Tripeptide-Assisted Gold Nanocluster Formation for Fe3+ and Cu2+ Sensing
Abstract
:1. Introduction
2. Results and Discussion
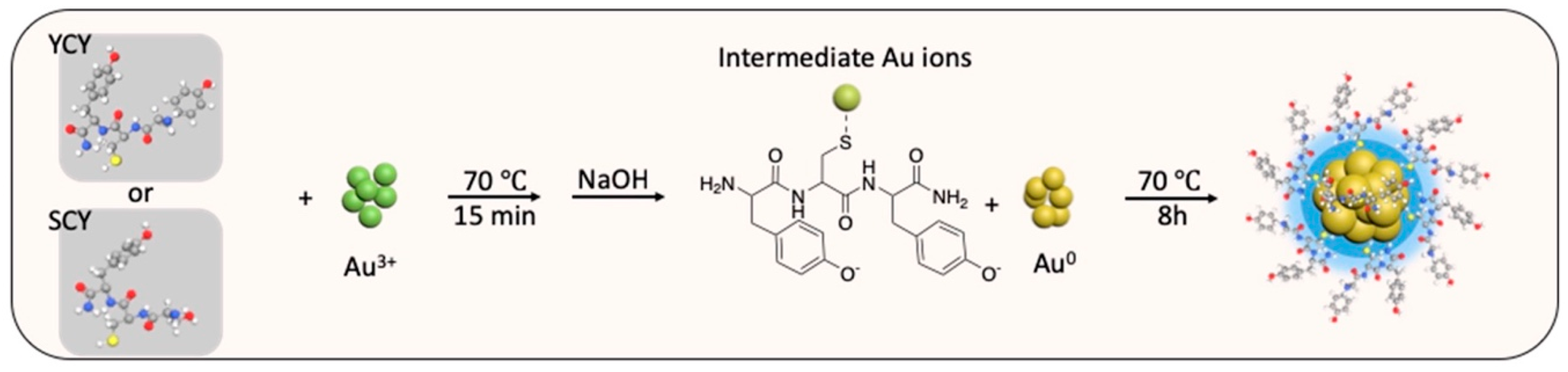
2.1. Preparation of AuNCs
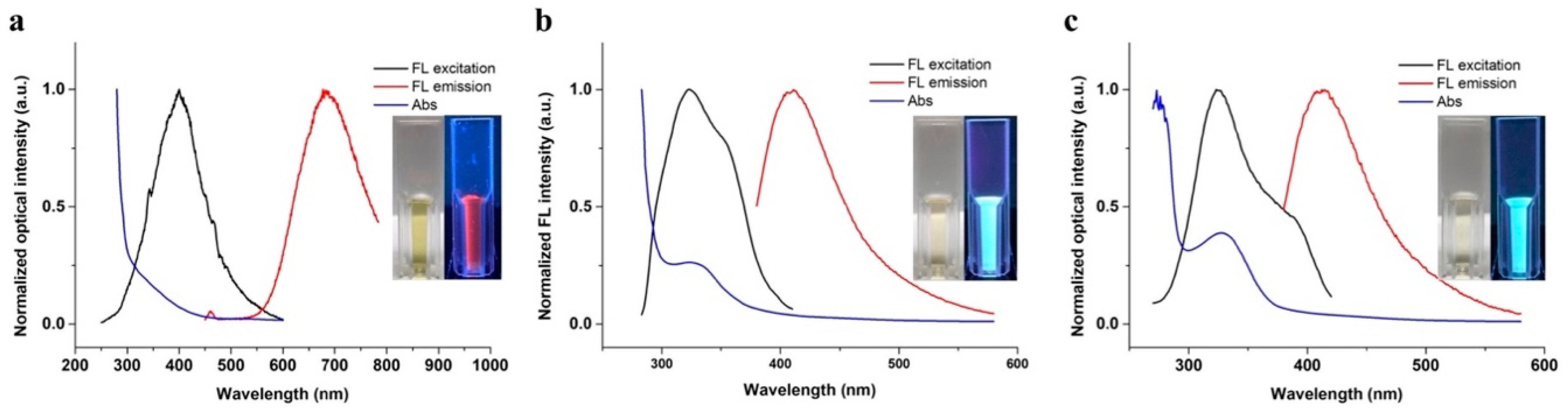
2.2. The Optical and Physical Characteristics of the AuNCs
2.3. Metal Selectivity and Sensitivity of AuNCs
2.4. Sensing Mechanism
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Tripeptides
3.3. Preparation of YCY- and SCY-Templated Fluorescent AuNCs
3.4. Characterization of AuNCs
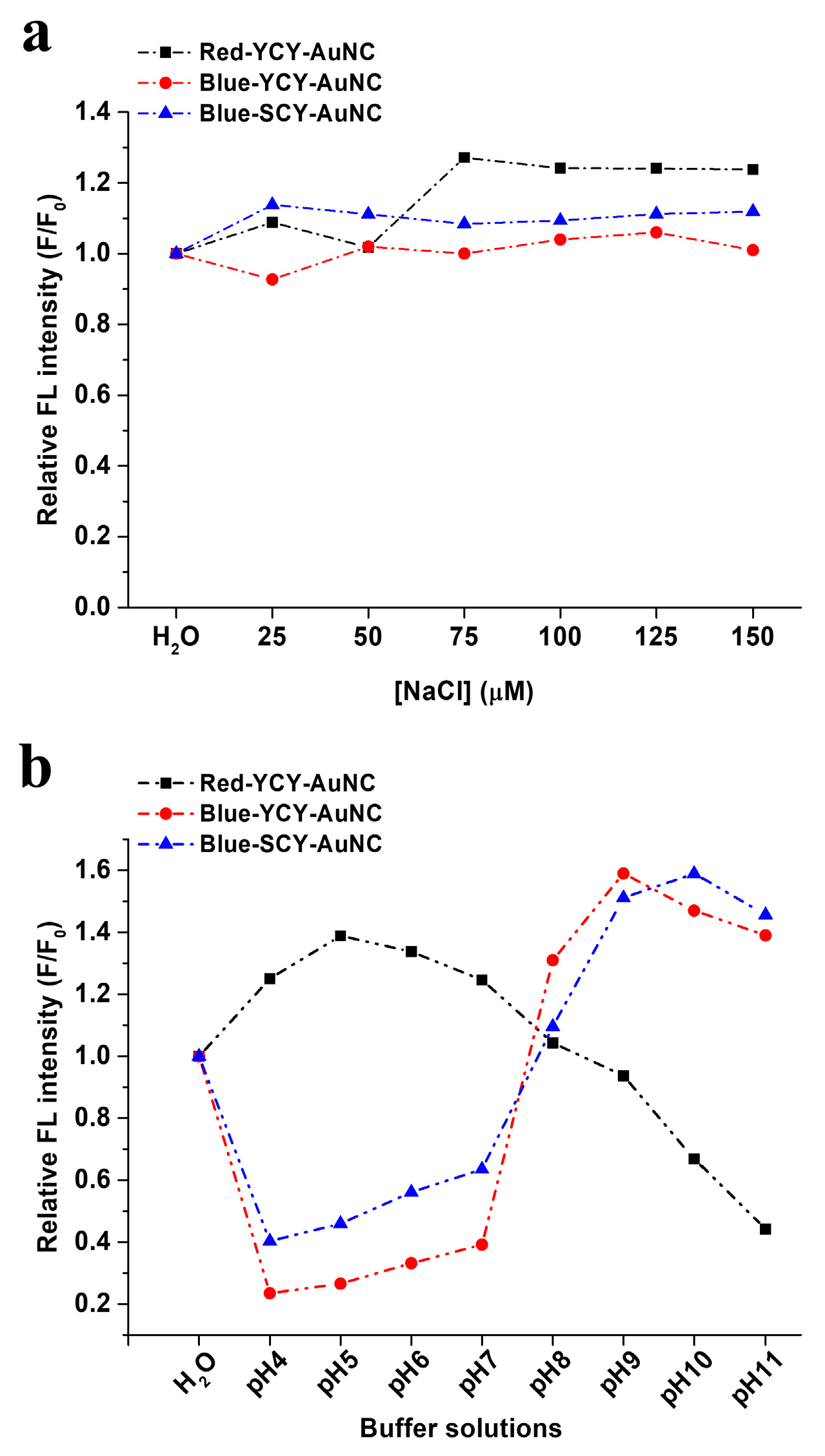
3.5. The Influence of pH and NaCl on Fluorescence Intensity
3.6. Fluorescent Detections of Metal Ions
3.7. Quantum Yield (QY) Measurement of AuNCs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fang, J.; Li, J.; Zhang, B.; Yuan, X.; Asakura, H.; Tanaka, T.; Teramura, K.; Xie, J.; Yan, N. The support effect on the size and catalytic activity of thiolated Au(2)(5) nanoclusters as precatalysts. Nanoscale 2015, 7, 6325–6333. [Google Scholar] [CrossRef] [PubMed]
- Yahia-Ammar, A.; Sierra, D.; Merola, F.; Hildebrandt, N.; Le Guevel, X. Self-Assembled Gold Nanoclusters for Bright Fluorescence Imaging and Enhanced Drug Delivery. ACS Nano 2016, 10, 2591–2599. [Google Scholar] [CrossRef] [PubMed]
- Ju, E.; Liu, Z.; Du, Y.; Tao, Y.; Ren, J.; Qu, X. Heterogeneous assembled nanocomplexes for ratiometric detection of highly reactive oxygen species in vitro and in vivo. ACS Nano 2014, 8, 6014–6023. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, C.; Liu, Y.; Zhang, J.; Bao, C.; Liang, S.; Wang, Q.; Yang, Y.; Fu, H.; Wang, K.; et al. Gold Nanoclusters-Based Nanoprobes for Simultaneous Fluorescence Imaging and Targeted Photodynamic Therapy with Superior Penetration and Retention Behavior in Tumors. Adv. Funct. Mater. 2015, 25, 1314–1325. [Google Scholar] [CrossRef]
- Sun, S.; Ning, X.; Zhang, G.; Wang, Y.C.; Peng, C.; Zheng, J. Dimerization of Organic Dyes on Luminescent Gold Nanoparticles for Ratiometric pH Sensing. Angew. Chem. Int. Ed. Engl. 2016, 55, 2421–2424. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, X.; Jiang, Y.W.; Ma, N.; Xia, L.Y.; Cheng, X.; Jia, H.R.; Liu, P.; Gu, N.; Chen, Z.; et al. Glutathione-Depleting Gold Nanoclusters for Enhanced Cancer Radiotherapy through Synergistic External and Internal Regulations. ACS Appl. Mater. Interfaces 2018, 10, 10601–10606. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Setyawati, M.I.; Leong, D.T.; Xie, J. Antimicrobial Gold Nanoclusters. ACS Nano 2017, 11, 6904–6910. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Gonzalez, J.I.; Zheng, J.; Dickson, R.M. Single-molecule optoelectronics. Acc. Chem. Res. 2005, 38, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Nicovich, P.R.; Dickson, R.M. Highly fluorescent noble-metal quantum dots. Annu. Rev. Phys. Chem. 2007, 58, 409–431. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, M.A. Some interesting properties of metals confined in time and nanometer space of different shapes. Acc. Chem. Res. 2001, 34, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Jin, R. Quantum sized, thiolate-protected gold nanoclusters. Nanoscale 2010, 2, 343–362. [Google Scholar] [CrossRef] [PubMed]
- Gui, Y.; Wang, Y.; He, C.; Tan, Z.; Gao, L.; Li, W.; Bi, H. Multi-optical signal channel gold nanoclusters and their application in heavy metal ions sensing arrays. J. Mater. Chem. C 2021, 9, 2833–2839. [Google Scholar] [CrossRef]
- Burratti, L.; Ciotta, E.; Bolli, E.; Kaciulis, S.; Casalboni, M.; De Matteis, F.; Garzón-Manjón, A.; Scheu, C.; Pizzoferrato, R.; Prosposito, P. Fluorescence enhancement induced by the interaction of silver nanoclusters with lead ions in water. Colloids Surf. A Physicochem. Eng. Asp. 2019, 579, 123634. [Google Scholar] [CrossRef]
- Luo, P.; Zheng, Y.; Qin, Z.; Li, C.; Jiang, H.; Wang, X. Fluorescence light up detection of aluminium ion and imaging in live cells based on the aggregation-induced emission enhancement of thiolated gold nanoclusters. Talanta 2019, 204, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Nain, A.; Tseng, Y.-T.; Lin, Y.-S.; Wei, S.-C.; Mandal, R.P.; Unnikrishnan, B.; Huang, C.-C.; Tseng, F.-G.; Chang, H.-T. Tuning the photoluminescence of metal nanoclusters for selective detection of multiple heavy metal ions. Sens. Actuators B Chem. 2020, 321, 128539. [Google Scholar] [CrossRef]
- Duan, H.; Nie, S. Etching colloidal gold nanocrystals with hyperbranched and multivalent polymers: A new route to fluorescent and water-soluble atomic clusters. J. Am. Chem. Soc. 2007, 129, 2412–2413. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.A.; Yang, T.Y.; Lee, C.H.; Huang, S.H.; Sperling, R.A.; Zanella, M.; Li, J.K.; Shen, J.L.; Wang, H.H.; Yeh, H.I.; et al. Synthesis, characterization, and bioconjugation of fluorescent gold nanoclusters toward biological labeling applications. ACS Nano 2009, 3, 395–401. [Google Scholar] [CrossRef]
- Chen, T.-H.; Nieh, C.-C.; Shih, Y.-C.; Ke, C.-Y.; Tseng, W.-L. Hydroxyl radical-induced etching of glutathione-capped gold nanoparticles to oligomeric AuI–thiolate complexes. RSC Adv. 2015, 5, 45158–45164. [Google Scholar] [CrossRef]
- Song, W.; Liang, R.P.; Wang, Y.; Zhang, L.; Qiu, J.D. Green synthesis of peptide-templated gold nanoclusters as novel fluorescence probes for detecting protein kinase activity. Chem. Commun. 2015, 51, 10006–10009. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Babanova, S.; Rocha, R.C.; Desireddy, A.; Artyushkova, K.; Boncella, A.E.; Atanassov, P.; Martinez, J.S. A Hybrid DNA-Templated Gold Nanocluster for Enhanced Enzymatic Reduction of Oxygen. J. Am. Chem. Soc. 2015, 137, 11678–11687. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Huang, H.; Feng, J.-J.; Luo, X.; Fang, K.-M.; Wang, Z.-G.; Wang, A.-J. A polypeptide-mediated synthesis of green fluorescent gold nanoclusters for Fe3+ sensing and bioimaging. J. Colloid. Interface Sci. 2017, 506, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Duan, R.; Liu, R.; Guan, M.; Chen, W.; Ma, J.; Chen, M.; Du, B.; Zhang, Q. Chitosan-Stabilized Self-Assembled Fluorescent Gold Nanoclusters for Cell Imaging and Biodistribution In Vivo. ACS Biomater. Sci. Eng. 2018, 4, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, H.; Li, D.; Li, L.; Lou, X.; Liu, H. Self-Nucleation and Self-Assembly of Highly Fluorescent Au5 Nanoclusters for Bioimaging. Chem. Mater. 2018, 30, 5507–5515. [Google Scholar] [CrossRef]
- Nandi, I.; Chall, S.; Chowdhury, S.; Mitra, T.; Roy, S.S.; Chattopadhyay, K. Protein Fibril-Templated Biomimetic Synthesis of Highly Fluorescent Gold Nanoclusters and Their Applications in Cysteine Sensing. ACS Omega 2018, 3, 7703–7714. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Si, Y.; Sun, Z.; Chen, L.; Li, R.; Qiao, Y.; Wang, H. Rapid, Selective, and Ultrasensitive Fluorimetric Analysis of Mercury and Copper Levels in Blood Using Bimetallic Gold–Silver Nanoclusters with “Silver Effect”-Enhanced Red Fluorescence. Anal. Chem. 2014, 86, 11714–11721. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.-H.; Tseng, W.-L. (Lysozyme Type VI)-Stabilized Au8 Clusters: Synthesis Mechanism and Application for Sensing of Glutathione in a Single Drop of Blood. Small 2012, 8, 1912–1919. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zheng, Y.; Ying, J.Y. Protein-directed synthesis of highly fluorescent gold nanoclusters. J. Am. Chem. Soc. 2009, 131, 888–889. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cai, C.; Razzaque, S.; Hussain, I.; Lu, Q.-W.; Tan, B. Synthesis of water-soluble and highly fluorescent gold nanoclusters for Fe3+ sensing in living cells using fluorescence imaging. J. Mater. Chem. B 2017, 5, 5608–5615. [Google Scholar] [CrossRef]
- Annie Ho, J.-a.; Chang, H.-C.; Su, W.-T. DOPA-Mediated Reduction Allows the Facile Synthesis of Fluorescent Gold Nanoclusters for Use as Sensing Probes for Ferric Ions. Anal. Chem. 2012, 84, 3246–3253. [Google Scholar] [CrossRef] [PubMed]
- Bothra, S.; Upadhyay, Y.; Kumar, R.; Ashok Kumar, S.K.; Sahoo, S.K. Chemically modified cellulose strips with pyridoxal conjugated red fluorescent gold nanoclusters for nanomolar detection of mercuric ions. Biosens. Bioelectron. 2017, 90, 329–335. [Google Scholar] [CrossRef]
- Wang, J.; Ma, S.; Ren, J.; Yang, J.; Qu, Y.; Ding, D.; Zhang, M.; Yang, G. Fluorescence enhancement of cysteine-rich protein-templated gold nanoclusters using silver(I) ions and its sensing application for mercury(II). Sens. Actuators B Chem. 2018, 267, 342–350. [Google Scholar] [CrossRef]
- Bain, D.; Maity, S.; Paramanik, B.; Patra, A. Core-Size Dependent Fluorescent Gold Nanoclusters and Ultrasensitive Detection of Pb2+ Ion. ACS Sustain. Chem. Eng. 2018, 6, 2334–2343. [Google Scholar] [CrossRef]
- Deng, L.; Zhou, Z.; Li, J.; Li, T.; Dong, S. Fluorescent silver nanoclusters in hybridized DNA duplexes for the turn-on detection of Hg2+ ions. Chem. Commun. 2011, 47, 11065–11067. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Chen, S.; Zhang, L.; Huang, H.; Zeng, Y.; Liu, F. Multiplex sensor for detection of different metal ions based on on–off of fluorescent gold nanoclusters. Anal. Chim. Acta 2014, 852, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zheng, Y.; Ying, J.Y. Highly selective and ultrasensitive detection of Hg2+ based on fluorescence quenching of Au nanoclusters by Hg2+–Au+ interactions. Chem. Commun. 2010, 46, 961–963. [Google Scholar] [CrossRef] [PubMed]
- Żamojć, K.; Kamrowski, D.; Zdrowowicz, M.; Wyrzykowski, D.; Wiczk, W.; Chmurzyński, L.; Makowska, J. A Pentapeptide with Tyrosine Moiety as Fluorecent Chmosensor for Selective Nanomolar-Level Detection of Copper(II) Ions. Int. J. Mol. Sci. 2020, 21, 743. [Google Scholar] [CrossRef]
- Remko, M.; Fitz, D.; Broer, R.; Rode, B.M. Effect of Metal Ions (Ni2+, Cu2+ and Zn2+) and Water Coordination on the Structure of L-phenylalanine, L-tyrosine, L-tryptophan and Their Zwitterionic Forms. J. Mol. Model. 2011, 17, 3117–3128. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kautu, A.; Singh, N.; Kumar, N.; Kumar, V.; Singh, R.; Kesharwani, K.; Swain, N.; Gupta, P.; Joshi, K.B. Metallopeptide-inspired Pyridine-bis-tyrosine Peptide Conjugated Mediated Facile Room Temperature Synthesis of Ultrafine Solid Mercury Nanoparticles for Plausible Applications. Next Mater. 2024, 4, 10118–10129. [Google Scholar]
- Sumiyoshi, S.; Suyama, K.; Tatsubo, D.; Tanak, N.; Tomohara, K.; Taniguchi, S.; Maeda, I.; Nose, T. Metal Ion Scavenging Activity of Elastin-like Peptide Analogues Containing a Cadmium Ion Binding Sequence. Sci. Rep. 2022, 12, 1861–1876. [Google Scholar] [CrossRef] [PubMed]
- Selvakannan, P.R.; Swami, A.; Srisathiyanarayanan, D.; Shirude, P.S.; Pasricha, R.; Mandale, A.B.; Sastry, M. Synthesis of Aqueous Au Core−Ag Shell Nanoparticles Using Tyrosine as a pH-Dependent Reducing Agent and Assembling Phase-Transferred Silver Nanoparticles at the Air−Water Interface. Langmuir 2004, 20, 7825–7836. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cui, Y.; Zhao, Y.; Liu, R.; Sun, Z.; Li, W.; Gao, X. Bifunctional peptides that precisely biomineralize Au clusters and specifically stain cell nuclei. Chem. Commun. 2012, 48, 871–873. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.-m.; Li, Y.; Xu, J.; Zhang, C.; Shuang, S.; Dong, C.; Choi, M.M.F. Glutathione-protected fluorescent gold nanoclusters for sensitive and selective detection of Cu2+. Sens. Actuators B Chem. 2013, 183, 583–588. [Google Scholar] [CrossRef]
- Vasimalai, N.; Fernández-Argüelles, M.T.; Espiña, B. Detection of Sulfide Using Mercapto Tetrazine-Protected Fluorescent Gold Nanodots: Preparation of Paper-Based Testing Kit for On-Site Monitoring. ACS Appl. Mater. Interfaces 2018, 10, 1634–1645. [Google Scholar] [CrossRef] [PubMed]
- Contino, A.; Maccarrone, G.; Zimbone, M.; Reitano, R.; Musumeci, P.; Calcagno, L.; Oliveri, I.P. Tyrosine capped silver nanoparticles: A new fluorescent sensor for the quantitative determination of copper(II) and cobalt(II) ions. J. Colloid. Interface Sci. 2016, 462, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Pettit, L.D.; Steel, I.; Kowalik, T.; Kozlowski, H.; Bataille, M. Specific binding of the tyrosine residue in copper(II) complexes of Tyr-Pro-Gly-Tyr and Tyr-Gly-Pro-Tyr. J. Chem. Soc. Dalton Trans. 1985, 6, 1201–1205. [Google Scholar] [CrossRef]
- Lu, W.; Qin, X.; Liu, S.; Chang, G.; Zhang, Y.; Luo, Y.; Asiri, A.M.; Al-Youbi, A.O.; Sun, X. Economical, Green Synthesis of Fluorescent Carbon Nanoparticles and Their Use as Probes for Sensitive and Selective Detection of Mercury(II) Ions. Anal. Chem. 2012, 84, 5351–5357. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Cheng, X.; Huo, L.; Lu, L. Gold-Nanocluster-Based Fluorescent Sensors for Highly Sensitive and Selective Detection of Cyanide in Water. Adv. Funct. Mater. 2010, 20, 951–956. [Google Scholar] [CrossRef]
- Gordon, D.J.; Fenske, R.F. Theoretical study of o-quinone complexes of iron. Inorg. Chem. 1982, 21, 2916–2923. [Google Scholar] [CrossRef]
- Dikalov, S.I.; Rumyantseva, G.V.; Piskunov, A.V.; Weiner, L.M. Role of quinone-iron(III) interaction in NADPH-dependent enzymatic generation of hydroxyl radicals. Biochemistry 1992, 31, 8947–8953. [Google Scholar] [CrossRef] [PubMed]





| Sample | Detection Metal Ion | Slope & Intercept | R2 | Linear Detection Range (μM) | Limit of Detection (μM) |
|---|---|---|---|---|---|
| Blue-YCY-AuNC | Fe3+ | Y = 0.128x − 0.314 | 0.995 | 0.25–100 | 3.2 |
| Cu2+ | Y = 0.101x + 0.0533 | 0.997 | 0.25–25 | 0.77 | |
| Blue-SCY-AuNC | Fe3+ | Y = 0.0646x + 0.144 | 0.965 | 0.25–37.5 | 4.1 |
| Cu2+ | Y = 0.0424x − 0.0144 | 0.993 | 0.25–25 | 1.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Youn, J.; Kang, P.; Crowe, J.; Thornsbury, C.; Kim, P.; Qin, Z.; Lee, J. Tripeptide-Assisted Gold Nanocluster Formation for Fe3+ and Cu2+ Sensing. Molecules 2024, 29, 2416. https://doi.org/10.3390/molecules29112416
Youn J, Kang P, Crowe J, Thornsbury C, Kim P, Qin Z, Lee J. Tripeptide-Assisted Gold Nanocluster Formation for Fe3+ and Cu2+ Sensing. Molecules. 2024; 29(11):2416. https://doi.org/10.3390/molecules29112416
Chicago/Turabian StyleYoun, Jonghae, Peiyuan Kang, Justin Crowe, Caleb Thornsbury, Peter Kim, Zhenpeng Qin, and Jiyong Lee. 2024. "Tripeptide-Assisted Gold Nanocluster Formation for Fe3+ and Cu2+ Sensing" Molecules 29, no. 11: 2416. https://doi.org/10.3390/molecules29112416







