Synthesis of Bodipy-Tagged Galactoconjugates and Evaluation of Their Antibacterial Properties
Abstract
:1. Introduction
2. Results and Discussion
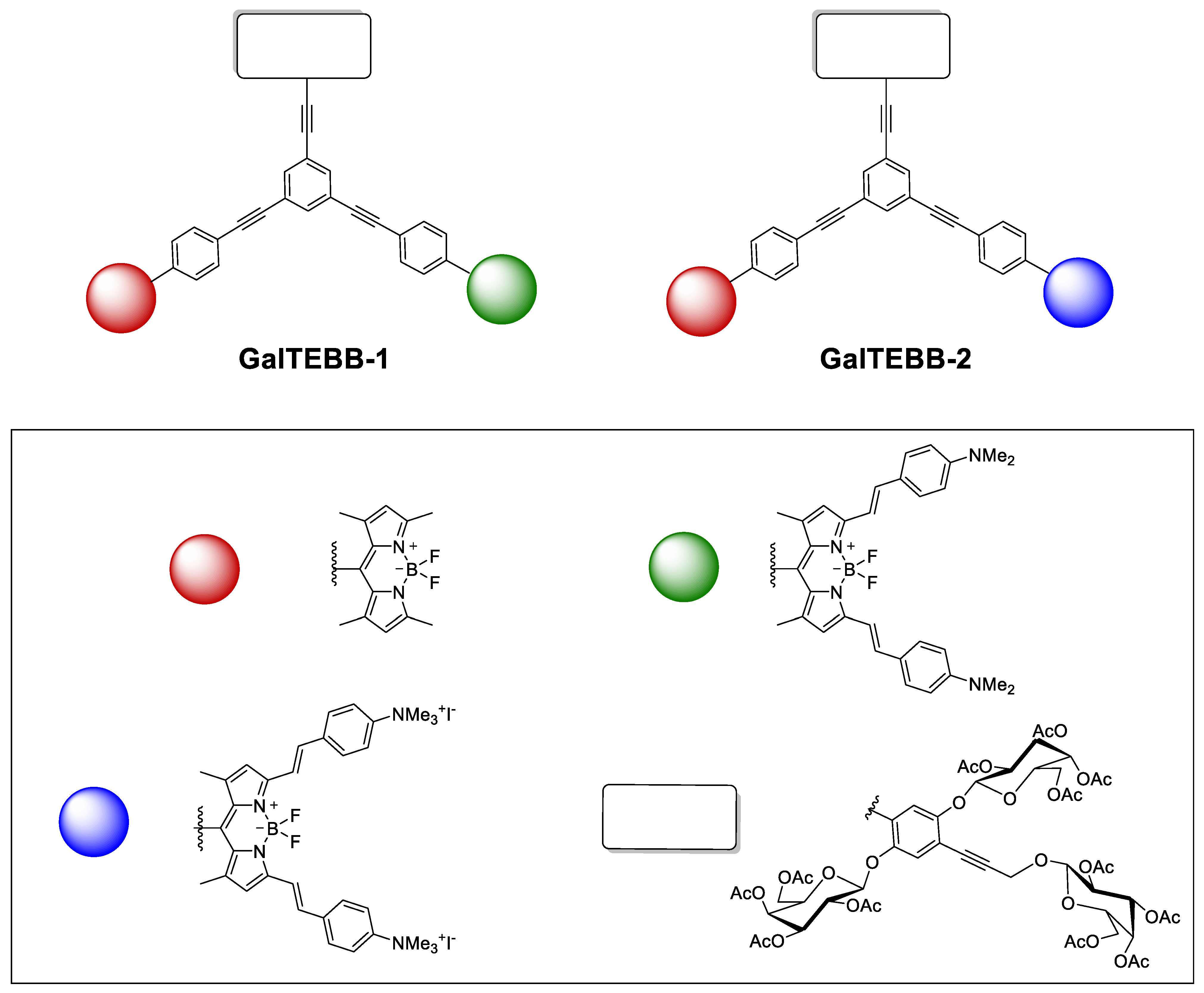
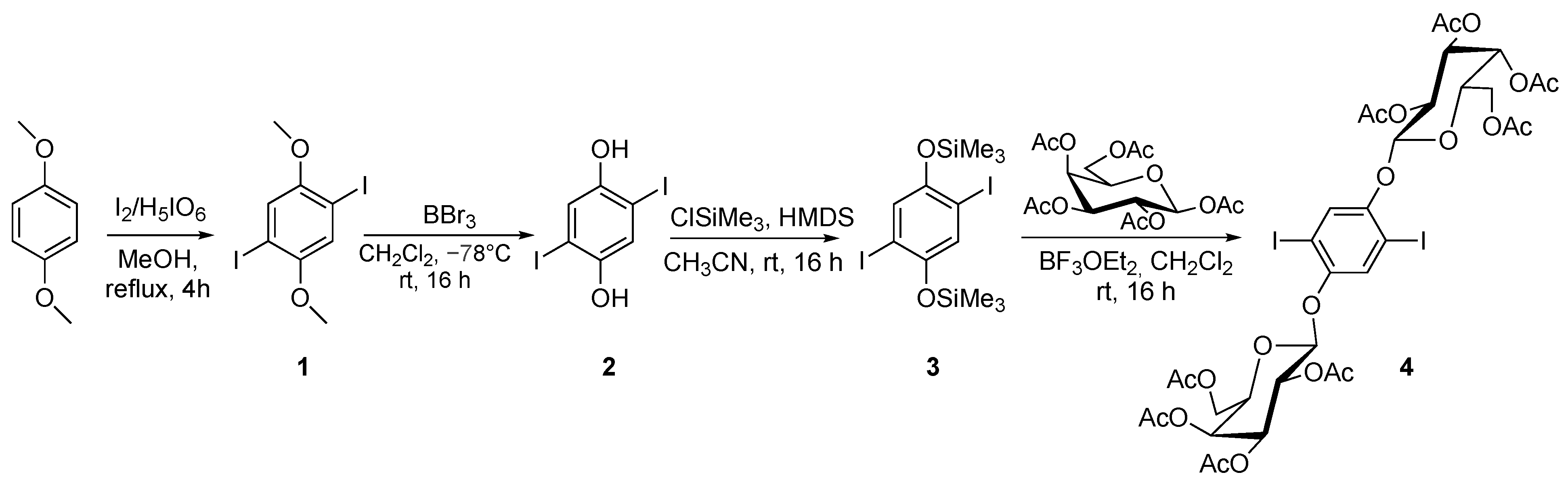
2.1. Synthesis
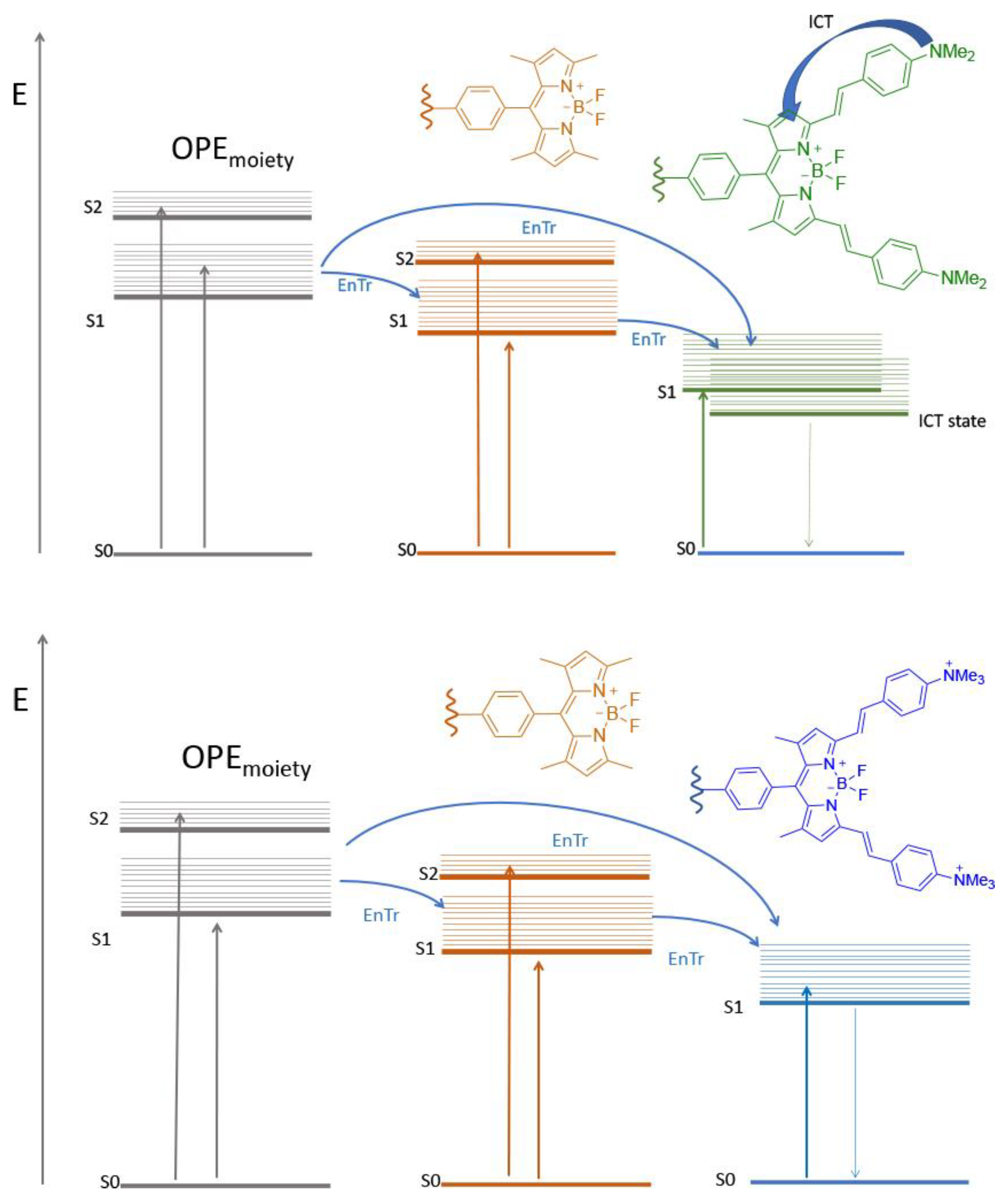
2.2. Photophysical Characterizations
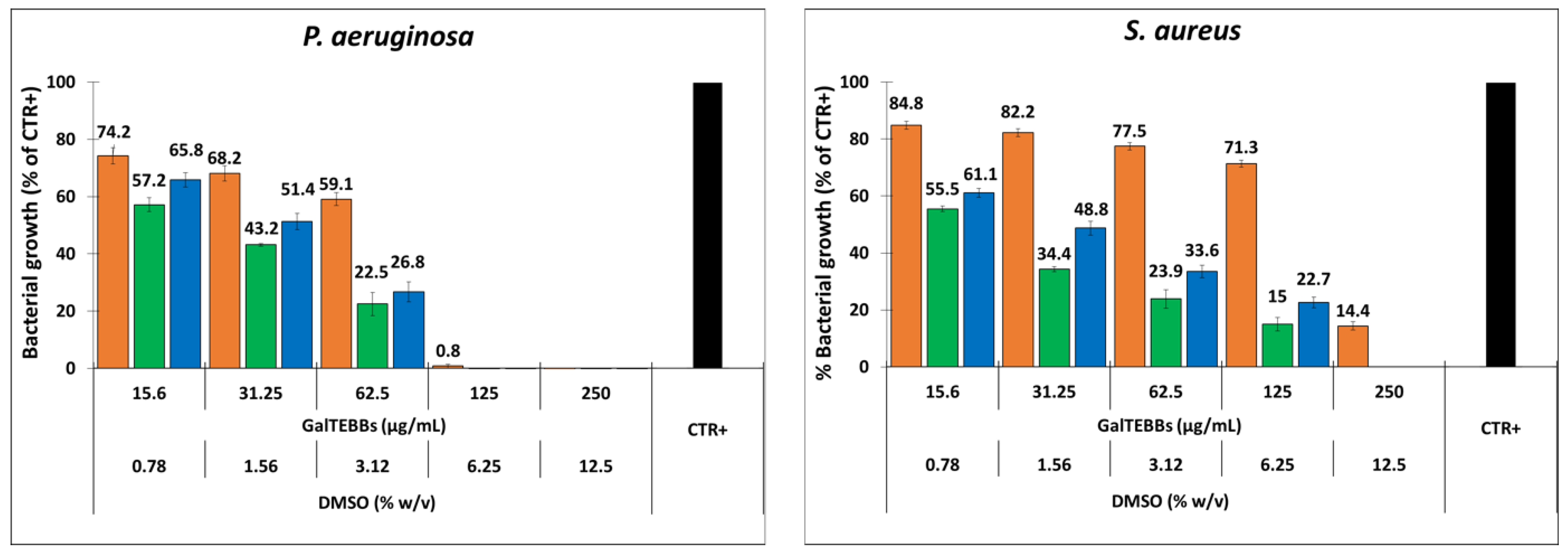
2.3. Biological Results
3. Conclusions
4. Experimental Compounds
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, Y.; Li, H.; Choi, J.; Boo, J.; Jo, H.; Hyun, J.Y.; Shin, I. Glycosidase-Targeting Small Molecules for Biological and Therapeutic Applications. Chem. Soc. Rev. 2023, 52, 7036–7070. [Google Scholar] [CrossRef] [PubMed]
- Kaltner, H.; Gabius, H.-J. Sensing Glycans as Biochemical Messages by Tissue Lectins: The Sugar Code at Work in Vascular Biology. Thromb. Haemost. 2019, 119, 517–533. [Google Scholar] [CrossRef]
- Thomas, B.; Yan, K.-C.; Hu, X.-L.; Donnier-Maréchal, M.; Chen, G.-R.; He, X.-P.; Vidal, S. Fluorescent Glycoconjugates and Their Applications. Chem. Soc. Rev. 2020, 49, 593–641. [Google Scholar] [CrossRef]
- Temperini, A.; Barattucci, A.; Bonaccorsi, P.M.; Rosati, O.; Minuti, L. Stereoselective Synthesis of Substituted Tetrahydropyrans and Isochromans by Cyclization of Phenylseleno Alcohols. J. Org. Chem. 2015, 80, 8102–8112. [Google Scholar] [CrossRef]
- Li, X.; Lv, P.; Du, Y.; Chen, X.; Liu, C. Emerging Roles of O-Glycosylation in Regulating Protein Aggregation, Phase Separation, and Functions. Curr. Opin. Chem. Biol. 2023, 75, 102314. [Google Scholar] [CrossRef]
- Arsić, A.; Hagemann, C.; Stajković, N.; Schubert, T.; Nikić-Spiegel, I. Minimal Genetically Encoded Tags for Fluorescent Protein Labeling in Living Neurons. Nat. Commun. 2022, 13, 314. [Google Scholar] [CrossRef]
- Kong, Y.; Jiang, Q.; Zhang, F.; Yang, Y. Small Molecular Fluorescent Probes: Application Progress of Specific Bacteria Detection and Antibacterial Phototherapy. Chem.—Asian J. 2023, 18, e202300178. [Google Scholar] [CrossRef] [PubMed]
- Barattucci, A.; Campagna, S.; Papalia, T.; Galletta, M.; Santoro, A.; Puntoriero, F.; Bonaccorsi, P. BODIPY on Board of Sugars: A Short Enlightened Journey up to the Cells. ChemPhotoChem 2020, 4, 647–658. [Google Scholar] [CrossRef]
- Mahanta, C.S.; Ravichandiran, V.; Swain, S.P. Recent Developments in the Design of New Water-Soluble Boron Dipyrromethenes and Their Applications: An Updated Review. ACS Appl. Bio Mater. 2023, 6, 2995–3018. [Google Scholar] [CrossRef]
- Cheng, H.-B.; Cao, X.; Zhang, S.; Zhang, K.; Cheng, Y.; Wang, J.; Zhao, J.; Zhou, L.; Liang, X.-J.; Yoon, J. BODIPY as a Multifunctional Theranostic Reagent in Biomedicine: Self-Assembly, Properties, and Applications. Adv. Mater. 2023, 35, e2207546. [Google Scholar] [CrossRef]
- Zou, Y.; Long, S.; Xiong, T.; Zhao, X.; Sun, W.; Du, J.; Fan, J.; Peng, X. Single-Molecule Förster Resonance Energy Transfer-Based Photosensitizer for Synergistic Photodynamic/Photothermal Therapy. ACS Cent. Sci. 2021, 7, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Pu, K. Molecular Probes for Autofluorescence-Free Optical Imaging. Chem. Rev. 2021, 121, 13086–13131. [Google Scholar] [CrossRef] [PubMed]
- Aversa, M.C.; Barattucci, A.; Bonaccorsi, P.; Temperini, A. Regio- and Stereocontrolled Synthesis of (Z)-α-(Phenylseleno)Sulfinyl and -Sulfonyl Alkenes via Sulfenic Acids, and a Study of Their Reactivity. Eur. J. Org. Chem. 2011, 2011, 5668–5673. [Google Scholar] [CrossRef]
- Deni, E.; Zamarrón, A.; Bonaccorsi, P.; Carmen Carreño, M.; Juarranz, Á.; Puntoriero, F.; Sciortino, M.T.; Ribagorda, M.; Barattucci, A. Glucose-Functionalized Amino-OPEs as Biocompatible Photosensitizers in PDT. Eur. J. Med. Chem. 2016, 111, 58–71. [Google Scholar] [CrossRef] [PubMed]
- de Matos, A.M. Recent Advances in the Development and Synthesis of Carbohydrate-Based Molecules with Promising Antibacterial Activity. Eur. J. Org. Chem. 2023, 26, e202200919. [Google Scholar] [CrossRef]
- Prasannan, D.; Raghav, D.; Sujatha, S.; Kumar, H.H.; Rathinasamy, K.; Arunkumar, C. Synthesis, Structure, Photophysical, Electrochemical Properties and Antibacterial Activity of Brominated BODIPYs. RSC Adv. 2016, 6, 80808–80824. [Google Scholar] [CrossRef]
- Santos, V.F.; Costa, M.S.; Campina, F.F.; Rodrigues, R.R.; Santos, A.L.E.; Pereira, F.M.; Batista, K.L.R.; Silva, R.C.; Pereira, R.O.; Rocha, B.A.M.; et al. The Galactose-Binding Lectin Isolated from Vatairea macrocarpa Seeds Enhances the Effect of Antibiotics Against Staphylococcus aureus-Resistant Strain. Probiotics Antimicrob. Proteins 2020, 12, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, H.; Zhang, J.; Tian, X.; Li, X. A Biocompatible Supramolecular Hydrogel with Multivalent Galactose Ligands Inhibiting Pseudomonas aeruginosa Virulence and Growth. RSC Adv. 2020, 10, 33642–33650. [Google Scholar] [CrossRef]
- Ho, L.K.; Daniel-Ivad, M.; Jeedigunta, S.P.; Li, J.; Iliadi, K.G.; Boulianne, G.L.; Hurd, T.R.; Smibert, C.A.; Nodwell, J.R. Chemical Entrapment and Killing of Insects by Bacteria. Nat. Commun. 2020, 11, 4608. [Google Scholar] [CrossRef]
- Calabrese, G.; Petralia, S.; Franco, D.; Nocito, G.; Fabbi, C.; Forte, L.; Guglielmino, S.; Squarzoni, S.; Traina, F.; Conoci, S. A New Ag-Nanostructured Hydroxyapatite Porous Scaffold: Antibacterial Effect and Cytotoxicity Study. Mater. Sci. Eng. C 2021, 118, 111394. [Google Scholar] [CrossRef]
- Franco, D.; Calabrese, G.; Petralia, S.; Neri, G.; Corsaro, C.; Forte, L.; Squarzoni, S.; Guglielmino, S.; Traina, F.; Fazio, E.; et al. Antimicrobial Effect and Cytotoxic Evaluation of Mg-Doped Hydroxyapatite Functionalized with Au-Nano Rods. Molecules 2021, 26, 1099. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Thian, E.S.; Wang, M.; Wang, Z.; Ren, L. Surface Design for Antibacterial Materials: From Fundamentals to Advanced Strategies. Adv. Sci. 2021, 8, e2100368. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Hou, J.; Tian, Y.; Zhao, J.; Sun, Q.; Zhou, S. Antibacterial Surfaces: Strategies and Applications. Sci. China Technol. Sci. 2022, 65, 1000–1010. [Google Scholar] [CrossRef] [PubMed]
- Tekdaş, D.A.; Viswanathan, G.; Topal, S.Z.; Looi, C.Y.; Wong, W.F.; Tan, G.M.Y.; Zorlu, Y.; Gürek, A.G.; Lee, H.B.; Dumoulin, F. Antimicrobial Activity of a Quaternized BODIPY against Staphylococcus Strains. Org. Biomol. Chem. 2016, 14, 2665–2670. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Kulkarni, S.S. Small Carbohydrate Derivatives as Potent Antibiofilm Agents. J. Med. Chem. 2022, 65, 8525–8549. [Google Scholar] [CrossRef] [PubMed]
- Alford, M.A.; Mann, S.; Akhoundsadegh, N.; Hancock, R.E.W. Competition between Pseudomonas aeruginosa and Staphylococcus Aureus Is Dependent on Intercellular Signaling and Regulated by the NtrBC Two-Component System. Sci. Rep. 2022, 12, 9027. [Google Scholar] [CrossRef]
- Chinchilla, R.; Nájera, C. The Sonogashira Reaction: A Booming Methodology in Synthetic Organic Chemistry. Chem. Rev. 2007, 107, 874–922. [Google Scholar] [CrossRef] [PubMed]
- Fernández, G.; García, F.; Sánchez, L. Morphological Changes in the Self-Assembly of a Radial Oligo-Phenylene Ethynylene Amphiphilic System. Chem. Commun. 2008, 6567–6569. [Google Scholar] [CrossRef]
- Kelly, T.L.; Lam, M.C.W.; Wolf, M.O. Carbohydrate-Labeled Fluorescent Microparticles and Their Binding to Lectins. Bioconjug. Chem. 2006, 17, 575–578. [Google Scholar] [CrossRef]
- Pawle, R.H.; Agarwal, A.; Malveira, S.; Smith, Z.C.; Thomas, S.W.I. Bandgap Engineering of Conjugated Materials with Nonconjugated Side Chains. Macromolecules 2014, 47, 2250–2256. [Google Scholar] [CrossRef]
- Babudri, F.; Colangiuli, D.; Lorenzo, P.A.D.; Farinola, G.M.; Omar, O.H.; Naso, F. Synthesis of Poly(Aryleneethynylene)s Bearing Glucose Units as Substituents. Chem. Commun. 2003, 130–131. [Google Scholar] [CrossRef]
- Spicer, C.D.; Davis, B.G. Rewriting the Bacterial Glycocalyx via Suzuki–Miyaura Cross-Coupling. Chem. Commun. 2013, 49, 2747–2749. [Google Scholar] [CrossRef]
- Ziessel, R.; Ulrich, G.; Haefele, A.; Harriman, A. An Artificial Light-Harvesting Array Constructed from Multiple Bodipy Dyes. J. Am. Chem. Soc. 2013, 135, 11330–11344. [Google Scholar] [CrossRef]
- Sutter, A.; Elhabiri, M.; Ulrich, G. Fluorescent pH-Responsive Probes Based on Water-Soluble Boron-Dipyrromethene (BODIPY) Derivatives, Featuring Long-Wavelength Emission. Chem.—Eur. J. 2018, 24, 11119–11130. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.-H.; Descalzo, A.B.; Shen, Z.; Röhr, H.; Liu, Q.; Wang, Y.-W.; Spieles, M.; Li, Y.-Z.; Rurack, K.; You, X.-Z. Mono- and Di(Dimethylamino)Styryl-Substituted Borondipyrromethene and Borondiindomethene Dyes with Intense near-Infrared Fluorescence. Chem. Asian J. 2006, 1, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Loudet, A.; Burgess, K. BODIPY Dyes and Their Derivatives: Syntheses and Spectroscopic Properties. Chem. Rev. 2007, 107, 4891–4932. [Google Scholar] [CrossRef]
- Rurack, K.; Kollmannsberger, M.; Daub, J. Molecular Switching in the Near Infrared (NIR) with a Functionalized Boron-Dipyrromethene Dye. Angew. Chem. Int. Ed. Engl. 2001, 40, 385–387. [Google Scholar] [CrossRef] [PubMed]
- Ansel, H.C.; Norred, W.P.; Roth, I.L. Antimicrobial Activity of Dimethyl Sulfoxide against Escherichia coli, Pseudomonas aeruginosa, and Bacillus megaterium. J. Pharm. Sci. 1969, 58, 836–839. [Google Scholar] [CrossRef] [PubMed]
- Kirkwood, Z.I.; Millar, B.C.; Downey, D.G.; Moore, J.E. Antimicrobial Effect of Dimethyl Sulfoxide and N, N-Dimethylformamide on Mycobacterium abscessus: Implications for Antimicrobial Susceptibility Testing. Int. J. Mycobacteriol. 2018, 7, 134. [Google Scholar] [CrossRef]
- Vereshchagin, A.N.; Frolov, N.A.; Egorova, K.S.; Seitkalieva, M.M.; Ananikov, V.P. Quaternary Ammonium Compounds (QACs) and Ionic Liquids (ILs) as Biocides: From Simple Antiseptics to Tunable Antimicrobials. Int. J. Mol. Sci. 2021, 22, 6793. [Google Scholar] [CrossRef]
- Bretti, C.; Cardiano, P.; Irto, A.; Lando, G.; Milea, D.; Sammartano, S. Interaction of N-Acetyl-l-Cysteine with Na+, Ca2+, Mg2+ and Zn2+. Thermodynamic Aspects, Chemical Speciation and Sequestering Ability in Natural Fluids. J. Mol. Liq. 2020, 319, 114164. [Google Scholar] [CrossRef] [PubMed]
- Bonaccorsi, P.; Aversa, M.C.; Barattucci, A.; Papalia, T.; Puntoriero, F.; Campagna, S. Artificial light-harvesting antenna systems grafted on a carbohydrate platform. Chem. Commun. 2012, 48, 10550–10552. [Google Scholar] [CrossRef] [PubMed]
- Nakamaru, N. Synthesis, Luminescence Quantum Yields, and Lifetimes of Trischelated Ruthenium(II) Mixed-ligand Complexes Including 3,3′-Dimethyl-2,2′-bipyridyl. Bull. Chem. Soc. Jpn. 1982, 55, 2697–2705. [Google Scholar] [CrossRef]
- Olmsted, J., III. Calorimetric determinations of absolute fluorescence quantum yields. J. Phys. Chem. 1979, 83, 2581–2584. [Google Scholar] [CrossRef]
- Serra, R.; Grande, R.; Butrico, L.; Rossi, A.; Settimio, U.F.; Caroleo, B.; Amato, B.; Gallelli, L.; de Franciscis, S. Chronic wound infections: The role of Pseudomonas aeruginosa and Staphylococcus aureus. Expert Rev. Anti. Infect. Ther. 2015, 13, 605–613. [Google Scholar] [CrossRef]
- Fischer, A.J.; Singh, S.B.; LaMarche, M.M.; Maakestad, L.J.; Kienenberger, Z.E.; Peña, T.A.; Stoltz, D.A.; Limoli, D.H. Sustained Coinfections with Staphylococcus aureus and Pseudomonas aeruginosa in Cystic Fibrosis. Am. J. Respir. Crit. Care Med. 2021, 203, 328–338. [Google Scholar] [CrossRef]


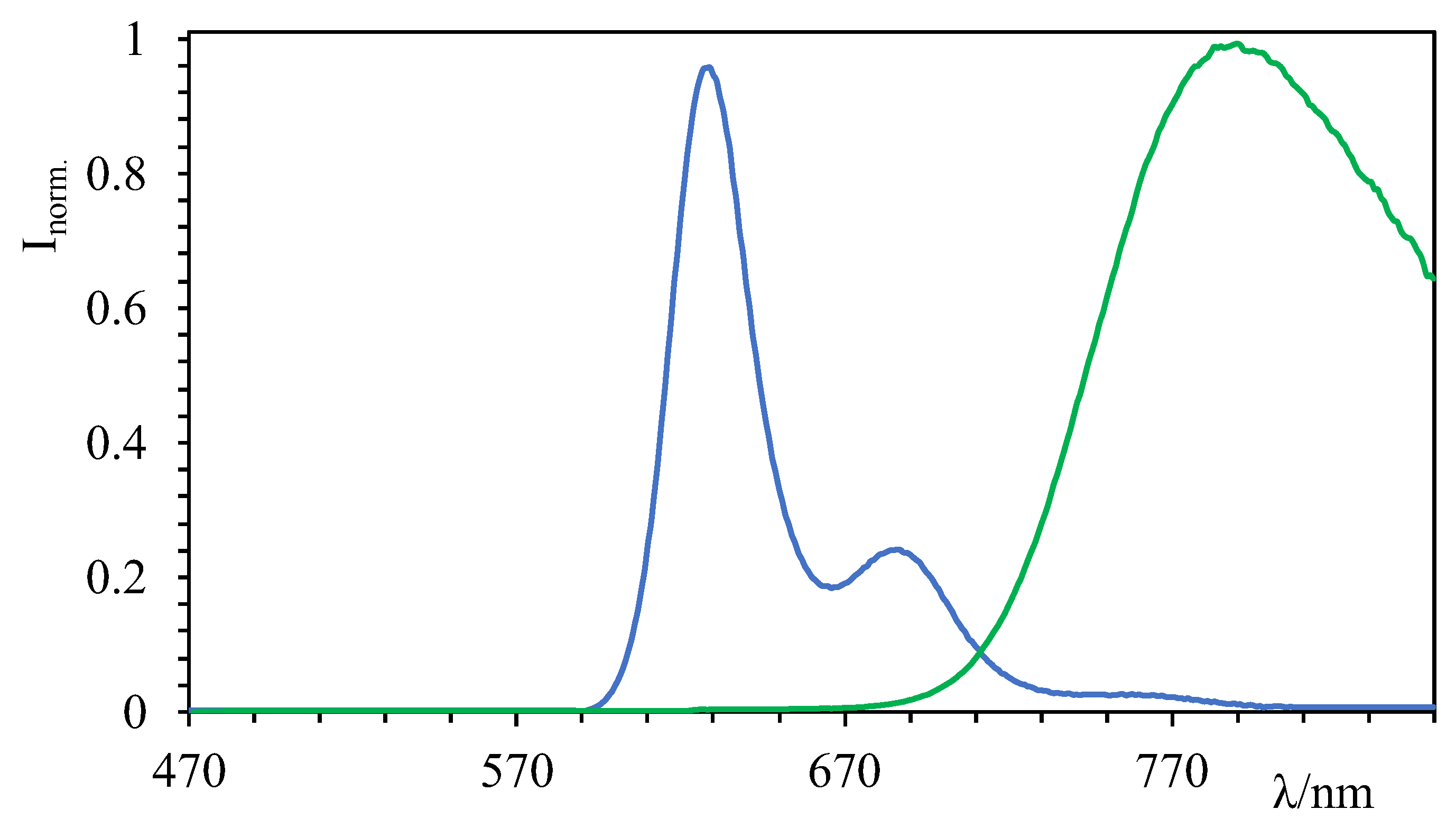
| Absorption | Emission | |||
|---|---|---|---|---|
| λmax/nm (ε/M−1 cm−1) | λmax/nm | τ/ns | Φ | |
| GalTEBB-1 | 500 (120,300) 700 (95,900) | 790 | 2.2 | 0.20 |
| GalTEBB-2 | 500 (102,600) 610 (122,300) | 625 | 5.6 | 0.7 |
| Compounds | P. aeruginosa | S. aureus | ||
|---|---|---|---|---|
| MIC | MBC | MIC | MBC | |
| DMSO alone | 6.25% | 12.5% | 25% | 25% |
| GalTEBB-1 | 125 µg/mL (6.25%) | 125 µg/mL (6.25%) | 250 µg/mL (12.5%) | 250 µg/mL (12.5%) |
| GalTEBB-2 | 125 µg/mL (6.25%) | 125 µg/mL (6.25%) | 250 µg/mL (12.5%) | 250 µg/mL (12.5%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gangemi, C.M.A.; Monforte, M.; Arrigo, A.; Bonaccorsi, P.M.; Conoci, S.; Iaconis, A.; Puntoriero, F.; Franco, D.; Barattucci, A. Synthesis of Bodipy-Tagged Galactoconjugates and Evaluation of Their Antibacterial Properties. Molecules 2024, 29, 2299. https://doi.org/10.3390/molecules29102299
Gangemi CMA, Monforte M, Arrigo A, Bonaccorsi PM, Conoci S, Iaconis A, Puntoriero F, Franco D, Barattucci A. Synthesis of Bodipy-Tagged Galactoconjugates and Evaluation of Their Antibacterial Properties. Molecules. 2024; 29(10):2299. https://doi.org/10.3390/molecules29102299
Chicago/Turabian StyleGangemi, Chiara Maria Antonietta, Maura Monforte, Antonino Arrigo, Paola Maria Bonaccorsi, Sabrina Conoci, Antonella Iaconis, Fausto Puntoriero, Domenico Franco, and Anna Barattucci. 2024. "Synthesis of Bodipy-Tagged Galactoconjugates and Evaluation of Their Antibacterial Properties" Molecules 29, no. 10: 2299. https://doi.org/10.3390/molecules29102299









