Copper-Nanoparticle-Induced Neurotoxic Effect and Oxidative Stress in the Early Developmental Stage of Zebrafish (Danio rerio)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Bioaccumulation of Cu
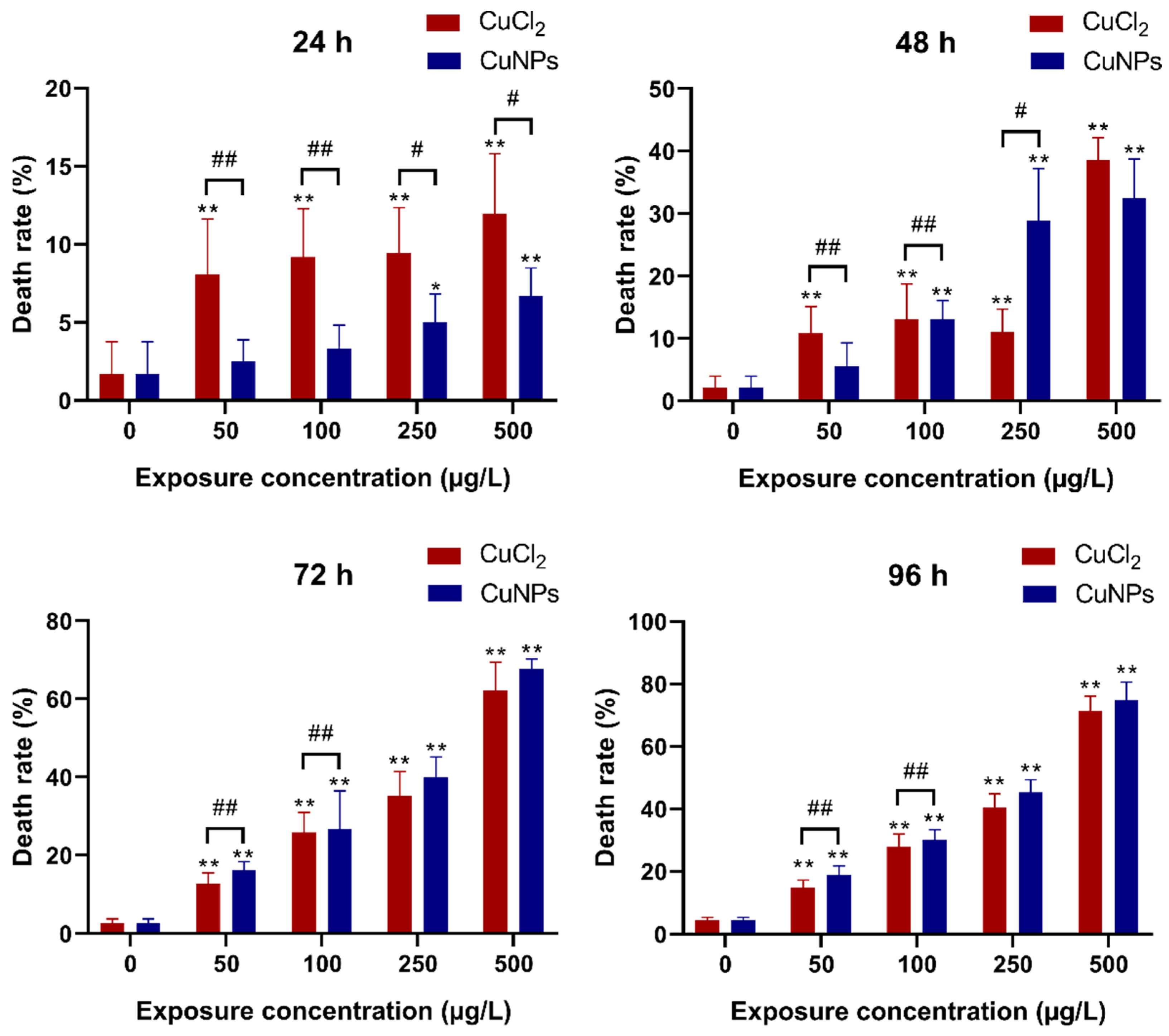
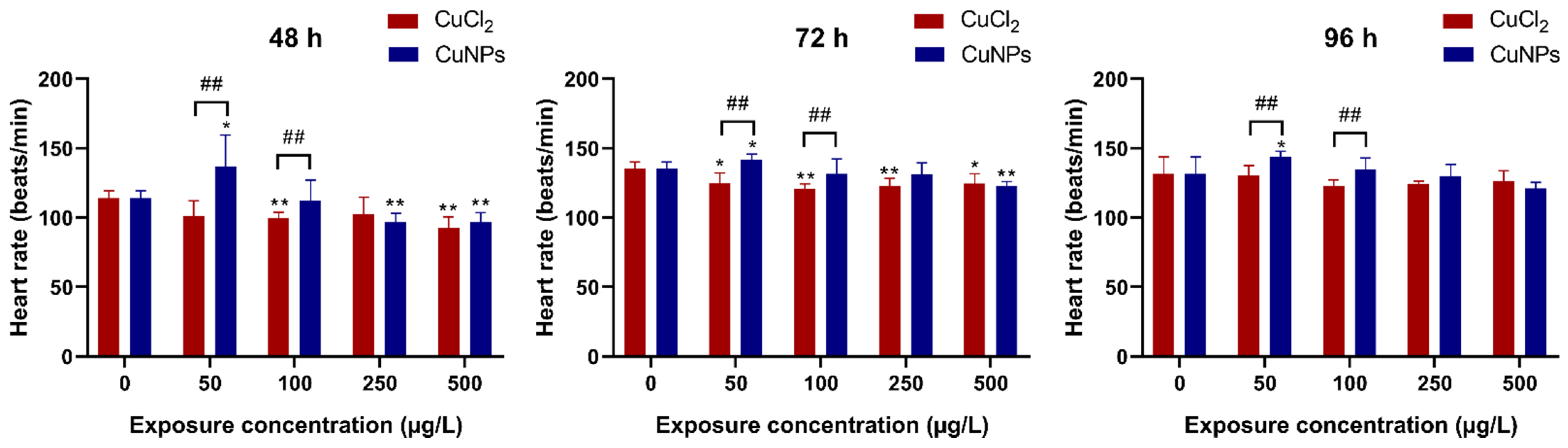
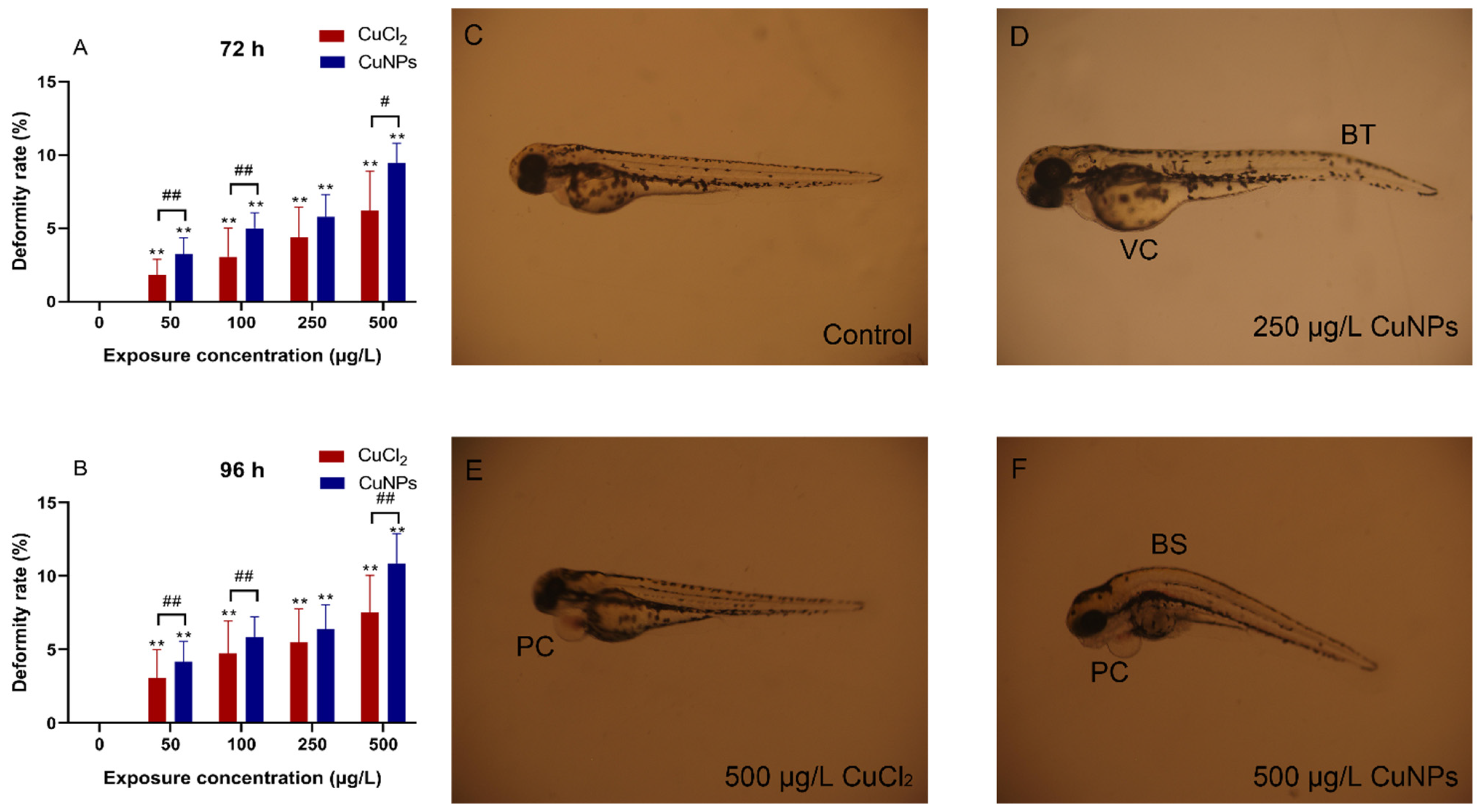
2.2. Embryotoxicity Test
2.3. Neurotoxicity Test
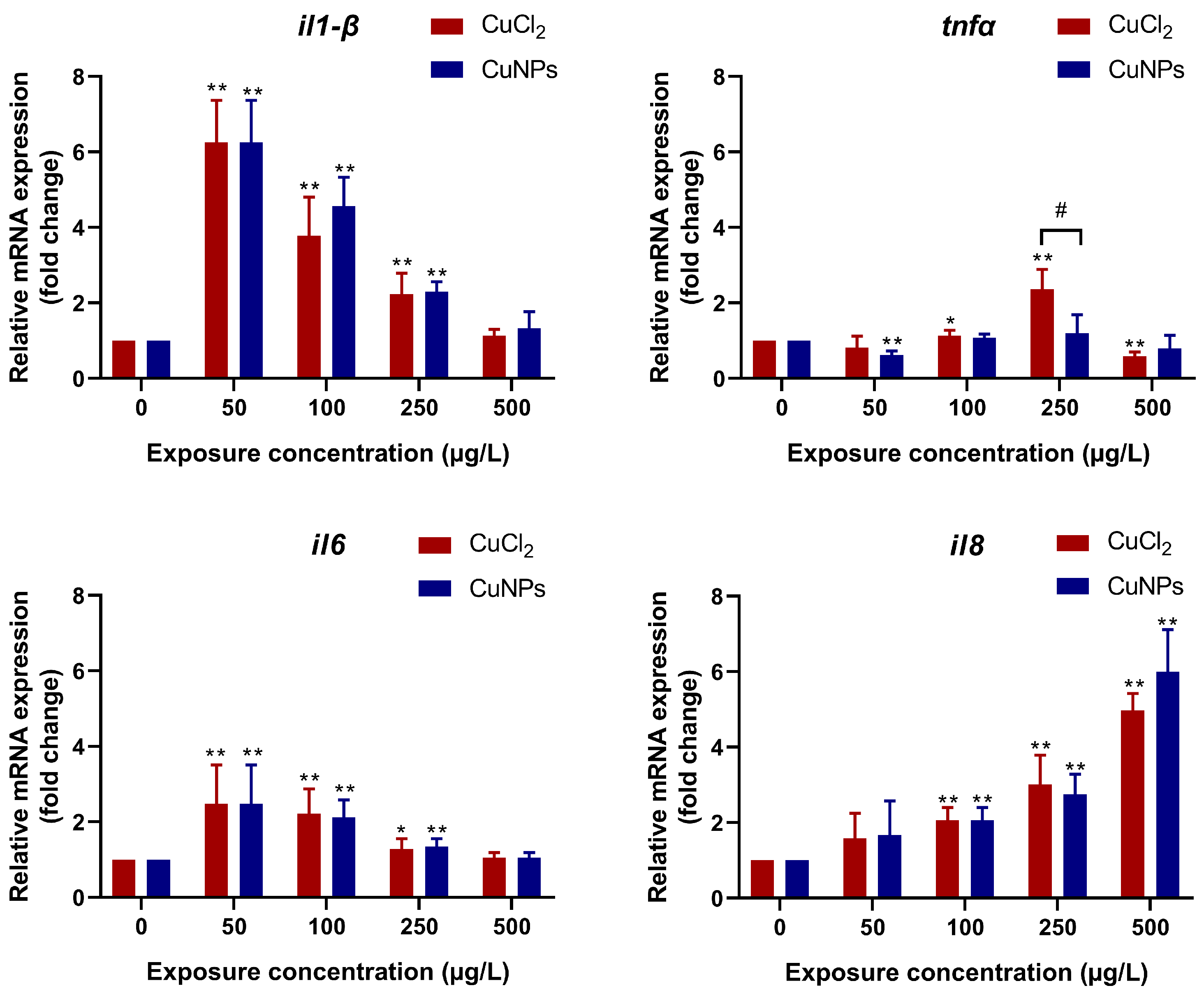
2.4. Oxidative Stress and Inflammatory Response
3. Materials and Methods
3.1. Zebrafish Breeding
3.2. Exposure Protocols
3.3. Measurement of Copper Bioaccumulation
3.4. Physiological Parameter Analysis
3.5. Enzymes Activity Analysis
3.6. mRNA Expression Analysis
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, J.; Hu, S.; Rao, M.; Hu, L.; Lei, H.; Wu, Y.; Wang, Y.; Ke, D.; Xia, W.; Zhu, C. Copper nanoparticle-induced ovarian injury, follicular atresia, apoptosis, and gene expression alterations in female rats. Int. J. Nanomed. 2017, 12, 5959–5971. [Google Scholar] [CrossRef] [PubMed]
- Deka, P.; Borah, B.J.; Saikia, H.; Bharali, P. Cu-based nanoparticles as emerging environmental catalysts. Chem. Rec. 2019, 19, 462–473. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, Y.; Sun, Q.; Xia, L.; Hu, J.; Cheng, K.; Wang, X.; Fu, X.; Gu, H. Copper nanoparticles show obvious in vitro and in vivo reproductive toxicity via ERK mediated signaling pathway in female mice. Int. J. Biol. Sci. 2018, 14, 1834–1844. [Google Scholar] [CrossRef] [PubMed]
- Ameh, T.; Sayes, C.M. The potential exposure and hazards of copper nanoparticles: A review. Environ. Toxicol. Pharmacol. 2019, 71, 103220. [Google Scholar] [CrossRef] [PubMed]
- Sirotkin, A.V.; Radosová, M.; Tarko, A.; Martín-García, I.; Alonso, F. Effect of morphology and support of copper nanoparticles on basic ovarian granulosa cell functions. Nanotoxicology 2020, 14, 683–695. [Google Scholar] [CrossRef] [PubMed]
- Crisan, M.C.; Teodora, M.; Lucian, M. Copper nanoparticles: Synthesis and characterization, physiology, toxicity and antimicrobial applications. Appl. Sci. 2022, 12, 141. [Google Scholar] [CrossRef]
- Chung, K.; Bang, J.; Thacharon, A.; Song, H.Y.; Kang, S.H.; Jang, W.; Dhull, N.; Thapa, D.; Ajmal, C.M.; Song, B.; et al. Non-oxidized bare copper nanoparticles with surface excess electrons in air. Nat. Nanotechnol. 2022, 17, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Cheng, G.; Xu, C.; Liu, H.; Wang, Y.; Li, N.; Fan, X.; Zhu, C.; Xia, W. Copper nanoparticles induce oxidative stress via the heme oxygenase 1 signaling pathway in vitro studies. Int. J. Nanomed. 2021, 16, 1565–1573. [Google Scholar] [CrossRef]
- Ahamed, M.; Lateef, R.; Akhtar, M.J.; Rajanahalli, P. Dietary antioxidant curcumin mitigates CuO nanoparticle-induced cytotoxicity through the oxidative stress pathway in human placental cells. Molecules 2022, 27, 7378. [Google Scholar] [CrossRef]
- Luisa, M.; Giacomo, C. Acute toxicity test of CuO nanoparticles using human mesenchymal stem cells. Toxicol. Mech. Methods 2014, 24, 449–454. [Google Scholar]
- Mahmood, R.I.; Kadhim, A.A.; Ibraheem, S.; Albukhaty, S.; Mohammed-Salih, H.S.; Abbas, R.H.; Jabir, M.S.; Mohammed, M.K.; Nayef, M.; AlMalki, F.A.; et al. Biosynthesis of copper oxide nanoparticles mediated Annona muricata as cytotoxic and apoptosis inducer factor in breast cancer cell lines. Sci. Rep. 2022, 12, 16165. [Google Scholar] [CrossRef] [PubMed]
- Anima, B.; Mondal, P.; Gurusubramanian, G.; Roy, V.K. Mechanistic study of copper nanoparticle (CuNP) toxicity on the mouse uterus via apelin signaling. Environ. Sci. Pollut. Res. Int. 2023, 30, 88824–88841. [Google Scholar] [CrossRef] [PubMed]
- Pohanka, M. Copper and copper nanoparticles toxicity and their impact on basic functions in the body. Bratisl. Med. J. 2019, 120, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A.; Ghorban, K.; Nojoomi, F.; Dadmanesh, M. Smaller copper oxide nanoparticles have more biological effects versus breast cancer and nosocomial infections bacteria. Asian Pac. J. Cancer Prev. 2021, 2, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Kim, B.Y.S.; Rutka, J.T.; Chan, W.C.W. Nanoparticle-mediated cellular response is size-dependent. Nat. Nanotech. 2008, 3, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Høgset, H.; Horgan, C.C.; Armstrong, J.P.K.; Bergholt, M.S.; Torraca, V.; Chen, Q.; Keane, T.J.; Bugeon, L.; Dallman, M.J.; Mostowy, S.; et al. In vivo biomolecular imaging of zebrafish embryos using confocal Raman spectroscopy. Nat. Commun. 2020, 11, 6172. [Google Scholar] [CrossRef] [PubMed]
- Haque, E.; Ward, A.C. Zebrafish as a model to evaluate nanoparticle toxicity. Nanomaterials 2018, 8, 561. [Google Scholar] [CrossRef] [PubMed]
- Strähle, U.; Scholz, S.; Geisler, R.; Greiner, P.; Hollert, H.; Rastegar, S.; Schumacher, A.; Selderslaghs, I.; Weiss, C.; Witters, H. Zebrafish embryos as an alternative to animal experiments—A commentary on the definition of the onset of protected life stages in animal welfare regulations. Reprod. Toxicol. 2012, 33, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Gagné, F.; André, C.; Skirrow, R.; Gélinas, M.; Auclair, J.; Aggelen, G.; Turcott, P.; Gagnon, C. Toxicity of silver nanoparticles to rainbow trout: A toxicogenomic approach. Chemosphere 2012, 89, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Cambier, S.; Rogeberg, M.; Georgantzopoulou, A.; Serchi, T.; Karlsson, C.; Verhaegen, S.; Iversen, T.G.; Guignard, C.; Kruszewski, M.; Hoffmann, L.; et al. Fate and effects of silver nanoparticles on early life-stage development of zebrafish (Danio rerio) in comparison to silver nitrate. Sci. Total Environ. 2018, 610–611, 972–982. [Google Scholar] [CrossRef]
- Yang, L.; He, Z.; Li, X.; Jiang, Z.; Xuan, F.; Tang, B.; Bian, X. Behavior and toxicity assessment of copper nanoparticles in aquatic environment: A case study on red swamp crayfish. J. Environ. Manag. 2022, 313, 114986. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, W. Comparative contributions of copper nanoparticles and ions to copper bioaccumulation and toxicity in barnacle larvae. Environ. Pollut. 2019, 249, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Long, X.H.; Cheng, Y.; Liu, Z.; Yan, S. The potential toxicity of copper nanoparticles and copper sulphate on juvenile Epinephelus coioides. Aquat. Toxicol. 2014, 152, 96–104. [Google Scholar] [CrossRef]
- Yang, Y.; Qin, Z.; Zeng, W.; Yang, T.; Cao, Y.; Mei, C.; Kuang, Y. Toxicity assessment of nanoparticles in various systems and organs. Nanotechnol. Rev. 2017, 6, 279–289. [Google Scholar] [CrossRef]
- Bostan, H.B.; Rezaee, R.; Valokala, M.G.; Tsarouhas, K.; Golokhvast, K.; Tsatsakis, A.M.; Karimi, G. Cardiotoxicity of nano-particles. Life Sci. 2016, 15, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Brun, N.; Varela, M.; Peijnenburg, W.J.G.M.; Spaink, H.P.; Koch, B.E.V.; Vijver, M.G. Nanoparticles induce dermal and intestinal innate immune system responses in zebrafish embryos. Environ. Sci. Nano 2018, 5, 904–916. [Google Scholar] [CrossRef]
- Ates, M.; Demir, V.; Adiguzel, R.; Arslan, Z. Bioaccumulation, subacute toxicity, and tissue distribution of engineered titanium dioxide nanoparticles in goldfish (Carassius auratus). J. Nanomater. 2013, 2013, 460518. [Google Scholar] [CrossRef] [PubMed]
- Stainier, D.Y.; Fishman, M.C. The zebrafish as a model system to study cardiovascular development. Trends Cardiovasc. Med. 1994, 4, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Dooley, K.; Zon, L.I. Zebrafish: A model system for the study of human disease. Curr. Opin. Genet. Dev. 2000, 10, 252–256. [Google Scholar] [CrossRef]
- Jurewicz, A.; Ilyas, S.; Uppal, J.K.; Ivandic, I.; Korsching, S.; Mathur, S. Evaluation of magnetite nanoparticle-based toxicity on embryo–larvae stages of zebrafish (Danio rerio). ACS Appl. Nano Mater. 2020, 3, 1621–1629. [Google Scholar] [CrossRef]
- Lee, W.S.; Cho, H.J.; Kim, E.; Huh, Y.H.; Kim, H.; Kim, B.; Kang, T.; Lee, J.S.; Jeong, J. Bioaccumulation of polystyrene nanoplastics and their effect on the toxicity of Au ions in zebrafish embryos. Nanoscale 2019, 11, 3173–3185. [Google Scholar] [CrossRef] [PubMed]
- Powers, C.M.; Yen, J.; Linney, E.A.; Seidler, F.J.; Slotkin, T.A. Silver exposure in developing zebrafish (Danio rerio): Persistent effects on larval behavior and survival. Neurotoxicol. Teratol. 2010, 32, 391–397. [Google Scholar] [CrossRef]
- Clemente, Z.; Castro, V.L.; Moura, M.A.; Jonsson, C.M.; Fraceto, L.F. Toxicity assessment of TiO2 nanoparticles in zebrafish embryos under different exposure conditions. Aquat. Toxicol. 2014, 147, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Liu, X.; Zhou, Y.; Yao, H. An in vivo evaluation of acute toxicity of cobalt ferrite (CoFe2O4) nanoparticles in larval-embryo Zebrafish (Danio rerio). Aquat. Toxicol. 2015, 166, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, J.; Liu, L.; Huang, C.; Zhou, D.; Fu, L. Characterization and toxicology evaluation of chitosan nanoparticles on the embryonic development of zebrafish, Danio rerio. Carbohydr. Polym. 2016, 141, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Samaee, S.M.; Rabbani, S.; Jovanović, B.; Mohajeri-Tehrani, M.R.; Haghpanah, V. Efficacy of the hatching event in assessing the embryo toxicity of the nano-sized TiO2 particles in zebrafish: A comparison between two different classes of hatching-derived variables. Ecotoxicol. Environ. Saf. 2015, 116, 121–128. [Google Scholar] [CrossRef]
- Caro, C.; Egea-Benavente, D.; Polvillo, R.; Royo, J.L.; Leal, P.M.; García-Martín, M.L. Comprehensive toxicity assessment of PEGylated magnetic nanoparticles for in vivo applications. Colloids. Surf. B Biointerfaces 2019, 177, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Priyam, A.; Singh, P.P.; Afonso, L.B.; Schultz, A.G. Exposure to biogenic phosphorus nano-agromaterials promotes early hatching and causes no acute toxicity in zebrafish embryos. Environ. Sci. Nano 2022, 9, 1364–1380. [Google Scholar] [CrossRef]
- De Luca, E.; Zaccaria, G.; Hadhoud, M.; Rizzo, G.; Ponzini, R.; Morbiducci, U.; Santoro, M.M. ZebraBeat: A flexible platform for the analysis of the cardiac rate in zebrafish embryos. Sci. Rep. 2014, 4, 4898. [Google Scholar] [CrossRef]
- Ganesan, S.; Thirumurthi, N.A.; Raghunath, A.; Vijayakumar, S.; Perumal, E. Acute and sub-lethal exposure to copper oxide nanoparticles causes oxidative stress and teratogenicity in zebrafish embryos. J. Appl. Toxicol. 2016, 36, 554–567. [Google Scholar] [CrossRef]
- Kalishwaralal, K.; Jeyabharathi, S.; Sundar, K.; Muthukumaran, A. A novel one-pot green synthesis of selenium nanoparticles and evaluation of its toxicity in zebrafish embryos. Artif. Cells Nanomed. Biotechnol. 2016, 44, 471–477. [Google Scholar] [CrossRef]
- Li, K.; Zhao, X.; Zhai, Y.; Chen, G.; Lee, E.H.; He, S. A study on the biocompatibility of surface-modified Au/Ag alloyed nanobox particles in zebrafish in terms of mortality rate, hatch rate and imaging of particle distribution behavior. Prog. Electromagn. Res. 2015, 150, 89–96. [Google Scholar] [CrossRef]
- Duan, J.; Yu, Y.; Shi, H.; Tian, L.; Guo, C.; Huang, P.; Zhou, X.; Peng, S.; Sun, Z. Toxic effects of silica nanoparticles on zebrafish embryos and larvae. PLoS ONE 2013, 8, e74606. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.A.S.; Brigante, T.A.V.; Oliveira, D.P. Tail coiling assay in zebrafish (Danio rerio) embryos: Stage of development, promising positive control candidates, and selection of an appropriate organic solvent for screening of developmental neurotoxicity (DNT). Water 2021, 13, 119. [Google Scholar] [CrossRef]
- Saint-Amant, L.; Drapeau, P. Time course of the development of motor behaviors in the zebrafish embryo. J. Neurobiol. 1998, 37, 622–632. [Google Scholar] [CrossRef]
- Chen, S.; Qin, Y.; Ye, X.; Liu, J.; Yan, X.; Zhou, L.; Wang, X.; Martyniuk, C.J.; Yan, B. Neurotoxicity of the Cu(OH)2 nanopesticide through perturbing multiple neurotransmitter pathways in developing zebrafish. Environ. Sci. Technol. 2023, 57, 19407–19418. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, A.R.; Sujatha, R.; Paul, V.; Asokan, C.; Govindasamy, S.; Jayakumar, R. Role of nitric oxide on GABA, glutamic acid, activities of GABA-T and GAD in rat brain cerebral cortex. Brain Res. 1999, 837, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Ghit, A.; Assal, D.; Al-Shami, A.S.; Hussein, D.E. GABAA receptors: Structure, function, pharmacology, and related disorders. J. Genet. Eng. Biotechnol. 2021, 19, 123. [Google Scholar] [CrossRef]
- Huang, Z.J. Activity-dependent development of inhibitory synapses and innervation pattern: Role of GABA signaling and beyond. J. Physiol. 2009, 587, 1881–1888. [Google Scholar] [CrossRef]
- Koch, U.; Magnusson, A.K. Unconventional GABA release: Mechanisms and function. Curr. Opin. Neurobiol. 2009, 19, 305–310. [Google Scholar] [CrossRef]
- Jin, X.; Galvan, A.; Wichmann, T.; Smith, Y. Localization and function of GABA transporters GAT-1 and GAT-3 in the basal ganglia. Front. Syst. Neurosci. 2011, 5, 63. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Xia, T.; Nel, A.E. The role of oxidative stress in ambient particulate matter-induced lung diseases and its implications in the toxicity of engineered nanoparticles. Free Radical Bio. Med. 2008, 44, 1689–1699. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Muralikrishnan, S.; Ng, C.T.; Yung, L.Y.; Bay, B.H. Nanoparticle-induced pulmonary toxicity. Exp. Biol. Med. 2010, 235, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Du, J.; Wang, S.; You, H. PFOS and ZnO nanoparticles induced oxidative stress in liver, gill and head of zebrafish. Sci. Discov. 2016, 4, 232–237. [Google Scholar]
- Manke, A.; Wang, L.; Rojanasakul, Y. Mechanisms of nanoparticle-induced oxidative stress and toxicity. BioMed Res. Int. 2013, 2013, 942916. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, K.; Poland, C.A. Inhaled nanoparticles and lung cancer—What we can learn from conventional particle toxicology. Swiss Med. Wkly. 2012, 142, w13547. [Google Scholar] [CrossRef] [PubMed]
- Lina, T.T.; Johnson, S.J.; Wagner, R.D. Intravaginal poly-(D, L-lactic-co-glycolic acid)-(polyethylene glycol) drug-delivery nanoparticles induce pro-inflammatory responses with Candida albicans infection in a mouse model. PLoS ONE 2020, 15, e0240789. [Google Scholar] [CrossRef] [PubMed]
- Marcellus, K.A.; Bugiel, S.; Nunnikhoven, A.; Curran, I.; Gill, S.S. Polystyrene nano- and microplastic particles induce an inflammatory gene expression profile in rat neural stem cell-derived astrocytes in vitro. Nanomaterials 2024, 14, 429. [Google Scholar] [CrossRef]
- Wang, X.; Ren, X.; He, H.; Li, F.; Liu, K.; Zhao, F.; Hu, H.; Zhang, P.; Huang, B.; Pan, X. Cytotoxicity and pro-inflammatory effect of polystyrene nano-plastic and micro-plastic on RAW264.7 cells. Toxicology 2023, 484, 153391. [Google Scholar] [CrossRef]
- Zuo, H.; Ma, X.; Yang, K.; Chen, Y.; Chen, J.; Guo, Y.; Zhao, J.; Wang, R.; Fang, F.; Liu, Y. distribution and risk assessment of metals in surface water and sediment in the upper reaches of the Yellow River, China. Soil. Sediment. Contam. 2016, 25, 917–940. [Google Scholar] [CrossRef]
- Čmelík, J.; Brovdyová, T.; Trögl, J.; Neruda, M.; Kadlečík, M.; Pacina, J.; Popelka, J.; Sirotkin, A. Changes in the content of heavy metals in Bílina River during 2012–2017: Effects of flood and industrial inputs. Water 2019, 11, 481. [Google Scholar] [CrossRef]
- Abdullah, S.N.S.; Subramaniam, K.A.; Zamani, Z.H.M.; Sarchio, S.N.E.; Yasin, F.M.; Shamsi, S. Biocompatibility study of curcumin-loaded pluronic F127 nanoformulation (NanoCUR) against the embryonic development of zebrafish (Danio rerio). Molecules 2022, 27, 4493. [Google Scholar] [CrossRef] [PubMed]
- Shamsi, S.; Alagan, A.A.; Sarchio, S.N.E.; Yasin, F.M. Synthesis, characterization, and toxicity assessment of Pluronic F127-functionalized graphene oxide on the embryonic development of zebrafish (Danio rerio). Int. J. Nanomed. 2020, 15, 8311–8329. [Google Scholar] [CrossRef] [PubMed]





| Gene | Primer (5′-3′) | Genbank No. | Product Length (bp) | |
|---|---|---|---|---|
| rpl7 | F | CAGAGGTATCAATGGTGTCAGCCC | NM_213213644.2 | 119 |
| R | TTCGGAGCATGTTGATGGAGGC | |||
| il-1β | F | TGATGAATGGGTGTTCGGCA | NM_012799.1 | 248 |
| R | TACCGATGCGTGCTACTTCC | |||
| tnf-α | F | CTCCATAAGACCCAGGGCAA | NM_212859.2 | 162 |
| R | CTGGTCCTGGTCATCTCTCC | |||
| il-6 | F | GCAAGAACGAAAACTGCAGC | NM_001079833.1 | 182 |
| R | CGATTCAGTCTGACCGGAGA | |||
| il-8 | F | TGTGTTATTGTTTTCCTGGCATTTC | XM_009306855.1 | 81 |
| R | GCGACAGCGTGGATCTACAG | |||
| gabra1 | F | TCAGGCAGAGCTGGAAGGAT | NM_001077326 | 117 |
| R | TGCCGTTGTGGAAGAACGT | |||
| abat | F | GCGTTCAGGCAAAGCTCT | NM_201498 | 204 |
| R | GCAGGACGGAAACGGAT | |||
| gad | F | AACTCAGGCGATTGTTGCAT | NM_194419 | 109 |
| R | TGAGGACATTTCCAGCCTTC | |||
| gat1 | F | GTGCGAGGACAAGTGCAAAG | NM_001007362 | 193 |
| R | ATGTTTGGAGGTTTGGGGCA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, N.; Tong, L.; Li, K.; Dong, Q.; Jing, J. Copper-Nanoparticle-Induced Neurotoxic Effect and Oxidative Stress in the Early Developmental Stage of Zebrafish (Danio rerio). Molecules 2024, 29, 2414. https://doi.org/10.3390/molecules29112414
Liu N, Tong L, Li K, Dong Q, Jing J. Copper-Nanoparticle-Induced Neurotoxic Effect and Oxidative Stress in the Early Developmental Stage of Zebrafish (Danio rerio). Molecules. 2024; 29(11):2414. https://doi.org/10.3390/molecules29112414
Chicago/Turabian StyleLiu, Na, Luyao Tong, Kunjie Li, Qiuxia Dong, and Jieying Jing. 2024. "Copper-Nanoparticle-Induced Neurotoxic Effect and Oxidative Stress in the Early Developmental Stage of Zebrafish (Danio rerio)" Molecules 29, no. 11: 2414. https://doi.org/10.3390/molecules29112414






