T.; Wani, I.A.; Ahmed, J.; Al-Hartomy, O.A. Effect of gold ion concentration on size and properties of gold nanoparticles in TritonX-100 based inverse microemulsions. Appl. Nanosci. 2014, 4, 491–498. [Google Scholar] [CrossRef]
- Guo, W.; Pleixats, R.; Shafir, A. Water-soluble gold nanoparticles: From catalytic selective Nitroarene reduction in water to refractive index sensing. Chem. Asian J. 2015, 10, 2437–2443. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, M.; Namvar, F.; Ahmad, M.B.; Mohamad, R. Green biosynthesis and characterization of magnetic iron oxide (Fe3O4) nanoparticles using seaweed (Sargassum muticum) aqueous extract. Molecules 2013, 18, 5954–5964. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Fang, Z.; Zheng, L.; Tsang, E.P. Biosynthesized iron nanoparticles in aqueous extracts of Eichhornia crassipes and its mechanism in the hexavalent chromium removal. Appl. Surf. Sci. 2017, 399, 322–329. [Google Scholar] [CrossRef]
- Ebrahiminezhad, A.; Zare-Hoseinabadi, A.; Berenjian, A.; Ghasemi, Y. Green synthesis and characterization of zero-valent iron nanoparticles using stinging nettle (Urtica dioica) leaf extract. Green Process. Synthesis 2017, 6, 469–475. [Google Scholar]
- Prasad, K.S.; Gandhi, P.; Selvaraj, K. Synthesis of green nano iron particles (GnIP) and their application in adsorptive removal of As(III) and As(V) from aqueous solution. Appl. Surf. Sci. 2014, 317, 1052–1059. [Google Scholar] [CrossRef]
- Mohanraj, S.; Kodhaiyolii, S.; Rengasamy, M.; Pugalenthi, V. Green synthesized iron oxide nanoparticles effect on fermentative hydrogen production by Clostridium acetobutylicum. Appl. Biochem. Biotechnol. 2014, 173, 318–331. [Google Scholar] [CrossRef]
- Essajai, R.; Benhouria, Y.; Rachadi, A.; Qjani, M.; Mzerd, A.; Hassanain, N. Shape-dependent structural and magnetic properties of Fe nanoparticles studied through simulation methods. RSC Adv. 2019, 9, 22057–22063. [Google Scholar] [CrossRef] [PubMed]
- Beyth, N.; Houri-Haddad, Y.; Domb, A.; Khan, W.; Hazan, R. Alternative antimicrobial approach: Nano-antimicrobial materials. Evid. Based Complement. Altern. Med. 2015, 2015, 246012. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X. Gold nanoparticles: Recent advances in the biomedical applications. Cell Biochem. Biophys. 2015, 72, 771–775. [Google Scholar] [CrossRef] [PubMed]
- Sharmin, S.; Rahaman, M.M.; Sarkar, C.; Atolani, O.; Islam, M.T.; Adeyemi, O.S. Nanoparticles as antimicrobial and antiviral agents: A literature-based perspective study. Heliyon 2021, 7, e06456. [Google Scholar] [CrossRef] [PubMed]
- Rajeshkumar, S.; Ponnanikajamideen, M.; Malarkodi, C.; Malini, M.; Annadurai, G. Microbe-mediated synthesis of antimicrobial semiconductor nanoparticles by marine bacteria. J. Nanostruct. Chem. 2014, 4, 96. [Google Scholar] [CrossRef]
- Maharani, V.; Sundaramanickam, A.; Balasubramanian, T.J.E. In vitro anticancer activity of silver nanoparticle synthesized by Escherichia coli VM1 isolated from marine sediments of Ennore southeast coast of India. Enzym. Microb. Technol. 2016, 95, 146–154. [Google Scholar] [CrossRef]
- Thomas, R.; Janardhanan, A.; Varghese, R.T.; Soniya, E.; Mathew, J.; Radhakrishnan, E. Antibacterial properties of silver nanoparticles synthesized by marine Ochrobactrum sp. Braz. J. Microbiol. 2014, 45, 1221–1227. [Google Scholar] [CrossRef] [PubMed]
- Qi, P.; Zhang, D.; Wan, Y. Sulfate-reducing bacteria detection based on the photocatalytic property of microbial synthesized ZnS nanoparticles. Anal. Chim. Acta 2013, 800, 65–70. [Google Scholar] [CrossRef]
- Mohanpuria, P.; Rana, N.K.; Yadav, S.K. Biosynthesis of nanoparticles: Technological concepts and future applications. J. Nanopart. Res. 2008, 10, 507–517. [Google Scholar] [CrossRef]
- Hulkoti, N.I.; Taranath, T. Biosynthesis of nanoparticles using microbes—A review. Colloids Surf. B Biointerfaces 2014, 121, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.; Ahmad, A.; Mandal, D.; Senapati, S.; Sainkar, S.R.; Khan, M.I.; Parishcha, R.; Ajaykumar, P.V.; Alam, M.; Kumar, R.; et al. Fungus-mediated synthesis of silver nanoparticles and their immobilization in the mycelial matrix: A novel biological approach to nanoparticle synthesis. Nano Lett. 2001, 1, 515–519. [Google Scholar] [CrossRef]
- Erasmus, M.; Cason, E.D.; Van Marwijk, J.; Botes, E.; Gericke, M.; Van Heerden, E. Gold nanoparticle synthesis using the thermophilic bacterium Thermus scotoductus SA-01 and the purification and characterization of its unusual gold reducing protein. Gold Bull. 2014, 47, 245–253. [Google Scholar] [CrossRef]
- Li, X.; Xu, H.; Chen, Z.S.; Chen, G. Biosynthesis of nanoparticles by microorganisms and their applications. J. Nanomater. 2011, 2011, 270974. [Google Scholar] [CrossRef]
- Mishra, A.; Tripathy, S.K.; Wahab, R.; Jeong, S.H.; Hwang, I.; Yang, Y.B.; Kim, Y.S.; Shin, H.S.; Yun, S.I. Microbial synthesis of gold nanoparticles using the fungus Penicillium brevicompactum and their cytotoxic effects against mouse mayo blast cancer C 2 C 12 cells. Appl. Microbiol. Biotechnol. Adv. 2011, 92, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Khanna, P.; Kaur, A.; Goyal, D. Algae-based metallic nanoparticles: Synthesis, characterization and applications. J. Microbiol Methods 2019, 163, 105656. [Google Scholar] [CrossRef] [PubMed]
- Hasan, S. A review on nanoparticles: Their synthesis and types. Res. J. Recent Sci. 2015, 2277, 2502. [Google Scholar]
- Eroglu, E.; Chen, X.; Bradshaw, M.; Agarwal, V.; Zou, J.; Stewart, S.G.; Duan, X.; Lamb, R.N.; Smith, S.M.; Raston, C.L.; et al. Biogenic production of palladium nanocrystals using microalgae and their immobilization on chitosan nanofibers for catalytic applications. RSC Adv. 2013, 3, 1009–1012. [Google Scholar] [CrossRef]
- Jena, J.; Pradhan, N.; Nayak, R.R.; Dash, B.P.; Sukla, L.B.; Panda, P.K.; Mishra, B.K. Microalga Scenedesmus sp.: A potential low-cost green machine for silver nanoparticle synthesis. J. Microbiol. Biotechnol. Adv. 2014, 24, 522–533. [Google Scholar] [CrossRef]
- Xia, Y.; Xiao, Z.; Dou, X.; Huang, H.; Lu, X.; Yan, R.; Gan, Y.; Zhu, W.; Tu, J.; Zhang, W.; et al. Green and facile fabrication of hollow porous MnO/C microspheres from microalgaes for lithium-ion batteries. ACS Nano 2013, 7, 7083–7092. [Google Scholar] [CrossRef]
- Li, N.; Zhao, P.; Astruc, D. Anisotropic gold nanoparticles: Synthesis, properties, applications, and toxicity. Angew. Chem. Int. Ed. 2014, 53, 1756–1789. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Yadav, S.K. Plant-mediated synthesis of silver and gold nanoparticles and their applications. J. Chem. Technol. Biotechnol. 2009, 84, 151–157. [Google Scholar] [CrossRef]
- Kar, P.; Banerjee, S.; Saleh-E-In, M.M.; Anandraj, A.; Kormuth, E.; Pillay, S.; Al-Ghamdi, A.A.; Ali, M.A.; Lee, J.; Sen, A.; et al. β-sitosterol conjugated silver nanoparticle-mediated amelioration of CCl4-induced liver injury in Swiss albino mice. J. King Saud Univ. Sci. 2022, 34, 102113. [Google Scholar] [CrossRef]
- Dhand, V.; Soumya, L.; Bharadwaj, S.; Chakra, S.; Bhatt, D.; Sreedhar, B. Green synthesis of silver nanoparticles using Coffea arabica seed extract and its antibacterial activity. Mater. Sci. Eng. C 2016, 58, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Kalaiyarasu, T.; Karthi, N.; Sharmila, G.V.; Manju, V. In vitro assessment of antioxidant and antibacterial activity of green synthesized silver nanoparticles from Digitaria radicosa leaves. Asian J. Pharm. Clin. Res. 2016, 9, 297302. [Google Scholar]
- Rao, N.H.; Lakshmidevi, N.; Pammi, S.; Kollu, P.; Ganapaty, S.; Lakshmi, P. Green synthesis of silver nanoparticles using methanolic root extracts of Diospyros paniculata and their antimicrobial activities. Mater. Sci. Eng. C 2016, 62, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Kharat, S.N.; Mendhulkar, V.D. Synthesis, characterization and studies on antioxidant activity of silver nanoparticles using Elephantopus scaber leaf extract. Mater. Sci. Eng. C 2016, 62, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Phull, A.R.; Abbas, Q.; Ali, A.; Raza, H.; Zia, M.; Haq, I.U. Antioxidant, cytotoxic and antimicrobial activities of green synthesized silver nanoparticles from crude extract of Bergenia ciliata. Future J. Pharm. Sci. 2016, 2, 31–36. [Google Scholar] [CrossRef]
- Velusamy, P.; Das, J.; Pachaiappan, R.; Vaseeharan, B.; Pandian, K. Greener approach for synthesis of antibacterial silver nanoparticles using aqueous solution of neem gum (Azadirachta indica L.). Indus. Crops Prod. 2015, 66, 103–109. [Google Scholar] [CrossRef]
- Patil, M.P.; Ngabire, D.; Thi, H.H.P.; Kim, M.D.; Kim, G.D. Ecofriendly synthesis of gold nanoparticles and evaluation of their cytotoxic activity on cancer cells. J. Clust. Sci. 2017, 28, 119–132. [Google Scholar] [CrossRef]
- Chakraborty, N.; Banerjee, A.; Lahiri, S.; Panda, A.; Ghosh, A.N.; Pal, R. Biorecovery of gold using cyanobacteria and an eukaryotic alga with special reference to nanogold formation–a novel phenomenon. J. Appl. Phycol. 2009, 21, 145–152. [Google Scholar] [CrossRef]
- Rajesh, S.; Raja, D.P.; Rathi, J.M.; Sahayaraj, K. Biosynthesis of silver nanoparticles using Ulva fasciata (Delile) ethyl acetate extract and its activity against Xanthomonas campestris pv. malvacearum. J. Biopestic. 2012, 5, 119. [Google Scholar]
- Rajeshkumar, S.; Malarkodi, C.; Gnanajobitha, G.; Paulkumar, K.; Vanaja, M.; Kannan, C.; Annadurai, G. Seaweed-mediated synthesis of gold nanoparticles using Turbinaria conoides and its characterization. J. Nanostruct. Chem. 2013, 3, 44. [Google Scholar] [CrossRef]
- Ghodake, G.; Lee, D.S. Biological synthesis of gold nanoparticles using the aqueous extract of the brown algae Laminaria japonica. J. Nanoelectron. Optoelectron. 2011, 6, 268–271. [Google Scholar] [CrossRef]
- Behera, M.; Behera, P.R.; Bhuyan, P.P.; Singh, L.; Pradhan, B. Algal Nanoparticles and Their Antibacterial Activity: Current Research Status and Future Prospectives. Drugs Drug Candidates 2023, 2, 554–570. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Kannan, C.; Annadurai, G. Synthesis and characterization of antimicrobial silver nanoparticles using marine brown seaweed Padina tetrastromatica. Drug Invent. Today 2012, 4, 511–513. [Google Scholar]
- Rao, M.D.; Pennathur, G. Green synthesis and characterization of cadmium sulphide nanoparticles from Chlamydomonas reinhardtii and their application as photocatalysts. Mater. Res. Bull. 2017, 85, 64–73. [Google Scholar] [CrossRef]
- Yousefzadi, M.; Rahimi, Z.; Ghafori, V. The green synthesis, characterization and antimicrobial activities of silver nanoparticles synthesized from green alga Enteromorpha flexuosa (wulfen) J. Agardh. Mater. Lett. 2014, 137, 1–4. [Google Scholar] [CrossRef]
- Wei, D.; Qian, W. Facile synthesis of Ag and Au nanoparticles utilizing chitosan as a mediator agent. Colloids Surf. B Biointerfaces 2008, 62, 136–142. [Google Scholar] [CrossRef]
- Niu, H.; Volesky, B. Gold-cyanide biosorption with L-cysteine. J. Chem. Technol. Biotechnol. 2000, 75, 436–442. [Google Scholar] [CrossRef]
- El-Shanshoury, A.R.; ElSilk, S.E.; Ebeid, M.E. Extracellular biosynthesis of Silver Nanoparticles using Escherichia coli ATCC 8739, Bacillus subtilis ATCC 6633 and Streptococcus thermophilus ESh1 and Their Antimicrobial Activities. ISRN Nanotechnol. 2011, 2011, 385480. [Google Scholar] [CrossRef]
- Saravanan, M.; Vemu, A.K.; Barik, S.K. Rapid biosynthesis of silver nanoparticles from Bacillus megaterium (NCIM 2326) and their antibacterial activity on multi drug resistant clinical pathogens. Colloids Surf. B Biointerfaces 2011, 88, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Kim, Y.K.; Zhang, D.; Yang, D.C. Biological Synthesis of Nanoparticles from Plants and Microorganisms. Trends Biotechnol. 2016, 34, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Mohanasrinivasan, V.; Subathra Devi, C.; Mehra, A.; Prakash, S.; Agarwal, A.; Selvarajan, E.; Jemimah Naine, S. Biosynthesis of MgO nanoparticles Using Lactobacillus spp. and its activity against human leukemia cell lines HL-60. BioNanoScience 2018, 8, 249–253. [Google Scholar] [CrossRef]
- Singh, A.K.; Rathod, V.; Singh, D.; Kulkarni, P. Effect of silver nanoparticles (AgNPs) produced by an endophytic fungus Fusarium semitectum isolated from a medicinal plant Withania somnifera (Ashwagandha) on seed germination. Int. J. Res. Stud. Agric. Sci. 2016, 2, 6–12. [Google Scholar]
- Jhansi, K.; Jayarambabu, N.; Reddy, K.P.; Reddy, N.M.; Suvarna, R.P.; Rao, K.V.; Kumar, V.R.; Rajendar, V. Biosynthesis of MgO nanoparticles using mushroom extract: Effect on peanut (Arachis hypogaea L.) seed germination. 3 Biotech 2017, 7, 263. [Google Scholar] [CrossRef] [PubMed]
- Gudikandula, K.; Vadapally, P.; Charya, M.S. Biogenic synthesis of silver nanoparticles from white rot fungi: Their characterization and antibacterial studies. OpenNano 2017, 2, 64–78. [Google Scholar] [CrossRef]
- Azmath, P.; Baker, S.; Rakshith, D.; Satish, S. Mycosynthesis of silver nanoparticles bearing antibacterial activity. Saudi Pharm. J. 2016, 24, 140–146. [Google Scholar] [CrossRef]
- Castro-Longoria, E.; Vilchis-Nestor, A.R.; Avalos-Borja, M. Biosynthesis of silver, gold and bimetallic nanoparticles using the filamentous fungus Neurospora crassa. Colloids Surf. B Biointerfaces 2011, 83, 42–48. [Google Scholar] [CrossRef]
- Das, S.K.; Dickinson, C.; Lafir, F.; Brougham, D.F.; Marsili, E. Synthesis, characterization and catalytic activity of gold nanoparticles biosynthesized with Rhizopus oryzae protein extract. Green Chem. 2012, 14, 1322–1334. [Google Scholar] [CrossRef]
- Ahluwalia, V.; Kumar, J.; Sisodia, R.; Shakil, N.A.; Walia, S. Green synthesis of silver nanoparticles by Trichoderma harzianum and their bio-efficacy evaluation against Staphylococcus aureus and Klebsiella pneumonia. Ind. Crop. Prod. 2014, 55, 202–206. [Google Scholar] [CrossRef]
- Kaul, R.K.; Kumar, P.; Burman, U.; Joshi, P.; Agrawal, A.; Raliya, R.; Tarafdar, J.C. Magnesium and iron nanoparticles production using microorganisms and various salts. Mater. Sci. Pol. 2012, 30, 254–258. [Google Scholar] [CrossRef]
- Chavan, R.R.; Bhinge, S.D.; Bhutkar, M.A.; Randive, D.S.; Wadkar, G.H.; Todkar, S.S.; Urade, M.N. Characterization, antioxidant, antimicrobial and cytotoxic activities of green synthesized silver and iron nanoparticles using alcoholic Blumea eriantha DC plant extract. Mater. Today Commun. 2020, 24, 101320. [Google Scholar] [CrossRef]
- Din, S.U.; Iqbal, H.; Haq, S.; Ahmad, P.; Khandaker, M.U.; Elansary, H.O.; Al-Harbi, F.F.; Abdelmohsen, S.A.; El-Abedin, T.K.Z. Investigation of the biological applications of biosynthesized nickel oxide nanoparticles mediated by Buxus wallichiana extract. Crystals 2022, 12, 146. [Google Scholar] [CrossRef]
- Bibi, I.; Kamal, S.; Ahmed, A.; Iqbal, M.; Nouren, S.; Jilani, K.; Nazar, N.; Amir, M.; Abbas, A.; Ata, S.; et al. Nickel nanoparticle synthesis using Camellia sinensis as reducing and capping agent: Growth mechanism and photo-catalytic activity evaluation. Int. J. Biol. Macromol. 2017, 103, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Thi, T.U.D.; Nguyen, T.T.; Thi, Y.D.; Thi, K.H.T.; Phan, B.T.; Pham, K.N. Green synthesis of ZnO nanoparticles using orange fruit peel extract for antibacterial activities. RSC Adv. 2020, 10, 23899–23907. [Google Scholar]
- Chan, J.Z.; Ali, R.R.; Shameli, K.; Tarmizi, Z.I.; Salleh, M.S.N. Biosynthesis of Gold Nanoparticles: A Simple Method of Size Controlled using Clitoria ternatea Flower Extract. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1051, 012090. [Google Scholar] [CrossRef]
- Khan, M.I.; Akhtar, M.N.; Ashraf, N.; Najeeb, J.; Munir, H.; Awan, T.I.; Tahir, M.B.; Kabli, M.R. Green synthesis of magnesium oxide nanoparticles using Dalbergia sissoo extract for photocatalytic activity and antibacterial efficacy. Appl. Nanosci. 2020, 10, 2351–2364. [Google Scholar] [CrossRef]
- Bindhu, M.R.; Umadevi, M.; Esmail, G.A.; Al-Dhabi, N.A.; Arasu, M.V. Green synthesis and characterization of silver nanoparticles from Moringa oleifera flower and assessment of antimicrobial and sensing properties. J. Photochem. Photobiol. B Biol. 2020, 205, 111836. [Google Scholar] [CrossRef]
- Rizwana, H.; Bokahri, N.A.; Alkhattaf, F.; Albasher, G.; Aldehaish, H.A. Antifungal, antibacterial, and cytotoxic activities of silver nanoparticles synthesized from aqueous extracts of macearils of Myristica fragrans. Molecules 2021, 26, 7709. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.M.; Ismail, E.H.; El-Baghdady, K.Z.; Mohamed, D. Green synthesis of silver nanoparticles using olive leaf extract and its antibacterial activity. Arab. J. Chem. 2014, 7, 1131–1139. [Google Scholar] [CrossRef]
- Ananda, A.; Ramakrishnappa, T.; Archana, S.; Yadav, L.R.; Shilpa, B.M.; Nagaraju, G.; Jayanna, B.K. Green synthesis of MgO nanoparticles using Phyllanthus emblica for Evans blue degradation and antibacterial activity. Mater. Today Proc. 2022, 49, 801–810. [Google Scholar] [CrossRef]
- Sharmila, G.; Muthukumaran, C.; Sangeetha, E.; Saraswathi, H.; Soundarya, S.; Kumar, N.M. Green fabrication, characterization of Pisonia alba leaf extract derived MgO nanoparticles and its biological applications. Nano-Struct. Nano-Objects 2019, 20, 100380. [Google Scholar] [CrossRef]
- Devi, H.S.; Boda, M.A.; Shah, M.A.; Parveen, S.; Wani, A.H. Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity. Green Process. Synth. 2019, 8, 38–45. [Google Scholar] [CrossRef]
- Rizwana, H.; Alwhibi, M.S.; Aldarsone, H.A.; Awad, M.A.; Soliman, D.A.; Bhat, R.S. Green synthesis, characterization, and antimicrobial activity of silver nanoparticles prepared using Trigonella foenum-graecum L. leaves grown in Saudi Arabia. Green Process. Synth. 2021, 10, 421–429. [Google Scholar] [CrossRef]
- Nami, S.A.A.; Arshad, M.; Khan, M.S.; Alam, M.; Lee, D.U.; Park, S.; Sarikavakli, N. Morphological, Structural, Molecular Docking and Biocidal Studies of Newly Synthesized Ppy-MA/TiO2 Nanocomposites. Polym. Adv. Technol. 2015, 26, 1627–1638. [Google Scholar] [CrossRef]
- Leifert, A.; Pan, Y.; Kinkeldey, A.; Schiefer, F.; Setzler, J.; Scheel, O.; Lichtenbeld, H.; Schmid, G.; Wenzel, W.; Jahnen-Dechent, W.; et al. Differential hERG Ion Channel Activity of Ultrasmall Gold Nanoparticles. Proc. Natl. Acad. Sci. USA 2013, 110, 8004–8009. [Google Scholar] [CrossRef]
- Skariyachan, S.; Parveen, A.; Garka, S. Nanoparticle Fullerene (C60) Demonstrated Stable Binding with Antibacterial Potential Towards Probable Targets of Drug Resistant Salmonella Typhi—A Computational Perspective and In Vitro Investigation. J. Biomol. Struct. Dyn. 2017, 35, 3449–3468. [Google Scholar] [CrossRef]
- Ledesma, A.E.; Chemes, D.M.; de los Angeles Frías, M.; Torres, M.D.P.G. Spectroscopic Characterization and Docking Studies of ZnO Nanoparticle Modified with BSA. Appl. Surf. Sci. 2017, 412, 177–188. [Google Scholar] [CrossRef]
- Ates, S.; Zor, E.; Akin, I.; Bingol, H.; Alpaydin, S.; Akgemci, E.G. Discriminative Sensing of DOPA Enantiomers by Cyclodextrin Anchored Graphene Nanohybrids. Anal. Chim. Acta 2017, 970, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Brancolini, G.; Kokh, D.B.; Calzolai, L.; Wade, R.C.; Corni, S. Docking of Ubiquitin to Gold Nanoparticles. ACS Nano 2012, 6, 9863–9878. [Google Scholar] [CrossRef] [PubMed]
- Panneerselvam, S.; Choi, S. Nanoinformatics: Emerging Databases and Available tools. Int. J. Mol. Sci. 2014, 15, 7158–7182. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.R.; Basheer, S.M.; Williams, L.; Joseph, A. Highly selective inhibition of α-glucosidase by green synthesised ZnO nanoparticles-In-vitro screening and in-silico docking studies. Int. J. Mol. Sci. 2019, 139, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Mehdizadeh, P.; Fesharaki, S.S.H.; Nouri, M.; Ale-Ebrahim, M.; Akhtari, K.; Shahpasand, K.; Saboury, A.A.; Falahati, M. Tau Folding and Cytotoxicity of Neuroblastoma Cells in the Presence of Manganese Oxide Nanoparticles: Biophysical, Molecular Dynamics, Cellular, and Molecular Studies. Int. J. Biol. Macromol. 2019, 125, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Vyshnava, S.S.; Kanderi, D.K.; Panjala, S.P.; Pandian, K.; Bontha, R.R.; Goukanapalle, P.K.R.; Banaganapalli, B. Effect of Silver Nanoparticles Against the Formation of Biofilm by Pseudomonas aeruginosa an in Silico Approach. Appl. Biochem. Biotechnol. 2016, 180, 426–437. [Google Scholar] [CrossRef]
- Shakir, M.; Khan, M.S.; Al-Resayes, S.I.; Baig, U.; Alam, P.; Khan, R.H.; Alam, M. In Vitro DNA Binding, Molecular Docking and Antimicrobial Studies on a Newly Synthesized Poly(o-Toluidine)-Titanium Dioxide Nanocomposite. RSC Adv. 2014, 4, 39174. [Google Scholar] [CrossRef]
- Malekshah, R.E.; Fahimirad, B.; Khaleghian, A. Synthesis, Characterization, Biomedical Application, Molecular Dynamic Simulation and Molecular Docking of Schiff Base Complex of Cu(II) Supported on Fe/APTS. Int. J. Nanomed. 2020, 15, 2583–2603. [Google Scholar] [CrossRef] [PubMed]
- Sabir, S.; Arshad, M.; Chaudhari, S.K. Zinc Oxide Nanoparticles for Revolutionizing Agriculture: Synthesis and Applications. Sci. World J. 2014, 2014, 925494. [Google Scholar] [CrossRef]
- Mohammed, Y.H.I.; Alghamdi, S.; Jabbar, B.; Marghani, D.; Beigh, S.; Abouzied, A.S.; Khalifa, N.E.; Khojali, W.M.; Huwaimel, B.; Alkhalifah, D.H.M.; et al. Green synthesis of zinc oxide nanoparticles using Cymbopogon citratus extract and its antibacterial activity. ACS Omega 2023, 8, 32027–32042. [Google Scholar] [CrossRef]
- Alrabayah, I.N.; Elhawary, S.S.; Kandil, Z.A.; El-Kadder, E.M.A.; Moemen, Y.S.; Saleh, A.M.; El Raey, M.A. Green Synthesized Zinc Oxide Nanoparticles Based on Cestrum diurnum L. of Potential Antiviral Activity against Human Corona 229-E Virus. Molecules 2022, 28, 266. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Chimal, R.; García-Pérez, V.I.; Álvarez-Pérez, M.A.; Tavera-Hernández, R.; Reyes-Carmona, L.; Martinez-Hernandez, M.; Arenas-Alatorre, J.Á. Influence of the particle size on the antibacterial activity of green synthesized zinc oxide nanoparticles using Dysphania ambrosioides extract, supported by molecular docking analysis. Arab. J. Chem. 2022, 15, 103804. [Google Scholar] [CrossRef]
- Giannousi, K.; Geromichalos, G.; Kakolyri, D.; Mourdikoudis, S.; Dendrinou-Samara, C. Interaction of ZnO Nanostructures with Proteins: In Vitro Fibrillation/Antifibrillation Studies and in Silico Molecular Docking Simulations. ACS Chem. Neurosci. 2020, 11, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.T.; Ayaz, M.; Ovais, M.; Wadood, A.; Ali, M.; Shinwari, Z.K.; Maaza, M. In Vitro Cholinesterase Enzymes Inhibitory Potential and in Silico Molecular Docking Studies of Biogenic Metal Oxides Nanoparticles. Inorg. Nano-Met. Chem. 2018, 48, 441–448. [Google Scholar] [CrossRef]
- Singh, K.P.; Dhasmana, A.; Rahman, Q. Elucidation the Toxicity Mechanism of Zinc Oxide Nanoparticle Using Molecular Docking Approach with Proteins. Asian J. Pharm. Clin. Res. 2018, 11, 441–446. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, K.; Xu, D.; Yang, G.; Huang, H.; Nie, F.; Liu, C.; Yang, S. CuO Nanostructures: Synthesis, Characterization, Growth Mechanisms, Fundamental Properties, and Applications. Prog. Mater. Sci. 2014, 60, 208–337. [Google Scholar] [CrossRef]
- Li, Y.; Hong, M.; Lin, Y.; Bin, Q.; Lin, Z.; Cai, Z.; Chen, G. Highly Sensitive Electrochemical Immunoassay for H1N1 Influenza Virus Based on Copper-Mediated Amplification. Chem. Commun. 2012, 48, 6562–6564. [Google Scholar] [CrossRef] [PubMed]
- Grigore, M.E.; Biscu, E.R.; Holban, A.M.; Gestal, M.C.; Grumezescu, A.M. Methods of Synthesis, Properties and Biomedical Applications of CuO Nanoparticles. Pharmaceuticals 2016, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Albert, J.; Mundy, R.; Yu, X.J.; Beuzon, C.R.; Holden, D.W. SseA is a Chaperone for the SseB and SseD Translocon Components of the Salmonella PathogenicityIsland-2-Encoded Type III Secretion System. Microbiology 2003, 149, 1103–1111. [Google Scholar] [CrossRef]
- Sazak, C.; Attar, A.; Yilmaz, A.; Altikatoglu Yapaoz, M. Biofabrication of Acer palmatum-Mediated Multifunctional CuO Nanoparticles for Dye Removal, Antibacterial–Antifungal Activity, and Molecular Docking. ACS Omega 2023, 8, 36835–36844. [Google Scholar] [CrossRef]
- Kocabas, B.B.; Attar, A.; Yuka, S.A.; Yapaoz, M.A. Biogenic synthesis, molecular docking, biomedical and environmental applications of multifunctional CuO nanoparticles mediated Phragmites australis. Bioorg. Chem. 2023, 133, 106414. [Google Scholar] [CrossRef] [PubMed]
- Chibber, S.; Ahmed, I. Molecular Docking, a Tool to Determine Interaction of CuO and TiO2 Nanoparticles with Human Serum Albumin. Biochem. Biophys. Rep. 2016, 31, 782–786. [Google Scholar]
- Kumari, P.; Panda, P.K.; Jha, E.; Kumari, K.; Nisha, K.; Mallick, M.A.; Verma, S.K. Mechanistic Insight to ROS and Apoptosis Regulated Cytotoxicity Inferred by Green Synthesized CuO Nanoparticles from Calotropis Gigantea to Embryonic Zebrafish. Sci. Rep. 2017, 7, 16284. [Google Scholar] [CrossRef] [PubMed]
- Abou El-Nour, K.M.; Eftaiha, A.A.; Al-Warthan, A.; Ammar, R.A. Synthesis and Applications of Silver Nanoparticles. Arabian J. Chem. 2010, 3, 135–140. [Google Scholar] [CrossRef]
- Tavakoli, M.; Malakooti, M.H.; Paisana, H.; Ohm, Y.; Green Marques, D.; Alhais Lopes, P.; Piedade, A.P.; De Almeida, A.T.; Majidi, C. EGaIn-Assisted Room-Temperature Sintering of Silver Nanoparticles for Stretchable. Adv. Mater. 2018, 30, 1801852. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Kar, P.; Sarkar, I.; Chhetri, A.; Mishra, D.K.; Dutta, A.; Kumar, A.; Sinha, B.; Sen, A. Structural elucidation and chemical characterization of underutilized fruit silverberry (Elaeagnus pyriformis) silver nanoparticles playing a dual role as anti-cancer agent by promoting apoptosis and inhibiting ABC transporters. S. Afr. J. Bot. 2022, 145, 243–257. [Google Scholar] [CrossRef]
- Banerjee, S.; Kar, P.; Islam, R.; Naidoo, D.; Roy, A.; Sarkar, I.; Sen, G.; Saha, T.; Yasmin, H.; Sen, A. Synthesis of silver nanoparticles from secondary metabolites of star gooseberry fruit (Phyllanthus acidus) and their nephroprotective efficiency. S. Afr. J. Bot. 2022, 151, 385–395. [Google Scholar] [CrossRef]
- Wasukan, N.; Kuno, M.; Maniratanachote, R. Molecular Docking as a Promising Predictive Model for Silver Nanoparticle-Mediated Inhibition of Cytochrome P450 Enzymes. J. Chem. Inf. Model. 2019, 59, 5126–5134. [Google Scholar] [CrossRef]
- Kohl, N.E.; Emini, E.A.; Schleif, W.A.; Davis, L.J.; Heimbach, J.C.; Dixon, R.A.; Scolnick, E.M.; Sigal, I.S. Active Human Immunodeficiency Virus Protease is Required for Viral infectivity. Proc. Natl. Acad. Sci. USA 1988, 85, 4686–4690. [Google Scholar] [CrossRef]
- Huang, H.; Shao, X.; Xie, Y.; Wang, T.; Zhang, Y.; Wang, X.; Deng, X. An Integrated Genomic Regulatory Network of Virulence-Related Transcriptional Factors in Pseudomonas aeruginosa. Nat. Commun. 2019, 10, 2931. [Google Scholar] [CrossRef]
- Ali, S.G.; Ansari, M.A.; Sajid Jamal, Q.M.; Khan, H.M.; Jalal, M.; Ahmad, H.; Mahdi, A.A. Antiquorum Sensing Activity of Silver Nanoparticles in P. aeruginosa: An in Silico Study. In Silico Pharmacol. 2017, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Elkashif, A.; Seleem, M.N. Investigation of Auranofin and Gold-Containing Analogues Antibacterial Activity Against Multidrug-Resistant Neisseria gonorrhoeae. Sci. Rep. 2020, 10, 5602. [Google Scholar] [CrossRef] [PubMed]
- Her, S.; Jaffray, D.A.; Allen, C. Gold Nanoparticles for Applications in Cancer Radiotherapy: Mechanisms and Recent Advancements. Adv. Drug Deliv. Rev. 2017, 109, 84–101. [Google Scholar] [CrossRef]
- Kaushik, A.C.; Wang, Y.J.; Wang, X.; Kumar, A.; Singh, S.P.; Pan, C.T.; Shiue, Y.L.; Wei, D.Q. Evaluation of Anti-EGFR-iRGD Recombinant Protein with GOLD Nanoparticles: Synergistic Effect on Antitumor Efficiency Using Optimized Deep Neural Networks. RSC Adv. 2019, 9, 19261–19270. [Google Scholar] [CrossRef]
- Al-Radadi, N.S. Single-step green synthesis of gold conjugated polyphenol nanoparticle using extracts of Saudi’s myrrh: Their characterization, molecular docking and essential biological applications. Saudi Pharm. J. 2022, 30, 1215–1242. [Google Scholar] [CrossRef]
- Ghazanfari, M.R.; Kashefi, M.; Shams, S.F.; Jaafari, M.R. Perspective of Fe3O4 Nanoparticles Role in Biomedical Applications. Biochem. Res. Int. 2016, 2016, 7840161. [Google Scholar] [CrossRef] [PubMed]
- Hola, K.; Markova, Z.; Zoppellaro, G.; Tucek, J.; Zboril, R. Tailored Functionalization of Iron Oxide Nanoparticles for MRI, Drug Delivery, Magnetic Separation and Immobilization of Biosubstances. Biotechnol. Adv. 2015, 33, 1162–1176. [Google Scholar] [CrossRef]
- Abo-Zeid, Y.; Ismail, N.S.; McLean, G.R.; Hamdy, N.M. A molecular docking study repurposes FDA approved iron oxide nanoparticles to treat and control COVID-19 infection. Eur. J. Pharm. Sci. 2020, 153, 105465. [Google Scholar] [CrossRef]
- Aghili, Z.; Taheri, S.; Zeinabad, H.A.; Pishkar, L.; Saboury, A.A.; Rahimi, A.; Falahati, M. Investigating the Interaction of Fe Nanoparticles with Lysozyme by Biophysical and Molecular Docking Studies. PLoS ONE 2016, 11, e0164878. [Google Scholar] [CrossRef]
| Nanoparticle | Natural Source | Size | Shape | Specific Surface Area | Solubility | Optical Properties |
|---|---|---|---|---|---|---|
| ZnO NPs | Sargassum muticum (Algae) Vigna mungo (Plant) Prunus bracteopadus (Plant) | 30–57 nm 65 nm 55 nm | hexagonal [17] Spherical [18] Spherical [19] | 88.89 m2/g [20] | 0.3–3.6 mg/L in aqueous medium [21] | Poorly conductive [22] |
| Cu NPs | Pseudomonas stutzeri (Bacteria) Bifurcaria bifurcate (Algae) Gloriosa superba (Plant) Coffea Arabica (Plant) Thymus vulgaris (Plant) Glycine max (Plant) | 50–150 nm 5–45 nm 5–10 nm 20–60 nm 23–94 nm 26.6 nm | Cubical [23] Spherical, Elongated [24] Monoclinic, Spherical [25] Monoclinic [26] Spherical [27] Spherical, Hexagonal [28] | 5–10 m2/g [29] | At pH 9–11, there is minimal Cu solubility; nevertheless, above pH 11, CuO complexes with hydroxide ions [30] | Highly conductive [22] |
| Ag NPs | Bacillus cereus (Bacteria) Pseudomonas deceptionensis (Bacteria) Aspergillus tamarii (Fungi) Fusariumo xysporum (Fungi) Pencillium ochrochloron (Fungi) Calliandra haemacephala (Plant) Musa paradisiaca (Plant) Alysicarpus monilifer (Plant) | 4–5 nm 10–30 nm 3–5 nm 5–15 nm 7.7 nm 70 nm 23.7 nm 5–45 nm | Spherical [31] Spherical [32] Spherical [33] Spherical [34] Spherical [33] Spherical [35] Spherical [36] Spherical, Hexagonal [37] | 23.81 m2/g [20] | Superior solubility in water and extended colloidal stability [38] | Highly reflective [22] |
| Au NPs | Chlorella vulgaris (Algae) Lemanea fluviatilis (Algae) Pogostemon benghalensis (Plant) Mentha (Plant) Lantana camara (Plant) Cannbis sativa (Plant) Mimusops elengi (Plant) | 2–10 nm 5–15 nm 10–50 nm 10–100 nm 4–12 nm 10–35 nm 9–14 nm | Spherical [39] Cubic [40] Spherical, Triangular [41] Spherical, Triangular [42] Spherical [43] Spherical [44] Spherical [45] | 5.8–107 m2/g [46] | AuNPs have great solubility in organic solvents such as toluene [47] | Highly reflective [22] |
| Fe NPs | Sargassum muticum (Algae) Eichhornia crassipes (Plant) Urtica dioica (Plant) Mentha spicata (Plant) Murraya koenigii (Plant) | 18 nm 20–80 nm 21–71 nm 20–45 nm 59 nm | Cubic [48] Spherical [49] Spherical [50] Crystalline [51] Spherical [52] | 14.42 m2/g [53] | Insoluble in water and inorganic solutions [53] | Poorly conductive [22] |
| Natural Source | Metallic Nanoparticles | Application | Reference |
|---|---|---|---|
| Algal extract based | |||
| Spirulina platensis Lyngbya majuscule Rhizoclonium hieroglyphicum | Au NPs | Bio-recovery of accumulated gold (industrial application) | [83] |
| Ulva fasciata | Ag NPs | Biopesticidal application | [84] |
| Turbinaria conoides | Ag NPs | Synthesis of valuable gold nanoparticles for biomedical application | [85] |
| Laminaria japonica | Ag NPs | Bio-recovery of accumulated gold (industrial application) | [86] |
| Gelidiella acerosa | Ag NPs | Biological and biomedical applications | [87,88] |
| Cystophora moniliformis | |||
| Desmarestia menziesii | |||
| Padina tetrastromatica | Ag NPs | Synthesis of antimicrobial Ag NPs (medicinal application) | [89] |
| Sargassum polycystum | |||
| Chlamydomonas reinhardtii | Cadmium sulfide (CdS) bimetallic NPs | Biosensors, photocatalysis, and light-emitting diodes (LEDs) | [90] |
| Enteromorpha flexuosa | Ag NPs | Antimicrobial therapy in modern medicine | [91] |
| Pithophora crispa | Au NPs | Production of semiconductor nanoparticles, including silicon nanoparticles that are employed as bio-indicators in various industrial waste products | [92] |
| Gracilaria edulis | Ag NPs ZnO NPs | Biological/medicinal application as an antimicrobial agent | [87] |
| Bacterial extract based | |||
| Bacillus subtilis | Au-CN2− | Biosorption removal and concentration of gold from solutions containing residual cyanide (industrial application). Antimicrobial agent | [93,94] |
| Bacillus megaterium | Ag NPs | Biological application as an antibacterial agent against drug-resistant clinical pathogens | [95] |
| Bhargavaea indica | Ag NPs Au NPs | Biotechnology application | [96] |
| Escherichia coli | Ag NPs | Biological application as an antimicrobial agent | [94] |
| Lactobacillus plantarum | MgO NPs | Biomedical and nanotechnology application—cytotoxicity against human leukemia cells | [97] |
| L. sporogenes | |||
| Nocadiopsis valliformis | Ag NPs | Biological application as an antibacterial and cytotoxic agent | [98] |
| Streptococcus thermophilus | Biological application as an antibacterial and antifungal agent | [94] | |
| Fungal extract based | |||
| Agaricus biosporus | MgO NPs | Useful to stimulate seed germination and the growth of peanut plants | [99] |
| Basidiomycetes sp. | Ag NPs | Biological application as an antibacterial agent | [100] |
| Colletotrichum sp. | Biological application—bactericidal activity against selected human pathogens | [101] | |
| Neurospora crassa | Alloy-type Au/Ag bimetallic NPs | NPs stabilization and facile and economical biomass handling | [102] |
| Rhizopus oryza | Gold-nano-bioconjugate | Production of important enzymes, including amylase, lipase, pectinolytic enzymes, and in biodiesel production | [103] |
| Trichoderma harzianum | Ag NPs | Biological application as an antimicrobial | [104] |
| Penicillium chrysogenum | Au-CN2− | Biosorption—removal and concentration of gold from solutions containing residual cyanide (industrial application) | [93] |
| Sargassum fluitans | |||
| Pochonia chlamydosporium | MgCl2 NPs | Potential nano-nutrients for plants | [105] |
| Aspergillus fumigatus | MgSO4 NPs | ||
| Aspergillus wentii | Fe2O3 NPs FeSO4 NPs | ||
| Curvularia lunata | Fe2O3 NPs FeSO4 NPs | ||
| Chaetomium globosum | Fe2O3 NPs | ||
| Plant extract based | |||
| Blumea eriantha | Ag NPs Fe2O3 NPs | Biological application as an antioxidant, antibacterial, and cytotoxic agent | [106] |
| Buxus wallichiana | NiO NPs | Biological application as an antioxidant and bactericidal agent | [107] |
| Camellia sinensis | Ni NPs | Industrial application—photocatalysis | [108] |
| Citrus sinensis | ZnO NPs | Biomedical application as an antibacterial agent | [109] |
| Clitoria ternatea | Au NPs | As a stabilizing and reducing agent to reduce the consumption of harmful substances | [110] |
| Coffea arabica | Ag NPs | Biological application as an antibacterial agent | [76] |
| Dalbergia sissoo | MgO NPs | Photocatalysis and biological application as an antibacterial agent | [111] |
| Hordeum vulgare | Ni NPs NiO NPs | Photocatalysis and biological application as an antioxidant agent | [107] |
| Moringa oleifera | Ag NPs | Its antimicrobial and optical properties make it potentially useful in water treatment | [112] |
| Myristica fragrans | Ag NPs | Antibacterial, antifungal, and anticancer activities, thus, may be utilized in the agrochemical and pharmaceutical industries, as well as for biomedical applications. | [113] |
| Olea europaea | Ag NPs | Synthesis of Ag NPs for antibacterial application | [114] |
| Phyllanthus emblica | MgO NPs | Photocatalysis—removal of dye from wastewater. Biological application antibacterial agent. | [115] |
| Pisonia alba | MgO NPs | Biological application as an antifungal agent | [116] |
| Platanus orientalis | Fe2O3 NPs | Biological application as an antifungal agent against Aspergillus niger and Mucor piriformis | [117] |
| Trigonella foenum-graecum | Ag NPs | Biological application as an antibacterial and antifungal agent | [118] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kar, P.; Oriola, A.O.; Oyedeji, A.O. Molecular Docking Approach for Biological Interaction of Green Synthesized Nanoparticles. Molecules 2024, 29, 2428. https://doi.org/10.3390/molecules29112428
Kar P, Oriola AO, Oyedeji AO. Molecular Docking Approach for Biological Interaction of Green Synthesized Nanoparticles. Molecules. 2024; 29(11):2428. https://doi.org/10.3390/molecules29112428
Chicago/Turabian StyleKar, Pallab, Ayodeji O. Oriola, and Adebola O. Oyedeji. 2024. "Molecular Docking Approach for Biological Interaction of Green Synthesized Nanoparticles" Molecules 29, no. 11: 2428. https://doi.org/10.3390/molecules29112428
APA StyleKar, P., Oriola, A. O., & Oyedeji, A. O. (2024). Molecular Docking Approach for Biological Interaction of Green Synthesized Nanoparticles. Molecules, 29(11), 2428. https://doi.org/10.3390/molecules29112428







