Markovnikov-Type Hydrotrifluoromethylchalcogenation of Unactivated Terminal Alkenes with [Me4N][XCF3] (X = S, Se) and TfOH
Abstract
:1. Introduction
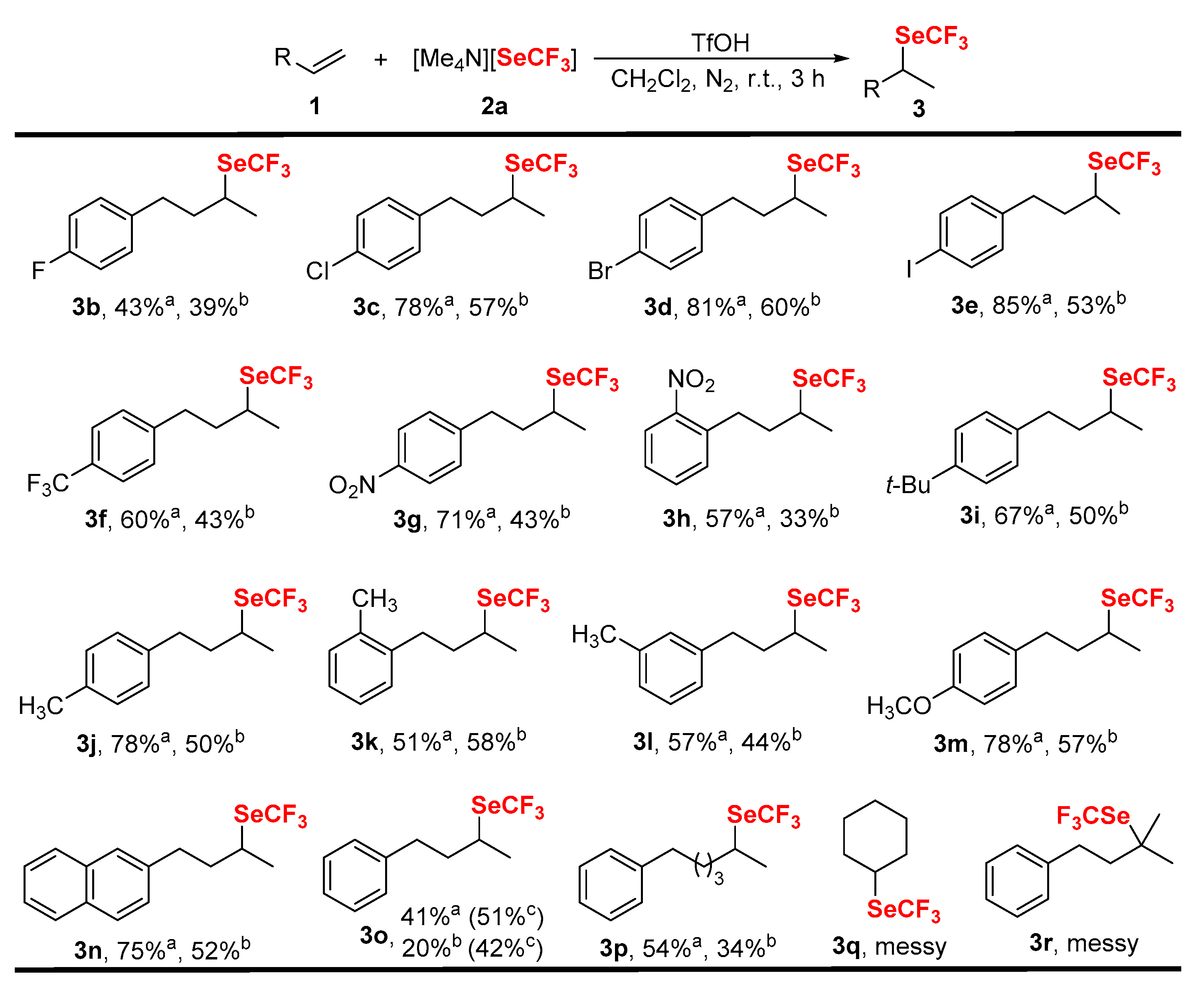
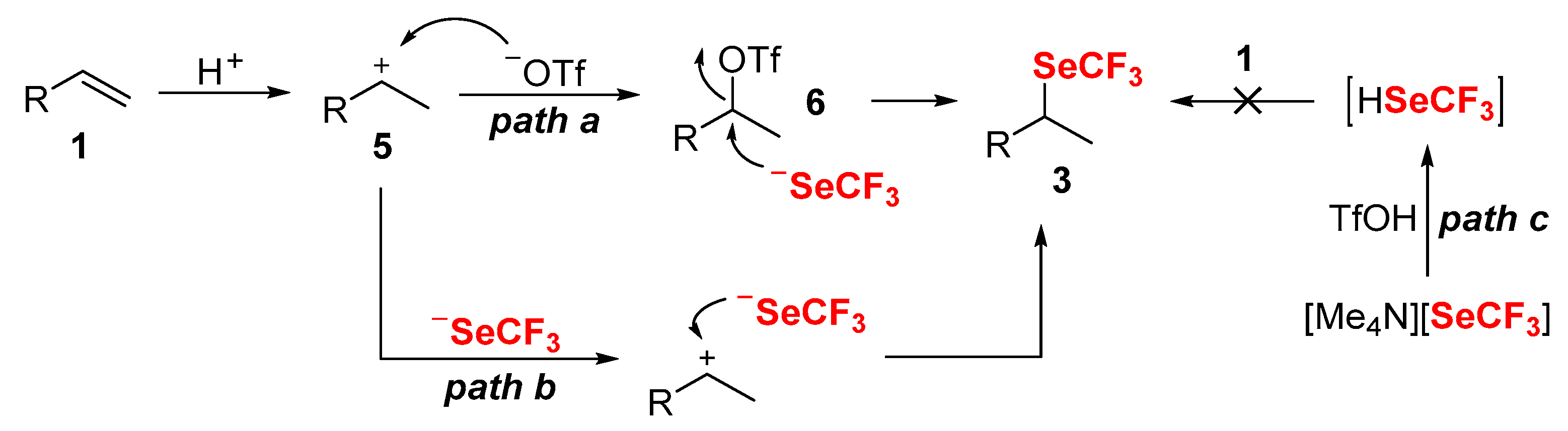
2. Results and Discussions
3. Materials and Methods
3.1. General Information
3.2. General Procedure for Hydrotrifluoromethylselenolation of Unactivated Terminal Alkenes
3.3. General Procedure for Hydrotrifluoromethylthiolation of Unactivated Terminal alkenes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kirsch, P. Modern Fluoroorganic Chemistry: Synthesis, Reactivity, Applications, 2nd ed.; Wiley-VCH: Wheineim, Germany, 2013. [Google Scholar]
- Shao, X.; Xu, C.; Lu, L.; Shen, Q. Shelf-Stable Electrophilic Reagents for Trifluoromethylthiolation. Acc. Chem. Res. 2015, 48, 1227–1236. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-H.; Matsuzaki, K.; Shibata, N. Synthetic Methods for Compounds Having CF3-S Units on Carbon by Trifluoromethylation, Trifluoromethylthiolation, Triflylation, and Related Reactions. Chem. Rev. 2015, 115, 731–764. [Google Scholar] [CrossRef] [PubMed]
- Barata-Vallejo, S.; Bonesi, S.; Postigo, A. Late Stage Trifluoromethylthiolation Strategies for Organic Compounds. Org. Biomol. Chem. 2016, 14, 7150–7182. [Google Scholar] [CrossRef] [PubMed]
- Tlili, A.; Toulgoat, F.; Billard, T. Synthetic Approaches to Trifluoromethoxy-Substituted Compounds. Angew. Chem. Int. Ed. 2016, 55, 11726–11735. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tang, P. Recent Advances in New Trifluoromethoxylation Reagents. Sci. China Chem. 2019, 62, 525–532. [Google Scholar] [CrossRef]
- Song, H.-X.; Han, Q.-Y.; Zhao, C.-L.; Zhang, C.-P. Fluoroalkylation Reactions in Aqueous Media: A Review. Green Chem. 2018, 20, 1662–1731. [Google Scholar] [CrossRef]
- Hardy, M.A.; Chachignon, H.; Cahard, D. Advances in Asymmetric Di-and Trifluoromethylthiolation, and Di- and Trifluoromethoxylation Reactions. Asian J. Org. Chem. 2019, 8, 591–609. [Google Scholar] [CrossRef]
- Wu, S.; Song, H.-X.; Zhang, C.-P. Fluoroalkylation of Diazo Compounds with Diverse Rfn Reagents. Chem. Asian J. 2020, 15, 1660–1677. [Google Scholar] [CrossRef]
- Han, Z.-Z.; Zhang, C.-P. Fluorination and Fluoroalkylation Reactions Mediated by Hypervalent Iodine Reagents. Adv. Synth. Catal. 2020. [CrossRef]
- Tlili, A.; Ismalaj, E.; Glenadel, Q.; Ghiazza, C.; Billard, T. Synthetic Approaches to Trifluoromethylselenolated Compounds. Chem. Eur. J. 2018, 24, 3659–3670. [Google Scholar] [CrossRef]
- Ghiazza, C.; Billard, T.; Tlili, A. Merging Visible-Light Catalysis for the Direct Late-Stage Group-16-Trifluoromethyl Bond Formation. Chem. Eur. J. 2019, 25, 6482–6495. [Google Scholar] [CrossRef] [PubMed]
- Ghiazza, C.; Monnereau, C.; Khrouz, L.; Medebielle, M.; Billard, T.; Tlili, A. New Avenues in Radical Trifluoromethylselenylation with Trifluoromethyl Tolueneselenosulfonate. Synlett 2019, 30, 777–782. [Google Scholar] [CrossRef]
- Peeler, J.C.; Weerapana, E. Chemical Biology Approaches to Interrogate the Selenoproteome. Acc. Chem. Res. 2019, 52, 2832–2840. [Google Scholar] [CrossRef] [PubMed]
- Robberecht, H.; De Bruyne, T.; Davioud-Charvet, E.; Mackrill, J.; Hermans, N. Selenium Status in Elderly People: Longevity and Age-Related Diseases. Curr. Pharm. Design 2019, 25, 1694–1706. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium and Human Health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Weekley, C.M.; Harris, H.H. Which Form is That? The Importance of Selenium Speciation and Metabolism in the Prevention and Treatment of Disease. Chem. Soc. Rev. 2013, 42, 8870–8894. [Google Scholar] [CrossRef]
- Misra, S.; Boylan, M.; Selvam, A.; Spallholz, J.E.; Bjoernstedt, M. Redox-Active Selenium Compounds—From Toxicity and Cell Death to Cancer Treatment. Nutrients 2015, 7, 3536–3556. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.-J.; Yi, W.-B.; Lu, G.-P.; Cai, C. Trifluoromethylation of Thiophenols and Thiols with Sodium Trifluoromethanesulfinate and Iodine Pentoxide. Catal. Sci. Technol. 2016, 6, 417–421. [Google Scholar] [CrossRef]
- Billard, T.; Langlois, B.R. A New Simple Access to Trifluoromethyl Thioethers or Selenoethers from Trifluoromethyl Trimethylsilane and Disulfides or Diselenides. Tetrahedron Lett. 1996, 37, 6865–6868. [Google Scholar] [CrossRef]
- Billard, T.; Large, S.; Langlois, B.R. Preparation of Trifluoromethyl Sulfides or Selenides from Trifluoromethyl Trimethylsilane and Thiocyanates or Selenocyanates. Tetrahedron Lett. 1997, 38, 65–68. [Google Scholar] [CrossRef]
- Large, S.; Roques, N.; Langlois, B.R. Nucleophilic Trifluoromethylation of Carbonyl Compounds and Disulfides with Trifluoromethane and Silicon-Containing Bases. J. Org. Chem. 2000, 65, 8848–8856. [Google Scholar] [CrossRef] [PubMed]
- Potash, S.; Rozen, S. General Synthesis of Trifluoromethyl Selenides Utilizing Selenocyanates and Fluoroform. J. Org. Chem. 2014, 79, 11205–11208. [Google Scholar] [CrossRef] [PubMed]
- Pooput, C.; Medebielle, M.; Dolbier Jr., W. R. A New and Efficient Method for the Synthesis of Trifluoromethylthio- and Selenoethers. Org. Lett. 2004, 6, 301–303. [Google Scholar] [CrossRef]
- Pooput, C.; Dolbier, W.R., Jr.; Médebielle, M. Nucleophilic Perfluoroalkylation of Aldehydes, Ketones, Imines, Disulfides, and Diselenides. J. Org. Chem. 2006, 71, 3564–3568. [Google Scholar] [CrossRef] [PubMed]
- Cherkupally, P.; Beier, P. Alkoxide-Induced Nucleophilic Trifluoromethylation Using Diethyl Trifluoromethylphosphonate. Tetrahedron Lett. 2010, 51, 252–255. [Google Scholar] [CrossRef]
- Blond, G.; Billard, T.; Langlois, B.R. New Stable Reagents for the Nucleophilic Trifluoromethylation. Part 4: Trifluoromethylation of Disulfides and Diselenides with Hemiaminals of Trifluoroacetaldehyde. Tetrahedron Lett. 2001, 42, 2473–2475. [Google Scholar] [CrossRef]
- Kondratenko, N.V.; Kolomeytsev, A.A.; Popov, V.I.; Yagupolskii, L.M. Synthesis and Reactions of Trifluoromethylthio(seleno)- and Pentafluorophenylthio(seleno)-Copper. Synthesis 1985, 1985, 667–669. [Google Scholar] [CrossRef]
- Feldhoff, R.; Haas, A.; Lieb, M. Darstellung und Eigenschaften Trifluormethyl- und Trifluormethylchalkogenyl-Substituierter Adamantane. J. Fluorine Chem. 1994, 67, 245–251. [Google Scholar] [CrossRef]
- Chen, C.; Hou, C.; Wang, Y.; Hor, T.S.A.; Weng, Z. Copper-Catalyzed Trifluoromethylselenolation of Aryl and Alkyl Halides: The Silver Effect in Transmetalation. Org. Lett. 2014, 16, 524–527. [Google Scholar] [CrossRef]
- Zhang, M.; Weng, Z. Palladium-Catalyzed Tandem Synthesis of 2-Trifluoromethylthio(seleno)-Substituted Benzofused Heterocycles. Org. Lett. 2019, 21, 5838–5842. [Google Scholar] [CrossRef]
- Glenadel, Q.; Ismalaj, E.; Billard, T. Benzyltrifluoromethyl (or Fluoroalkyl) Selenide: Reagent for Electrophilic Trifluoromethyl (or Fluoroalkyl) Selenolation. J. Org. Chem. 2016, 81, 8268–8275. [Google Scholar] [CrossRef] [PubMed]
- Ghiazza, C.; Debrauwer, V.; Monnereau, C.; Khrouz, L.; Medebielle, M.; Billard, T.; Tlili, A. Visible-Light-Mediated Metal-Free Synthesis of Trifluoromethylselenolated Arenes. Angew. Chem. Int. Ed. 2018, 57, 11781–11785. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-L.; Zhou, S.-H.; Lin, J.-H.; Deng, Q.-H.; Xiao, J.-C. Difluorocarbene-Derived Trifluoromethylselenolation of Benzyl Halides. Chem. Commun. 2019, 55, 1410–1413. [Google Scholar] [CrossRef] [PubMed]
- Dix, S.; Jakob, M.; Hopkinson, M.N. Deoxytrifluoromethylthiolation and Selenylation of Alcohols by Using Benzothiazolium Reagents. Chem. Eur. J. 2019, 25, 7635–7639. [Google Scholar] [CrossRef] [PubMed]
- Modak, A.; Pinter, E.N.; Cook, S.P. Copper-Catalyzed, N-Directed Csp3-H Trifluoromethylthiolation (-SCF3) and Trifluoromethylselenation (-SeCF3). J. Am. Chem. Soc. 2019, 141, 18405–18410. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, Q.; Pluta, R.; Rueping, M. Copper Catalyzed Oxidative Coupling Reactions for Trifluoromethylselenolations - Synthesis of R-SeCF3 Compounds Using Air Stable Tetramethylammonium Trifluoromethylselenate. Chem. Commun. 2015, 51, 4394–4397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aufiero, M.; Sperger, T.; Tsang, A.S.K.; Schoenebeck, F. Highly Efficient C-SeCF3 Coupling of Aryl Iodides Enabled by an Air-Stable Dinuclear PdI Catalyst. Angew. Chem., Int. Ed. 2015, 54, 10322–10326. [Google Scholar] [CrossRef] [PubMed]
- Matheis, C.; Wagner, V.; Goossen, L.J. Sandmeyer-Type Trifluoromethylthiolation and Trifluoromethylselenolation of (Hetero)Aromatic Amines Catalyzed by Copper. Chem. Eur. J. 2016, 22, 79–82. [Google Scholar] [CrossRef]
- Matheis, C.; Krause, T.; Bragoni, V.; Goossen, L.J. Trifluoromethylthiolation and Trifluoromethylselenolation of α-Diazo Esters Catalyzed by Copper. Chem. Eur. J. 2016, 22, 12270–12273. [Google Scholar] [CrossRef]
- Fang, W.-Y.; Dong, T.; Han, J.-B.; Zha, G.-F.; Zhang, C.-P. Expeditious Trifluoromethylthiolation and Trifluoromethylselenolation of Alkynyl(phenyl)iodoniums by [XCF3]− (X = S, Se) Anions. Org. Biomol. Chem. 2016, 14, 11502–11509. [Google Scholar] [CrossRef]
- Han, J.-B.; Dong, T.; Vicic, D.A.; Zhang, C.-P. Nickel-Catalyzed Trifluoromethylselenolation of Aryl Halides Using the Readily Available [Me4N][SeCF3] Salt. Org. Lett. 2017, 19, 3919–3922. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.; He, J.; Li, Z.-H.; Zhang, C.-P. Catalyst- and Additive-Free Trifluoromethylselenolation with [Me4N][SeCF3]. ACS Sustainable Chem. Eng. 2018, 6, 1327–1335. [Google Scholar] [CrossRef]
- Han, Q.-Y.; Zhao, C.-L.; Dong, T.; Shi, J.; Tan, K.-L.; Zhang, C.-P. Metal-Free Oxidative Trifluoromethylselenolation of Electron-Rich (Hetero)arenes with the Readily Available [Me4N][SeCF3] Reagent. Org. Chem. Front. 2019, 6, 2732–2737. [Google Scholar] [CrossRef]
- Han, Q.-Y.; Tan, K.-L.; Wang, H.-N.; Zhang, C.-P. Organic Photoredox-Catalyzed Decarboxylative Trifluoromethylselenolation of Aliphatic Carboxylic Acids with [Me4N][SeCF3]. Org. Lett. 2019, 21, 10013–10017. [Google Scholar] [CrossRef]
- Tan, K.-L.; Dong, T.; Zhang, X.-Q.; Zhang, C.-P. Oxidative Trifluoromethylselenolation of 1,3-Dicarbonyls with [Me4N][SeCF3]. Org. Biomol. Chem. 2020, 18, 1769–1779. [Google Scholar] [CrossRef]
- Wu, S.; Jiang, T.-H.; Zhang, C.-P. CaCl2-Promoted Dehydroxytrifluoromethylselenolation of Alcohols with [Me4N][SeCF3]. Org. Lett. 2020, 22, 6016–6020. [Google Scholar] [CrossRef]
- Tyrra, W.; Naumann, D.; Yagupolskii, Y.L. Stable Trifluoromethylselenates(0), [A]SeCF3 - Synthesis, Characterizations and Properties. J. Fluorine Chem. 2003, 123, 183–187. [Google Scholar] [CrossRef]
- Petrone, D.A. Stereoselective Heterocycle Synthesis via Alkene Difunctionalization. Springer International Publishing: Cham, Switzerland, 2018. [Google Scholar]
- Kissin, Y.V. Alkene Polymerization Reactions with Transition Metal Catalysts; Studies in Surface Science and Catalysis 173; Elsevier Academic Press: Waltham, MA, USA, 2007. [Google Scholar]
- Zhao, H.-P.; Liang, G.-C.; Nie, S.-M.; Lu, X.; Pan, C.-X.; Zhong, X.-X.; Su, G.-F.; Mo, D.-L. Metal-Free Graphene Oxide-Catalyzed Aza-Semipinacol Rearrangement to Prepare 2-(Indol-2-yl)phenols and Benzofuro[3,2-b]indolines Containing Quaternary Carbon Centers. Green Chem. 2020, 22, 404–410. [Google Scholar] [CrossRef]
- Ma, X.-P.; Nong, C.-M.; Zhao, J.; Lu, X.; Liang, C.; Mo, D.-L. Yb(OTf)3-Catalyzed Cycloaddition/[3,3]-Rearrangement of N-Vinyl-α,β-Unsaturated Ketonitrones with Methylenecyclopropanes: Stereoselective Synthesis of Nine-Membered Nitrogen Heterocycles. Adv. Synth. Catal. 2020, 362, 478–486. [Google Scholar] [CrossRef]
- Zhang, Y.; Briski, J.; Zhang, Y.; Rendy, R.; Klumpp, D.A. Superacid-Catalyzed Reactions of Olefinic Pyrazines: An Example of Anti-Markovnikov Addition Involving Superelectrophiles. Org. Lett. 2005, 7, 2505–2508. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Chu, L.; Qing, F.-L. Silver-Catalyzed Hydrotrifluoromethylation of Unactivated Alkenes with CF3SiMe3. Angew. Chem. Int. Ed. 2013, 52, 2198–2202. [Google Scholar] [CrossRef] [PubMed]
- Mizuta, S.; Verhoog, S.; Engle, K.M.; Khotavivattana, T.; O’Duill, M.; Wheelhouse, K.; Rassias, G.; Mdebielle, M.; Gouverneur, V. Catalytic Hydrotrifluoromethylation of Unactivated Alkenes. J. Am. Chem. Soc. 2013, 135, 2505–2508. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Wang, L.-S.; Li, B.; Fu, B.; Zhang, C.-P.; Li, W. Operationally Simple Hydrotrifluoromethylation of Alkenes with Sodium Triflinate Enabled by Ir Photoredox Catalysis. Chem. Commun. 2016, 52, 6371–6374. [Google Scholar] [CrossRef] [PubMed]
- Straathof, N.J.W.; Cramer, S.E.; Hessel, V.; Noël, T. Practical Photocatalytic Trifluoromethylation and Hydrotrifluoromethylation of Styrenes in Batch and Flow. Angew. Chem. Int. Ed. 2016, 55, 15549–15553. [Google Scholar] [CrossRef] [Green Version]
- Lonca, G.H.; Ong, D.Y.; Tran, T.M.H.; Tejo, C.; Chiba, S.; Gagosz, F. Anti-Markovnikov Hydrofunctionalization of Alkenes: Use of a Benzyl Group as a Traceless Redox-Active Hydrogen Donor. Angew. Chem. Int. Ed. 2017, 56, 11440–11444. [Google Scholar] [CrossRef]
- Zhang, W.; Zou, Z.; Wang, Y.; Wang, Y.; Liang, Y.; Wu, Z.; Zheng, Y.; Pan, Y. Leaving Group Assisted Strategy for Photoinduced Fluoroalkylations Using N-Hydroxybenzimidoyl Chloride Esters. Angew. Chem. Int. Ed. 2019, 58, 624–627. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Wan, W.; Li, J.; Hu, Q.; Jiang, H.; Zhu, S.; Wang, J.; Hao, J. An Efficient Regioselective Hydrodifluoromethylation of Unactivated Alkenes with TMSCF2CO2Et at Ambient Temperature. Chem. Commun. 2014, 50, 9749–9752. [Google Scholar] [CrossRef]
- Yu, C.; Iqbal, N.; Park, S.; Cho, E.J. Selective Difluoroalkylation of Alkenes by Using Visible Light Photoredox Catalysis. Chem. Commun. 2014, 50, 12884–12887. [Google Scholar] [CrossRef]
- Huang, W.; Chen, W.; Wang, G.; Li, J.; Cheng, X.; Li, G. Thiyl-Radical-Catalyzed Photoreductive Hydrodifluoroacetamidation of Alkenes with Hantzsch Ester as a Multifunctional Reagent. ACS Catal. 2016, 6, 7471–7474. [Google Scholar] [CrossRef]
- Nie, X.; Cheng, C.; Zhu, G. Palladium-Catalyzed Remote Aryldifluoroalkylation of Alkenyl Aldehydes. Angew. Chem. Int. Ed. 2017, 56, 1898–1902. [Google Scholar] [CrossRef]
- Wang, H.; Jui, N.T. Catalytic Defluoroalkylation of Trifluoromethylaromatics with Unactivated Alkenes. J. Am. Chem. Soc. 2018, 140, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Lin, J.-H.; Cao, Y.-C.; Xiao, J.-C. Visible-Light-Induced Radical Hydrodifluoromethylation of Alkenes. Org. Chem. Front. 2019, 6, 3580–3583. [Google Scholar] [CrossRef]
- Barthelemy, A.-L.; Magnier, E.; Dagousset, G. Direct Trifluoromethylthiolation Reactions Involving Radical Processes. Synthesis 2018, 50, 4765–4776. [Google Scholar]
- Harris, J.F., Jr.; Stacey, F.W. The Free Radical Addition of Trifluoromethanethiol to Fluoroölefins. J. Am. Chem. Soc. 1961, 83, 840–845. [Google Scholar] [CrossRef]
- Yang, T.; Lu, L.; Shen, Q. Iron-Mediated Markovnikov-Selective Hydro-Trifluoromethylthiolation of Unactivated Alkenes. Chem. Commun. 2015, 51, 5479–5481. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Hong, X.; Lu, L.; Shen, Q. Cobalt-Catalyzed Hydro-Difluoromethylthiolation/Hydro-Trifluoromethylthiolation of Unactivated Alkenes. Tetrahedron 2019, 75, 4156–4166. [Google Scholar] [CrossRef]
- Ouyang, Y.; Xu, X.-H.; Qing, F.-L. Hydrotrifluoromethylthiolation of Unactivated Alkenes and Alkynes with Trifluoromethanesulfonic Anhydride through Deoxygenative Reduction and Photoredox Radical Processes. Angew. Chem. Int. Ed. 2019, 58, 18508–18512. [Google Scholar] [CrossRef]
- Ghiazza, C.; Glenadel, Q.; Tlili, A.; Billard, T. Trifluoromethylselenolation and Fluoroalkylselenolation of Alkenes by Electrophilic Addition. Eur. J. Org. Chem. 2017, 3812–3814. [Google Scholar] [CrossRef]
- Ghiazza, C.; Khrouz, L.; Monnereau, C.; Billard, T.; Tlili, A. Visible-Light Promoted Fluoroalkylselenolation: Toward the Reactivity of Unsaturated Compounds. Chem. Commun. 2018, 54, 9909–9912. [Google Scholar] [CrossRef]
- Zhang, B.-S.; Gao, L.-Y.; Zhang, Z.; Wen, Y.-H.; Liang, Y.-M. Three-Component Difluoroalkylation and Trifluoromethylthiolation/Trifluoromethylselenolation of π-Bonds. Chem. Commun. 2018, 54, 1185–1188. [Google Scholar] [CrossRef]
- Roesky, H.W. Efficient Preparations of Fluorine Compounds; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013. [Google Scholar]
- Qi, X.-X.; Chen, P.-H.; Liu, G.-S. Catalytic Oxidative Trifluoromethoxylation of Allylic C-H Bonds Using a Palladium Catalyst. Angew. Chem. Int. Ed. 2017, 56, 9517–9521. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Lu, Z.-H.; Hu, X.-H.; Li, J.-L.; Yang, X.-J. Monocarboxylation and Intramolecular Coupling of Butenylated Arenes via Palladium-Catalyzed C–H Activation Process. Org. Lett. 2015, 17, 1489–1492. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.J.; Brown, R.C.D.; Raja, R. Heterogenisation of Ketonecatalysts within Mesoporous Supports for Asymmetric Epoxidation. RSC Adv. 2013, 3, 843–850. [Google Scholar] [CrossRef] [Green Version]
- Meng, Q.-Y.; Schirmer, T.E.; Katou, K.; König, B. Controllable Isomerization of Alkenes by Dual Visible-Light-Cobalt Catalysis. Angew. Chem. Int. Ed. 2019, 58, 5723–5728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armarego, W.L.F.; Chai, C.L.L. Purification of Laboratory Chemicals, 5th ed.; Butterworth Heinemann: Oxford, UK, 2003. [Google Scholar]
Sample Availability: Samples of the compounds 2a–b, CsOCF3, 1a–r, 3a–p, and 4a–h are available from the authors. |


| Entry a | Solvent | Acid | 1a:2a:Acid | Time (h) | Yields (3a, %) b |
|---|---|---|---|---|---|
| 1 | CH2Cl2 | TfOH | 1:1.5:1.5 | 6 | 45 |
| 2 | CH2Cl2 | CF3COOH | 1:1.5:1.5 | 6 | 0 |
| 3 | CH2Cl2 | conc. H2SO4 | 1:1.5:1.5 | 6 | 0 |
| 4 | CH2Cl2 | anhydrous HCl | 1:1.5:1 | 3 | 0 |
| 5 | CH2Cl2 | H3PO4 (85%) | 1:1.5:1.5 | 6 | 0 |
| 6 | CH2Cl2 | Et3N•3HF | 1:1.5:1.5 | 6 | 0 |
| 7 | CH2Cl2 | Et2O•HBF4 (85%) | 1:1.5:1.5 | 6 | 0 |
| 8 | CH2Cl2 | TsOH•H2O | 1:1.5:1.5 | 6 | 0 |
| 9 | CH2Cl2 | (CF3SO2)2NH | 1:1.5:1.5 | 6 | 0 |
| 10 | ClCH2CH2Cl | TfOH | 1:1.5:1.5 | 6 | 33 |
| 11 | toluene | TfOH | 1:1.5:1.5 | 6 | 19 |
| 12 | PhCl | TfOH | 1:1.5:1.5 | 6 | 37 |
| 13 | CF2ClCFCl2 | TfOH | 1:1.5:1.5 | 6 | 24 |
| 14 | MeCN | TfOH | 1:1.5:1.5 | 6 | 0 |
| 15 | (CF3)2CHOH | TfOH | 1:1.5:1.5 | 6 | 0 |
| 16 | CH2Cl2 | TfOH | 1:1.5:1.5 | 3 | 48 |
| 17 | CH2Cl2 | TfOH | 1:1.5:1 | 3 | 71 (68) |
| 18 | CH2Cl2 | TfOH | 1:1.5:2 | 6 | 15 |
| 19 | CH2Cl2 | TfOH | 1:2:1 | 3 | 57 |
| 20 | CH2Cl2 | TfOH | 1:1:1 | 3 | 39 |
| 21 | CH2Cl2 | TfOH | 1.5:1:1 | 3 | 42 |
| 22 | CH2Cl2 | TfOH | 2:2:1 | 3 | 83 (79) |

| Method A | Method B | Method C | |
|---|---|---|---|
| Yield (3a, %) a | 83 c, 65 d | 47 c, 40 d | 0 c, 0 d |
| Yield (3a, %) b | 71 c, 11 d | 45 c, 42 d | 0 c, 0 d |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, J.; Zhang, C.-P. Markovnikov-Type Hydrotrifluoromethylchalcogenation of Unactivated Terminal Alkenes with [Me4N][XCF3] (X = S, Se) and TfOH. Molecules 2020, 25, 4535. https://doi.org/10.3390/molecules25194535
Shi J, Zhang C-P. Markovnikov-Type Hydrotrifluoromethylchalcogenation of Unactivated Terminal Alkenes with [Me4N][XCF3] (X = S, Se) and TfOH. Molecules. 2020; 25(19):4535. https://doi.org/10.3390/molecules25194535
Chicago/Turabian StyleShi, Jin, and Cheng-Pan Zhang. 2020. "Markovnikov-Type Hydrotrifluoromethylchalcogenation of Unactivated Terminal Alkenes with [Me4N][XCF3] (X = S, Se) and TfOH" Molecules 25, no. 19: 4535. https://doi.org/10.3390/molecules25194535







