Rational Design of Enzymatic Electrodes: Impact of Carbon Nanomaterial Types on the Electrode Performance
Abstract
:1. Introduction
2. Results and Discussion
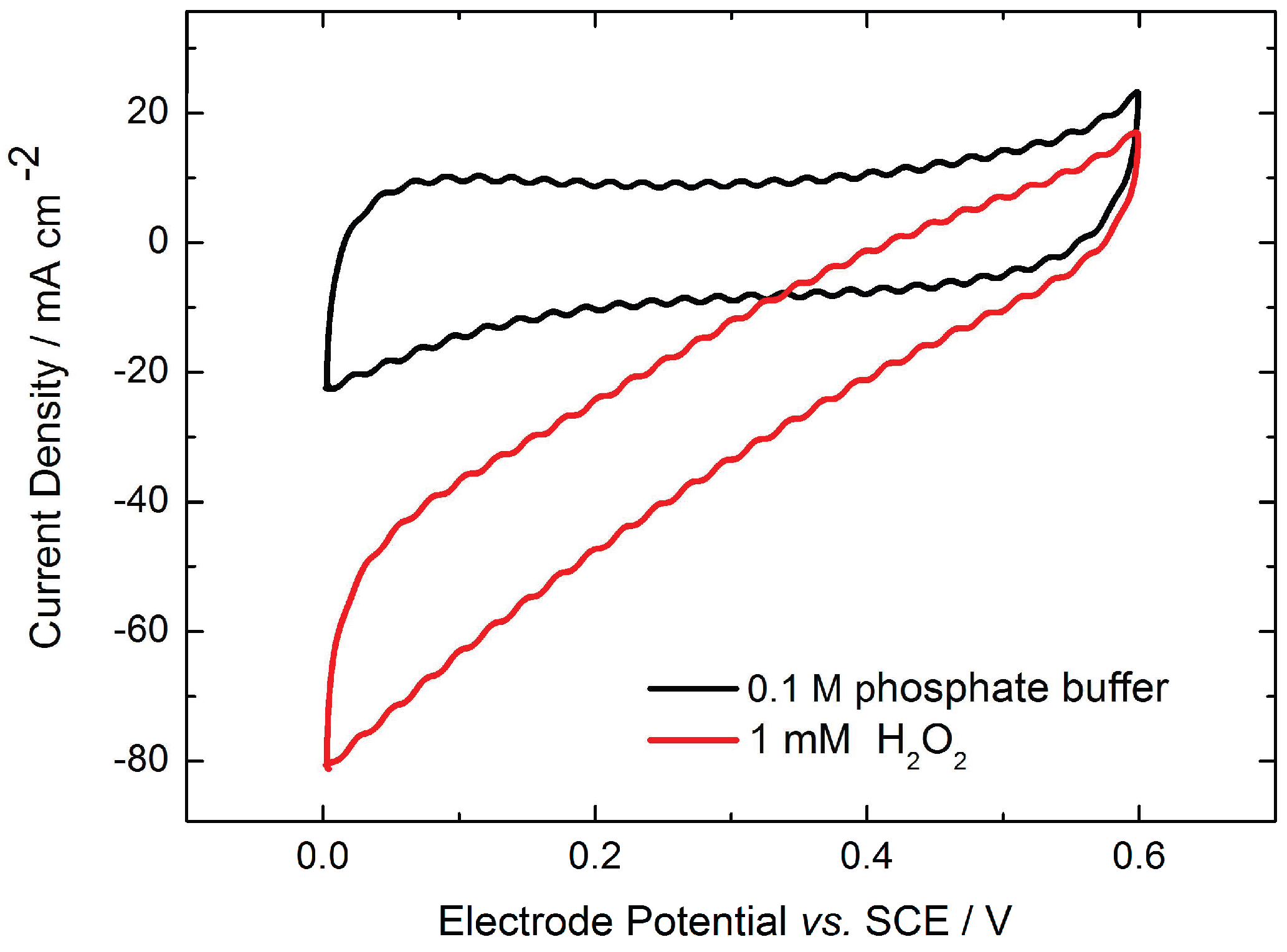
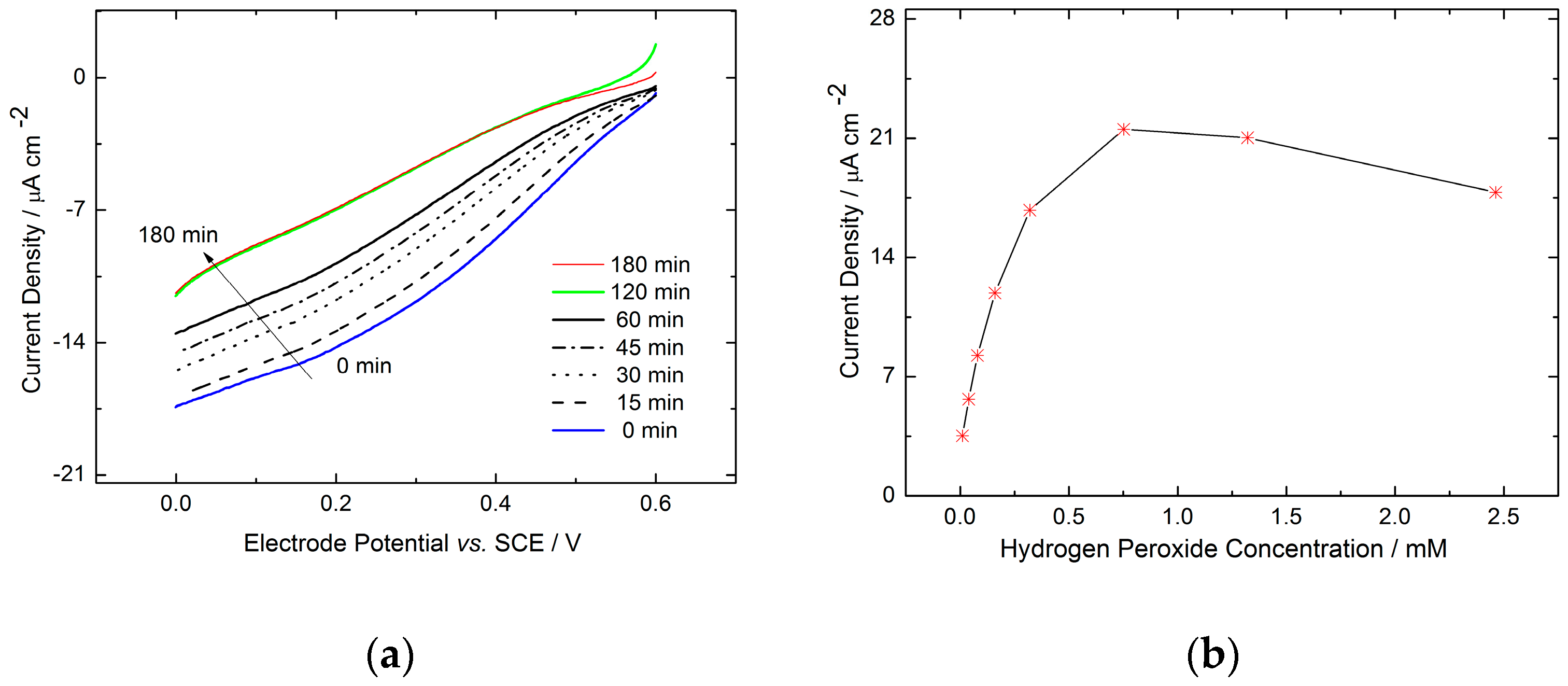
2.1. Monolayer Electrode Design
2.2. Three-Dimensional Electrode Design: Influence of Different Types of Nanomaterials on the Enzymatic Electrode Performance
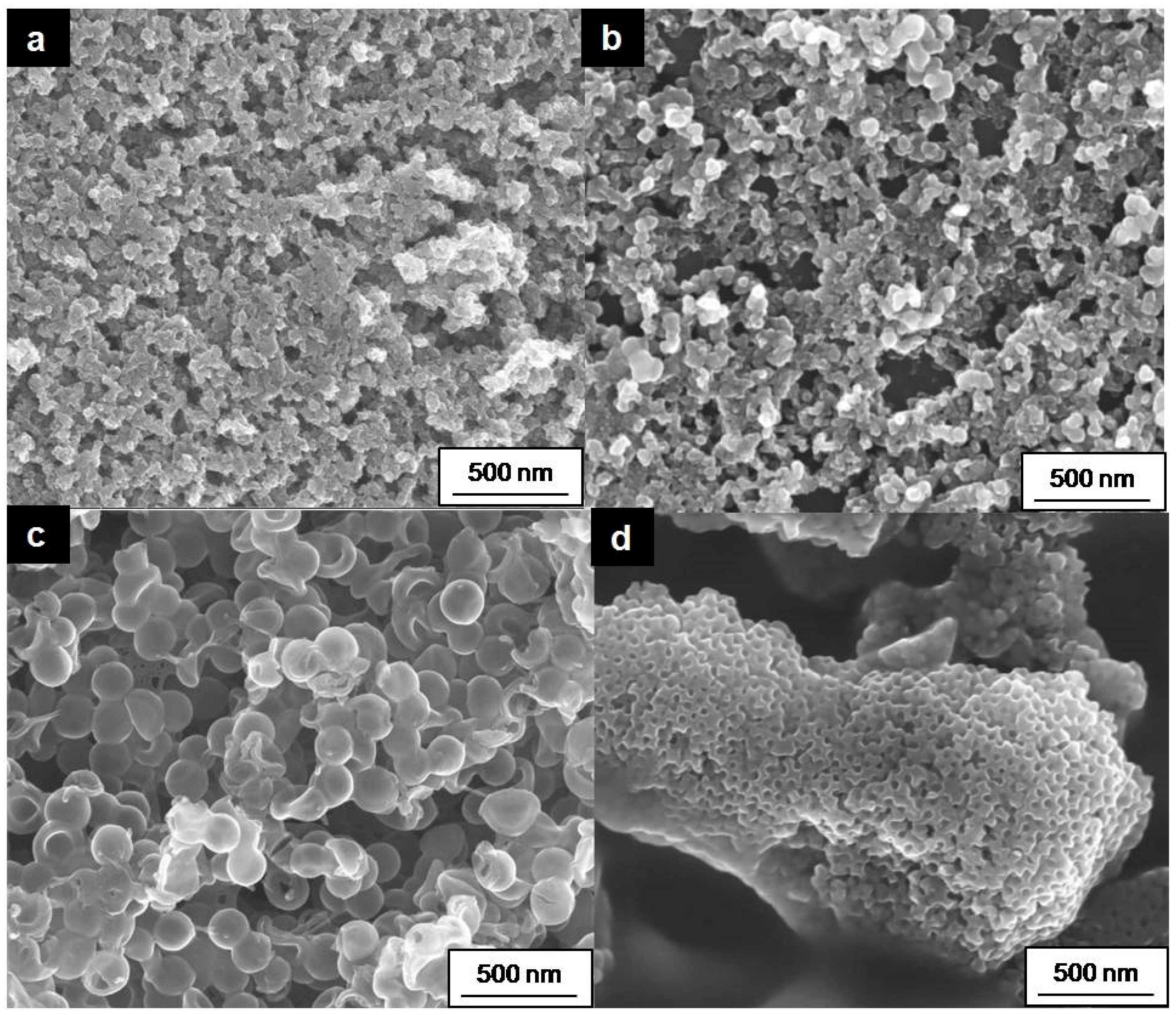
2.2.1. Material Characterization
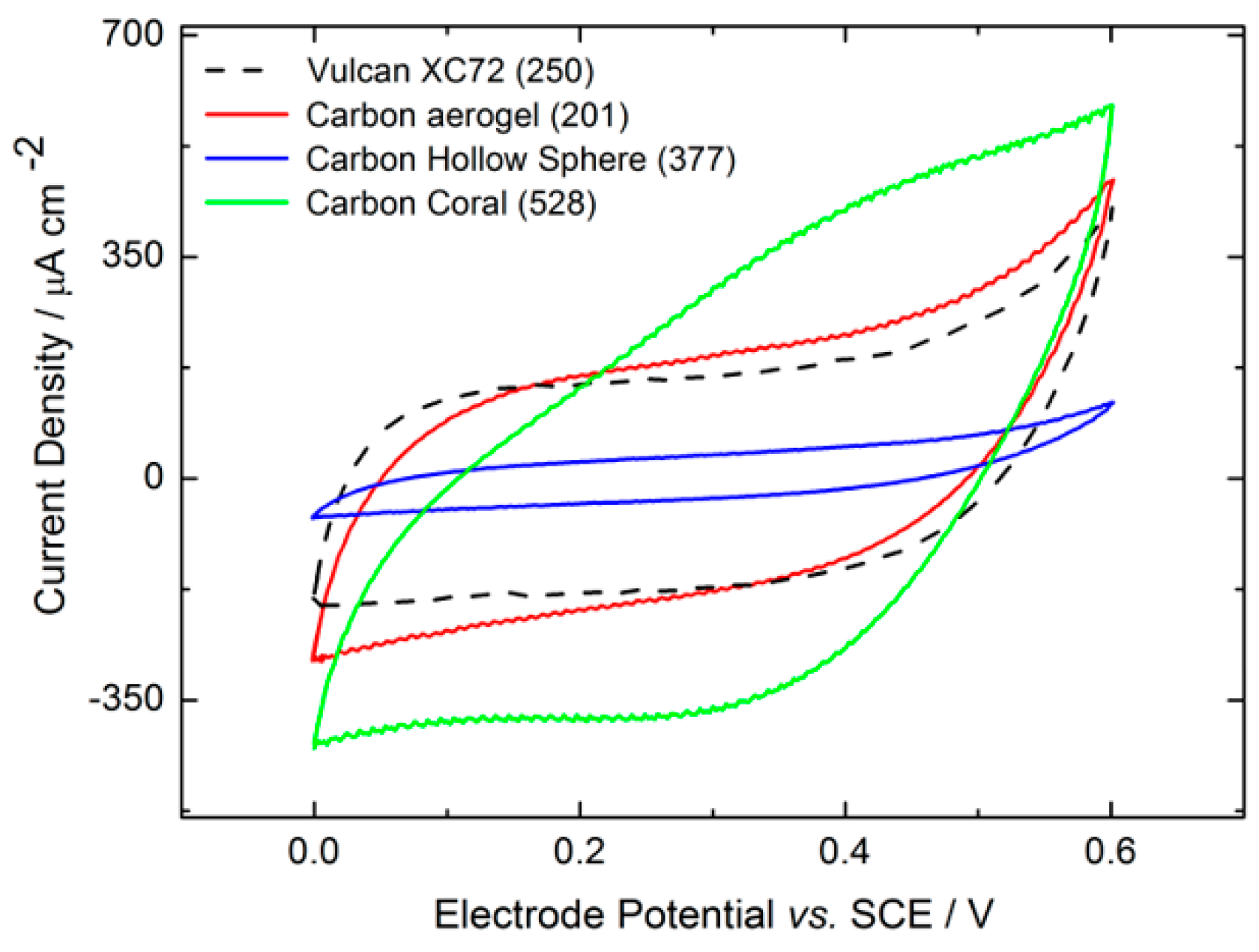
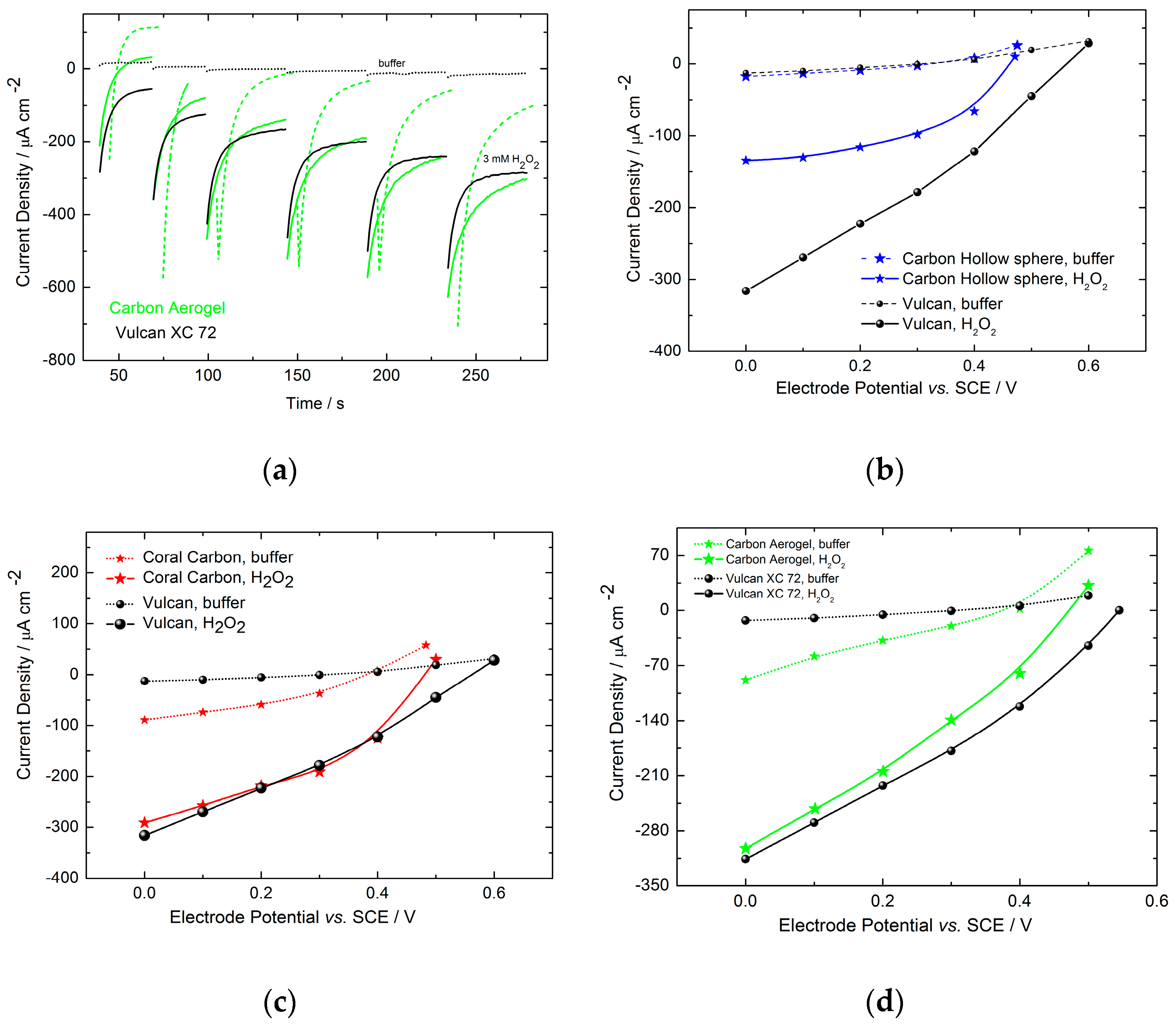
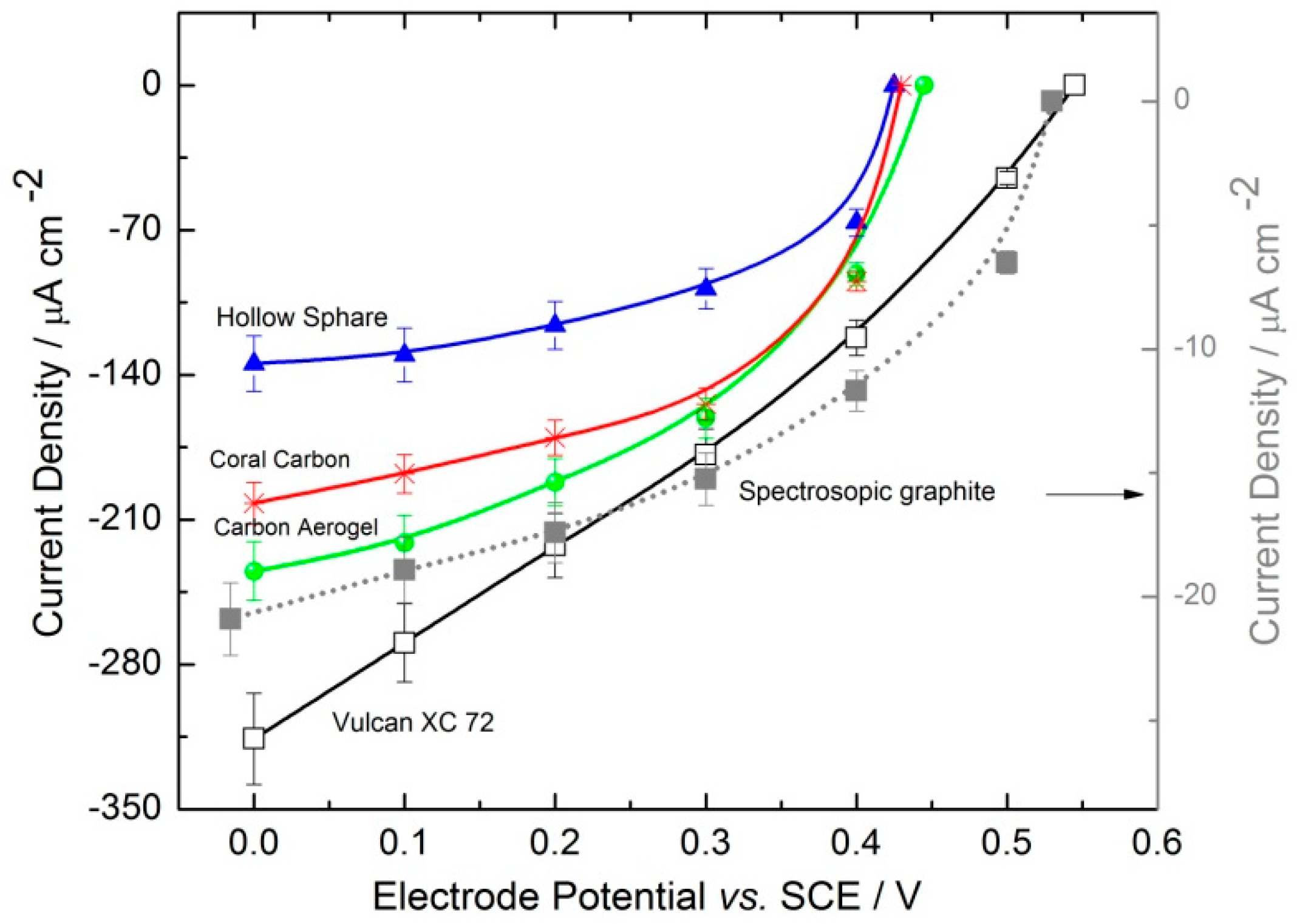
2.2.2. Electrochemical Electrode Characterization
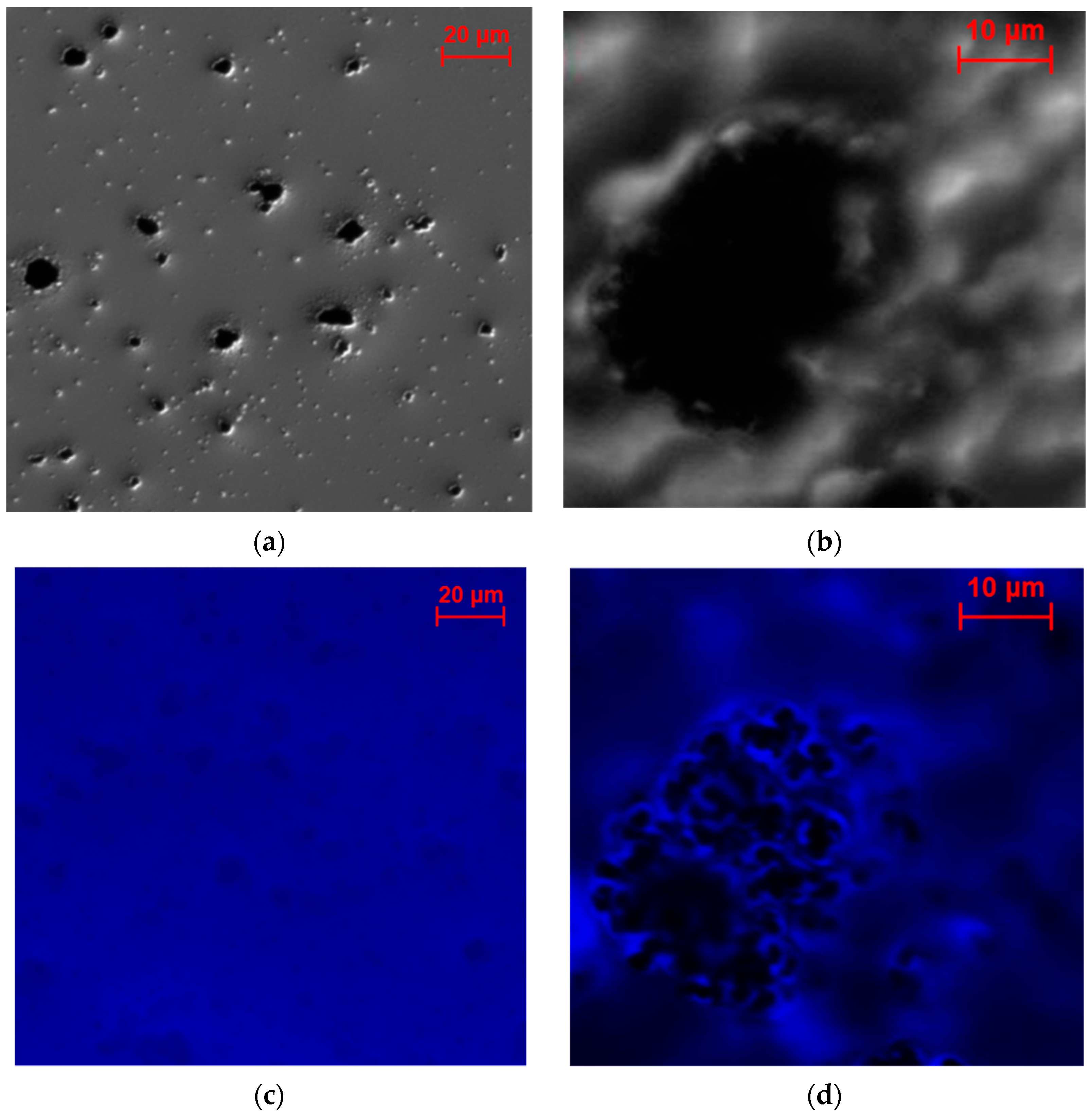
2.2.3. Investigation of the Electrode Cross-Sections
2.3. Characterization of the Enzyme Distribution
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Carbon Nanomaterial Synthesis
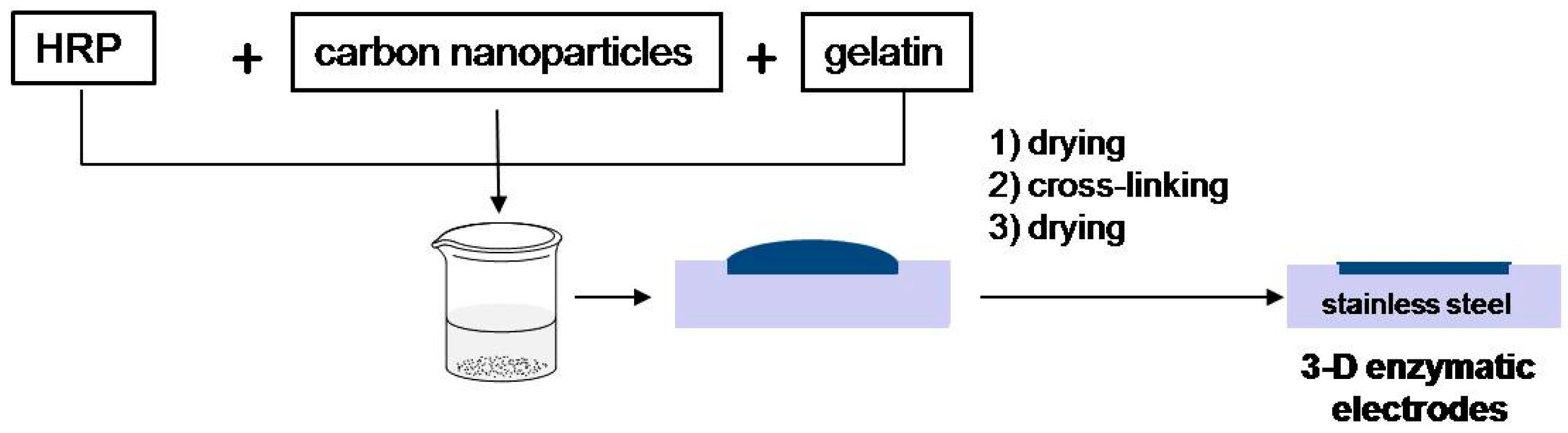
3.3. Enzymatic Electrode Preparations
3.4. Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cooney, M.J.; Svoboda, V.; Lau, C.; Martin, G.; Minteer, S.D. Enzyme catalysed biofuel cells. Energy Environ. Sci. 2008, 1, 320–337. [Google Scholar] [CrossRef]
- Kim, J.; Jia, H.F.; Wang, P. Challenges in biocatalysis for enzyme-based biofuel cells. Biotechnol. Adv. 2006, 24, 296–308. [Google Scholar] [CrossRef]
- Minteer, S.D. Nanobioelectrocatalysis and Its Applications in Biosensors, Biofuel Cells and Bioprocessing. Top. Catal. 2012, 55, 1157–1161. [Google Scholar] [CrossRef]
- Ivanov, I.; Vidakovic-Koch, T.; Sundmacher, K. Recent Advances in Enzymatic Fuel Cells: Experiments and Modeling. Energies 2010, 3, 803–846. [Google Scholar] [CrossRef]
- Osman, M.H.; Shah, A.A.; Walsh, F.C. Recent progress and continuing challenges in bio-fuel cells. Part I: Enzymatic cells. Biosens. Bioelectron. 2011, 26, 3087–3102. [Google Scholar] [CrossRef]
- Tamaki, T. Enzymatic Biofuel Cells Based on Three-Dimensional Conducting Electrode Matrices. Top. Catal. 2012, 55, 1162–1180. [Google Scholar] [CrossRef]
- Mazurenko, I.; Hitaishi, V.P.; Lojou, E. Recent advances in surface chemistry of electrodes to promote direct enzymatic bioelectrocatalysis. Curr. Opin. Electrochem. 2020, 19, 113–121. [Google Scholar] [CrossRef]
- Kim, J.; Grate, J.W.; Wang, P. Nanostructures for enzyme stabilization. Chem. Eng. Sci. 2006, 61, 1017–1026. [Google Scholar] [CrossRef]
- Kim, J.B. Nanobiocatalysis and its potential applications. J. Biotechnol. 2010, 150, S71. [Google Scholar] [CrossRef]
- Hwang, E.T.; Gu, M.B. Enzyme stabilization by nano/microsized hybrid materials. Eng. Life Sci. 2013, 13, 49–61. [Google Scholar] [CrossRef]
- Liu, Y.; Du, Y.; Li, C. Direct Electrochemistry Based Biosensors and Biofuel Cells Enabled with Nanostructured Materials. Electroanalysis 2013, 25, 815–831. [Google Scholar] [CrossRef]
- Balamurugan, T.; Ganesh, V.; Shin, J.H. Emerging Trends of Bilirubin Oxidases at the Bioelectrochemical Interface: Paving the Way for Self-Powered Electrochemical Devices and Biosensors. ACS Appl. Bio Mater. 2024, 7, 1381–1399. [Google Scholar]
- Habrioux, A.; Napporn, T.; Servat, K.; Tingry, S.; Kokoh, K.B. Electrochemical characterization of adsorbed bilirubin oxidase on Vulcan XC 72R for the biocathode preparation in a glucose/O-2 biofuel cell. Electrochim. Acta 2010, 55, 7701–7705. [Google Scholar] [CrossRef]
- Gao, F.; Guo, X.; Yin, J.; Zhao, D. Electrocatalytic activity of carbon spheres towards NADH oxidation at low overpotential and its applications in biosensors and biofuel cells. RSC Adv. 2011, 1, 1301–1309. [Google Scholar] [CrossRef]
- Tsujimura, S.; Kamitaka, Y.; Kano, K. Diffusion-controlled oxygen reduction on multi-copper oxidase-adsorbed carbon aerogel electrodes without mediator. Fuel Cells 2007, 7, 463–469. [Google Scholar] [CrossRef]
- Ferapontova, E.E.; Grigorenko, V.G.; Egorov, A.M.; Börchers, T.; Ruzgas, T.; Gorton, L. Direct Electron Transfer in the System Gold Electrode-Recombinant Horseradish Peroxidases. J. Electroanal. Chem. 2001, 509, 19–26. [Google Scholar] [CrossRef]
- Jeerapan, I.; Ma, N. Challenges and Opportunities of Carbon Nanomaterials for Biofuel Cells and Supercapacitors: Personalized Energy for Futuristic Self-Sustainable Devices. J. Carbon Res. C 2019, 5, 62. [Google Scholar] [CrossRef]
- Jönsson, G.; Gorton, L. An electrochemical sensor for hydrogen peroxide based on peroxidase adsorbed on a spectrographic graphite electrode. Electroanalysis 1989, 1, 465–468. [Google Scholar] [CrossRef]
- Ferapontova, E.E.; Puganova, E. Effect of pH on direct electron transfer between graphite and horseradish peroxidase. J. Electroanal. Chem. 2002, 518, 20–26. [Google Scholar] [CrossRef]
- Yamazaki, I.; Tamura, M.; Nakajima, R. Horseradish peroxidase-C. Mol. Cell. Biochem. 1981, 40, 143–153. [Google Scholar] [CrossRef]
- Farhangrazi, Z.S.; Fossett, M.E.; Powers, L.S.; Ellis, W.R., Jr. Variable-temperature spectroelectrochemical study of horseradish peroxidase. Biochemistry 1995, 34, 2866–2871. [Google Scholar] [CrossRef] [PubMed]
- Varnicic, M.; Bettenbrock, K.; Hermsdorf, D.; Vidaković-Koch, T.; Sundmacher, K. Combined electrochemical and microscopic study of porous enzymatic electrodes with direct electron transfer mechanism. RSC Adv. 2014, 4, 36471–36479. [Google Scholar] [CrossRef]
- Xia, H.; Kitazumi, Y.; Shirai, O.; Kano, K. Direct Electron Transfer-type Bioelectrocatalysis of Peroxidase at Mesoporous Carbon Electrodes and Its Application for Glucose Determination Based on Bienzyme System. Anal Sci. 2017, 33, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Seredych, M.; László, K.; Bandosz, T.J. Sulfur-Doped Carbon Aerogel as a Metal-Free Oxygen Reduction Catalyst. ChemCatChem 2015, 7, 2924–2931. [Google Scholar] [CrossRef]
- Varničić, M.; Vidaković-Koch, T.; Sundmacher, K. Gluconic Acid Synthesis in an Electroenzymatic Reactor. Electrochim. Acta 2015, 174, 480–487. [Google Scholar] [CrossRef]
- Bocanegra-Rodríguez, S.; Molins-Legua, C.; Campíns-Falcó, P.; Giroud, F.; Gross, A.; Cosnier, S. Monofunctional pyrenes at carbon nanotube electrodes for direct electron transfer H2O2 reduction with HRP and HRP-bacterial nanocellulose. Biosens. Bioelectron. 2021, 187, 113304. [Google Scholar] [CrossRef]
- Do, T.Q.N.; Varničić, M.; Hanke-Rauschenbach, R.; Vidaković-Koch, T.; Sundmacher, K. Mathematcal Modeling of Porous Enzymatic Electrode with Direct Electron Transfer Mechanism. Electrochim. Acta 2014, 137, 616–626. [Google Scholar] [CrossRef]
- Technical Data Sheet: NC7000™, Multiwall Carbon Nanotubes. Available online: https://www.nanocyl.com/wp-content/uploads/2016/07/DM-TI-02-TDS-NC7000-V08.pdf (accessed on 13 May 2024).
- Ketjenblack EC-300J Properties. Available online: https://www.nouryon.com/product/ketjenblack-ec-300j/ (accessed on 13 May 2024).



| Type of Material | Name | BET, m2 g−1 | Particle/Pore Size, nm |
|---|---|---|---|
| Particles | Carbon Aerogel | 201 | 14/- |
| Vulcan XC72 | 250 | 80/- | |
| Carbon Hollow Sphere | 377 | 168/- | |
| Porous | Coral Carbon | 528 | -/14 |
| Carbon Nanomaterial | BET Surface Area/m2 g−1 | Immobilization Procedure | Enzyme and Carbon Loadings, mg cm−2 | Scan Rate/mV s−1 | Rotation Rate/rpm | jmax/ mA cm−2 | Ref. |
|---|---|---|---|---|---|---|---|
| MWCNT | 250–300 [28] | Adsorption | 2.8 and 0.7 | 10 | no | 0.3 * | [26] |
| KB | 800 [29] | Adsorption/cross-linking with GA | 1.4 and N.A. | 5 | 4000 | ca. 7.5 * | [23] |
| Vulcan XC72 | 250 | Adsorption | 0.31 and 1 | SS | 400 | 1.0 | [22] |
| Vulcan XC72 | 250 | Entrapment | 0.52 and 1.05 | SS | 400 | 0.31 | This work |
| Carbon Aerogel | 201 | Entrapment | 0.52 and 1.05 | SS | 400 | 0.24 | This work |
| Coral Carbon | 528 | Entrapment | 0.52 and 1.05 | SS | 400 | 0.21 | This work |
| Carbon Hollow Spheres | 377 | Entrapment | 0.52 and 1.05 | SS | 400 | 0.14 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varničić, M.; Fellinger, T.-P.; Titirici, M.-M.; Sundmacher, K.; Vidaković-Koch, T. Rational Design of Enzymatic Electrodes: Impact of Carbon Nanomaterial Types on the Electrode Performance. Molecules 2024, 29, 2324. https://doi.org/10.3390/molecules29102324
Varničić M, Fellinger T-P, Titirici M-M, Sundmacher K, Vidaković-Koch T. Rational Design of Enzymatic Electrodes: Impact of Carbon Nanomaterial Types on the Electrode Performance. Molecules. 2024; 29(10):2324. https://doi.org/10.3390/molecules29102324
Chicago/Turabian StyleVarničić, Miroslava, Tim-Patrick Fellinger, Maria-Magdalena Titirici, Kai Sundmacher, and Tanja Vidaković-Koch. 2024. "Rational Design of Enzymatic Electrodes: Impact of Carbon Nanomaterial Types on the Electrode Performance" Molecules 29, no. 10: 2324. https://doi.org/10.3390/molecules29102324






