1. Introduction
Seasonal influenza virus epidemics continue to cause millions of hospitalizations, and hundreds of thousands of deaths globally each year [
1]. Attack rates for influenza virus infections are consistently highest among children, and those under 5 years of age are considered to be especially vulnerable to severe outcomes [
2]. Indeed, a recent systematic analysis estimated that influenza virus infections are responsible for a worldwide average of 870,000 hospitalizations of children <5 years old each year [
3]. Furthermore, children are also thought to be a significant source of viral transmission [
4,
5,
6,
7]. Seasonal vaccination continues to be the most effective way to prevent influenza virus infection, and many countries have adopted government-sponsored influenza immunization programs to protect their populations.
While inactivated influenza vaccines (IIV) formulations have been available for several decades, the first live-attenuated influenza vaccines (LAIV) widely approved for use in humans was manufactured by MedImmune (FluMist
®/Fluenz™) and became available in 2003 [
8]. In several early randomized clinical trials, trivalent LAIV demonstrated superior efficacy relative to trivalent inactivated influenza virus vaccine (TIV) in children [
9,
10]. As a result, the U.S. Advisory Committee on Immunization Practices (ACIP) preferentially recommended the use of LAIV for healthy children aged 2 to 8 years during the 2014–2015 season [
11]. This preferential recommendation was lifted during the 2015–2016 season, when the ACIP advised that either IIV or LAIV was appropriate for healthy individuals 2 through 49 years [
12]. However, in their 2016–2017 report, the ACIP recommended against using the use of LAIV, citing data for the U.S. Vaccine Effectiveness Network that indicated no significant vaccine effectiveness of quadrivalent LAIV against all influenza A and B strains for children aged 2 to 17 [
13]. In contrast, a recent cluster randomized blinded trial conducted in children aged 3 to 15 years by members of this group reported equivalent efficacy of IIV and LAIV across the three influenza seasons spanning October 2012 to May 2015 for all strains tested [
14]. Most recently, the ACIP has again recommended quadrivalent LAIV for use in populations for whom it is appropriate, but without preference relative to IIV [
15].
Here, we compare the immunogenicity of IIV and LAIV after vaccination of Hutterite children with the 2014–2015 Northern Hemisphere trivalent vaccines. Hemagglutination inhibition (HAI) titers, serum IgA endpoint titers, and mucosal IgA titers against the influenza A virus (IAV) components (H1N1 and H3N2) of the vaccines were evaluated.
2. Materials and Methods
2.1. Study Participants
In this study, we assessed 618 healthy Hutterite children and adolescents aged 3 to 15 years in the 2014–2015 flu season who participated in a cluster randomized clinical trial (ClinicalTrials.gov: NCT01653015) that compared the direct and indirect protectiveness of trivalent IIV and trivalent LAIV, as reported previously [
14]. The 2014–2015 season was chosen because this is the only season during which both serum and mucosal samples were collected. In this trial, participants were randomly assigned by colonies to two study groups—IIV group and LAIV group. Healthy children and adolescents aged 3 to 15 years in these two study groups received either IIV or LAIV depending on the group to which their Hutterite colony was randomized. In both LAIV and IIV colonies, previously unvaccinated children aged less than 9 years of age at the time of immunization received a second dose of the same vaccine four weeks following the first dose. The administration of each vaccine formulation occurred over a similar time-frame (average vaccination date for IIV was 2014-11-21; average vaccination date for LAIV was 2014-11-22). For the current study, we used previously obtained specimens from 618 children and adolescent participants from this trial. Of these, 278 were from IIV group and 340 from LAIV group. The IAV antigens included in the both IIV and LAIV were A/California/7/2009 (H1N1) pdm09-like virus, A/Texas/50/2012 (H3N2)-like virus, and B/Massachusetts/2/2012-like virus, all of which were well-matched with circulating strains in Canada during the 2014–2015 influenza season.
2.2. Sample Collection
Blood samples were drawn from participants at baseline and between 3 and 5 weeks post-vaccination. For previously unvaccinated children under 9 years of age who received two doses of vaccine, samples were collected after the second dose. Active surveillance was conducted twice weekly during the influenza season (28 December 2014, to 20 May 2015) and those with two or more signs or symptoms compatible with influenza were tested by RT-PCR of nasal swabs. We collected nasal swabs to test for mucosal IgA antibodies at baseline and three weeks after vaccination with either IIV or LAIV.
2.3. HAI
The HAI assay was performed as previously described using turkey erythrocytes and reference antigens for A/California/07/2009 (H1N1) pdm09-like virus (Cal/09 H1N1) and A/Texas/50/2012 (H3N2)-like (Tex/50 H3N2) [
16].
2.4. Enzyme-Linked Immunosorbent Assays (ELISAs)
ELISAs were performed on 96-well Nunc-immuno maxisorp plates (Thermo Scientific, Waltham, MA, USA). The plates were coated overnight with 2 ug/mL per well of purified recombinant HA (A/California/07/2009 H1 or A/Texas/50/2012 H3) in bicarbonate–carbonate coating buffer (100 mM, pH 9.4). The plates were blocked with 5% skim milk for 1 h. Serum samples were serially diluted starting with 1:50 dilution in 5% skim milk and then incubated for 1 h at room temperature. Mucosal samples were undiluted and were incubated for 1 h at room temperature. Plates were washed three times with PBS-T (0.1% Tween 20). Secondary goat anti-human IgA-horseradish peroxidase (HRP) (Santa Cruz Biotechnology, Dallas, TX, USA) at 1:4000 dilution was added in 5% skim milk for 1 h at room temperature, followed by 3x PBS-T wash prior to addition of HRP substrate (Sigmafast OPD, Sigma Aldrich, St. Louis, MO, USA). Reactions were stopped after 10 min by the addition of 3 M HCl, and the optical densities were read at 490 nm on a Spectramax I3 (Molecular Devices, San Jose, CA, USA). Endpoint titers of serum IgA were defined as the lowest dilution whose optical density remained three standard deviations above the mean of negative control wells. Since mucosal IgA could only be reliably measured using undiluted samples, only normalized optical density (O.D.) values were reported. We chose to favor specificity over sensitivity, and therefore, normalization was performed by subtracting the mean O.D. plus three standard deviations of blank samples from each experimental sample.
2.5. Statistical Analyses
We compared pre- and post-vaccination antibody titers in terms of three types of antibody measures through paired t tests for both the IIV and LAIV groups. A Mann–Whitney U test was used to compare the changes of the three antibody titers between the IIV and LAIV groups. We used linear regression to estimate the associations between antibody changes and their related factors. A backward stepwise selection method was used to determine the variables in the final model. Finally, we evaluated the correlation of protection against influenza by calculating protective effectiveness using Cox’s proportional hazards model. The protective effectiveness was defined as (1-hazard ratio) X 100%. All analyses were performed in R version 3.4.2.
4. Discussion
Children represent an extremely important group when it comes to the prevention of influenza virus infection by vaccination due to their elevated risk for severe illness, and their role as a major source of viral transmission [
22,
23]. Indeed, vaccination of children alone can have profound effects on the ‘herd/population immunity’ elicited by influenza vaccines [
22]. Therefore, ensuring that children receive influenza vaccine formulations that provide optimal vaccine efficacy is of paramount importance.
Despite observational data from the U.S. Vaccine Effectiveness network that led ACIP to recommend against the use of LAIV during the 2016–2017 influenza season, members of our group previously published the results of a cluster-randomized control trial in which the efficacy of IIV and LAIV was equivalent across the three influenza seasons spanning October 2012 to May 2015 for all strains present in the trivalent vaccine [
14]. However, the immunogenicity of IIV and LAIV in this cohort had not been analyzed, nor were immunological correlates of protection identified. Therefore, we performed an immunogenicity analysis of the humoral responses of that same cohort using serum and mucosal samples collected from the 2014–2015 influenza season (since this was the only season during which both serum and mucosal samples were collected).
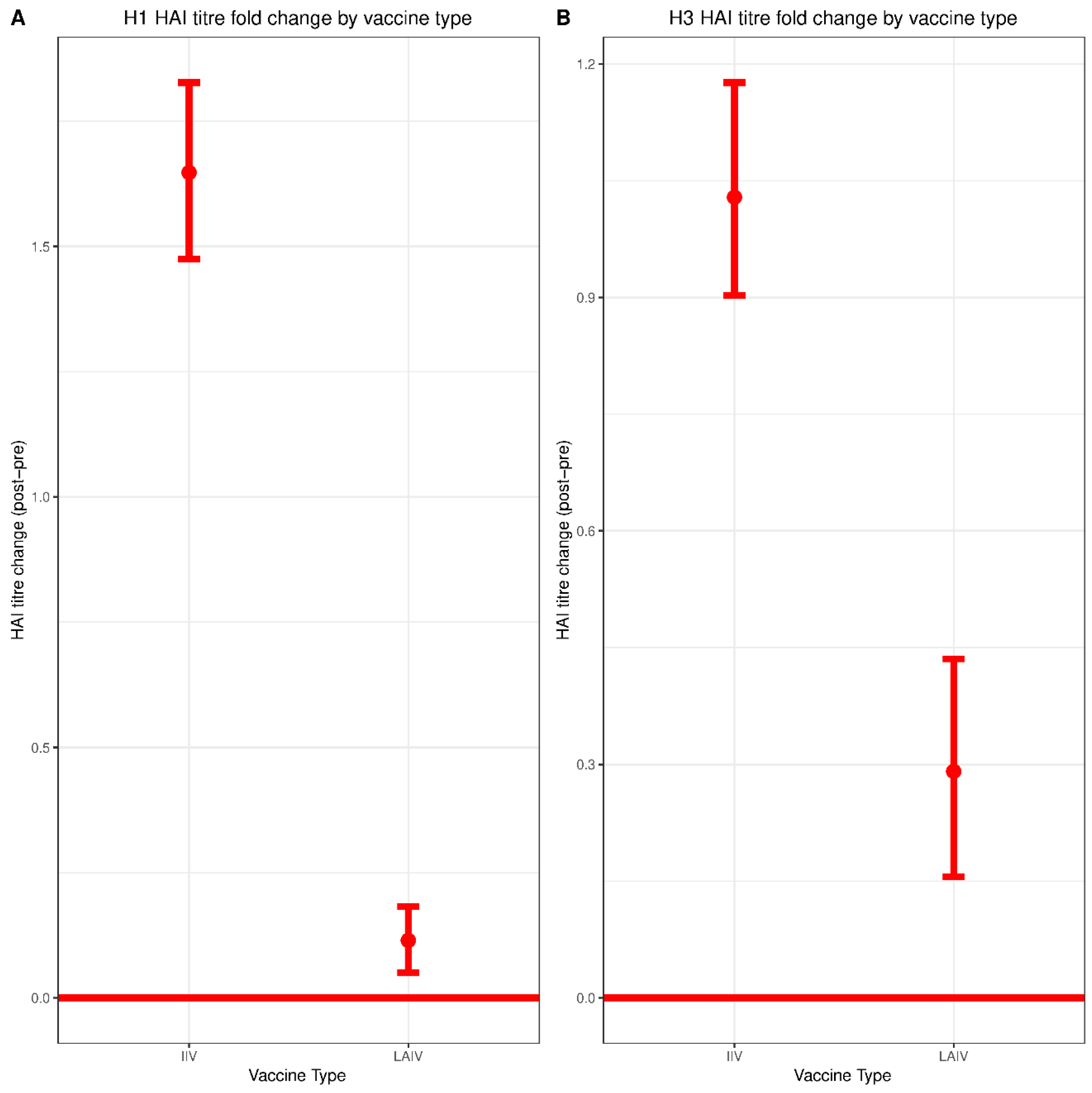
Both IIV and LAIV induced significant increases in serum HAI titers against both H1N1 and H3N2 however, the magnitude of seroconversion induced by IIV was much greater—consistent with prior studies [
24]. HAI titers of 1:80 and 1:160 correlated with protection against H3N2 infection for the group immunized with IIV. This observation is in line with previous studies reporting HAI titers of greater than 1:40 as being required for protection in children immunized with IIV [
16,
25].
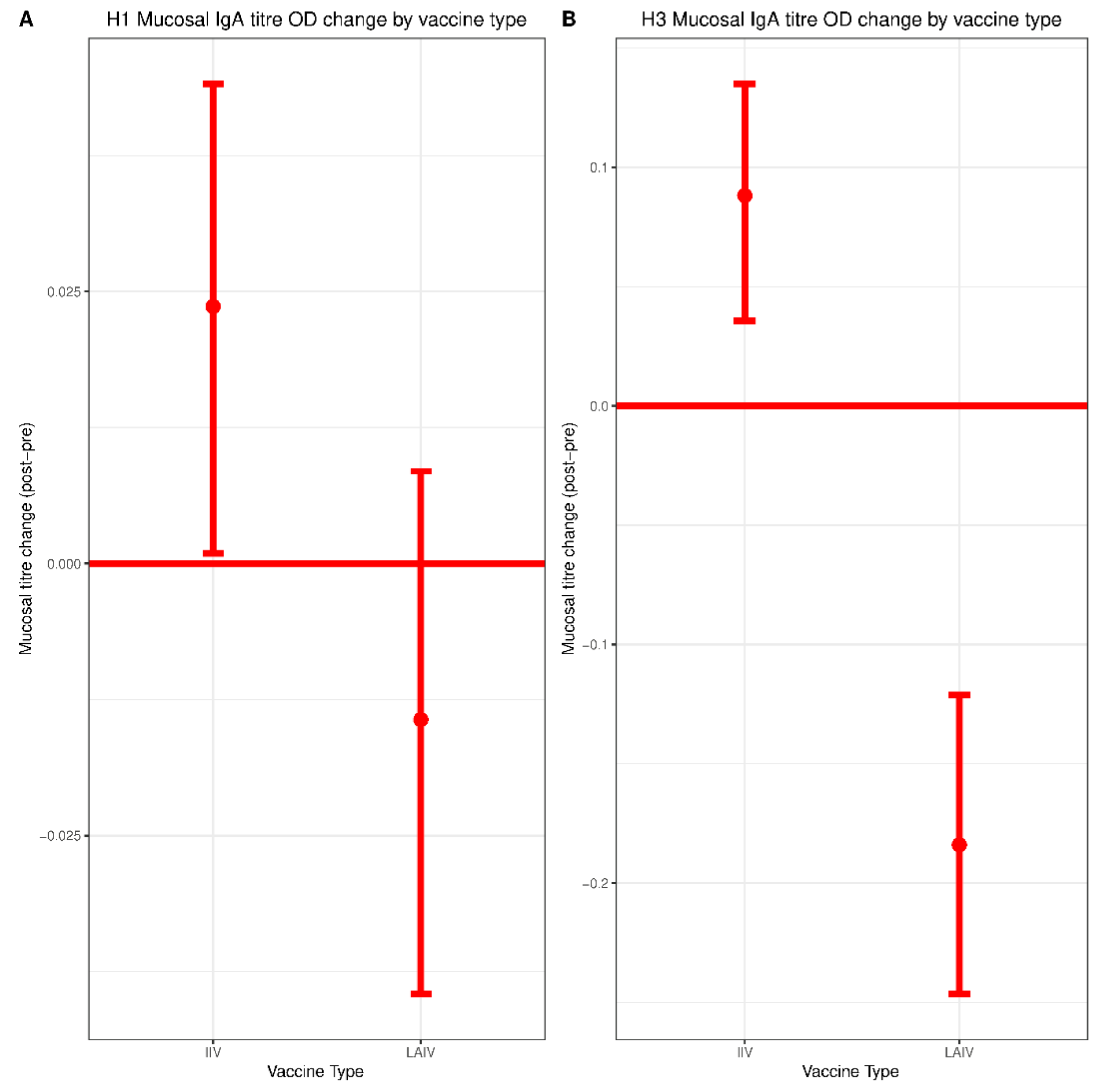
IIV recipients experienced a small but significant boost in mucosal IgA titers against both H1N1 and H3N2 vaccine components. No increase in mucosal IgA was observed in the LAIV group. However, consistent with an earlier H1N1 challenge study of children vaccinated with LAIV, detection of any mucosal IgA correlated with protection against H3N2 infection [
20]. Given the similarity in mucosal IgA titers when comparing IIV and LAIV groups, as well as the fact that the IIV group tended to mount stronger post-vaccination responses, it is possible that mucosal IgA might also serve as a correlate of protection for IIV. However, limitations in sample size precluded the generation of estimates for IIV in our model. This issue should be explored more thoroughly in future studies of larger cohorts.
The lack of a detectable boost in mucosal IgA titers in the LAIV group was surprising, since LAIV has been reported to boost mucosal IgA titers in several previous studies [
21,
26,
27,
28]. Several possibilities might explain this discrepancy. Firstly, pre-vaccination mucosal IgA titers against H3N2 were much higher in the LAIV group when compared to the IIV group. This likely reflects the fact that most children in the LAIV group had also been vaccinated with LAIV for the previous two seasons. High pre-vaccination antibody titers are known to correlate negatively with response to vaccination, which we also observed in response to H3—although the effect was modest [
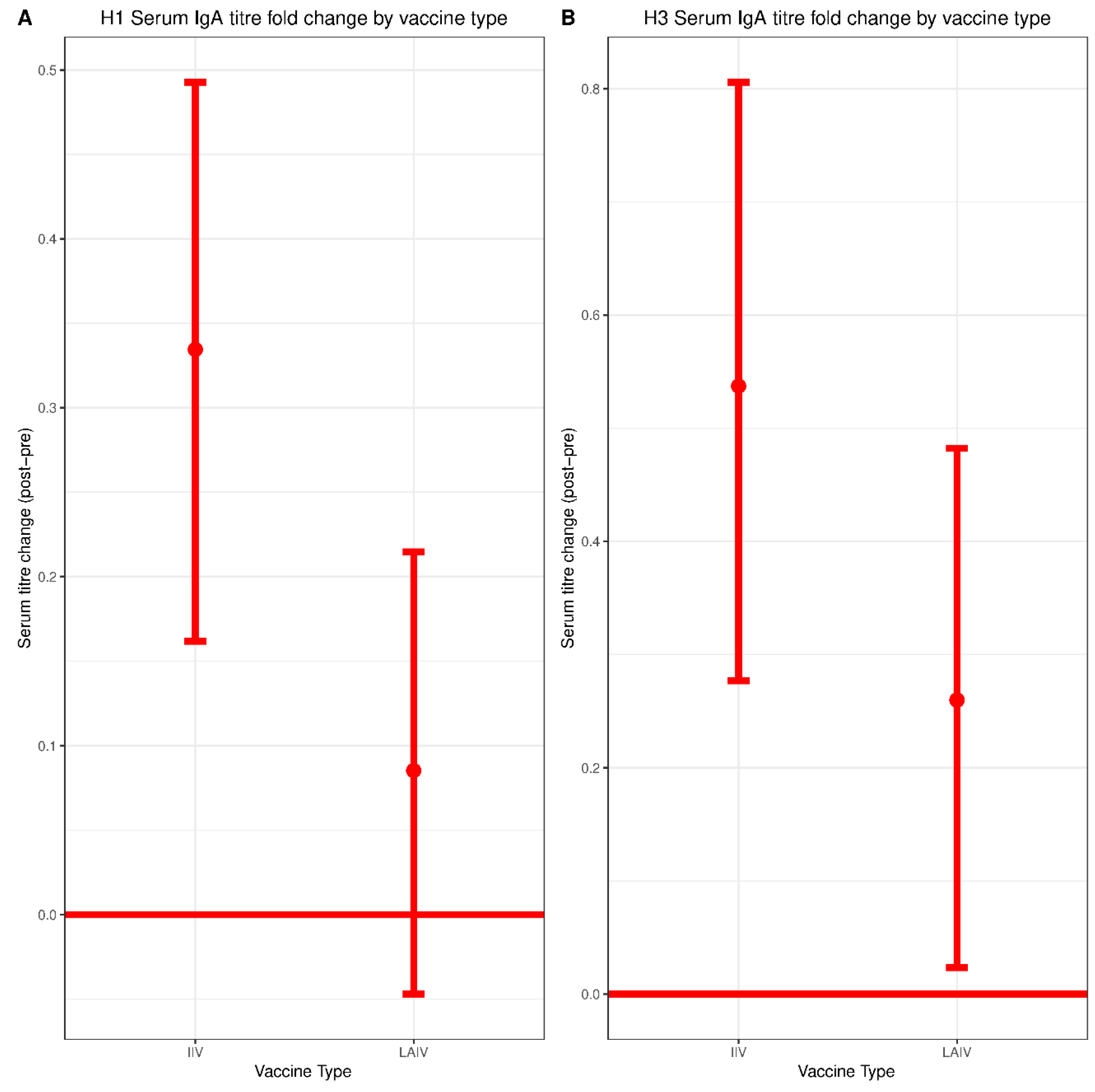
29]. This could also be due to differences in sampling methodologies. Most prior studies measured mucosal IgA collected in nasal washes whereas we detected mucosal IgA collected in nasal swabs, which are likely to have lower concentrations of antibody. Indeed, we were only able to reliably measure mucosal IgA above our limit of detection using undiluted samples, which precluded titration-based measurements. Notably, serum IgA did not correlate with protection in either the IIV or the LAIV group, highlighting the distinct composition of serum and mucosal IgA pools.
The influence of pre-existing immunity on the vaccine-induced responses during the 2014–2015 influenza season is also an important consideration in this study—since most of the participants had been enrolled in the study and vaccinated for each of the two previous seasons [
14]. Indeed, considerable pre-vaccination HAI titers against H1N1 and H3N2 vaccine strains were observed in both the IIV and LAIV groups. Pre-existing immunity has, in certain instances, been shown to limit responses to LAIV—probably by restricting replication of the attenuated virus [
28,
30]. High titers of pre-existing antibodies have also been identified as a negative correlate of serological responses in multiple systems biology studies of influenza virus vaccination [
29,
31]. Consistent with these reports, we found that pre-existing antibody titers were negatively correlated with HAI responses, and that this relationship was strongest in the IIV group. A weak, but significant negative correlation between H3 pre-vaccination mucosal IgA titers and post-vaccination response was also found for both IIV and LAIV. Interestingly, a study using a mouse model of influenza previously reported that influenza virus-specific B cells are sensitized to viral infection and subsequently, cell death. This led the authors to speculate that killing of these cells during early infection may allow the virus to establish initial replication and delay the memory antibody response [
32]. This has never been established in humans, to our knowledge. However, the small (but significant) decreases in mucosal IgA observed only in the LAIV group would be consistent with such a phenomenon and warrants further investigation. Given the modest magnitude of this decrease, it is also possible that while statistically significant (due to the relatively large number of samples analyzed), the decrease is not biologically meaningful.
A notable limitation of this study is that peripheral blood mononuclear cells were not collected and thus, T cell responses were not measured. LAIV is known to effectively boost T cell responses, including cross-reactive T cells that may help to protect against drifted or novel strains [
28,
33,
34]. Granzyme B levels have also been associated with protection against laboratory diagnosed influenza after vaccination of older adults [
35]. In addition, microneutralization titers capture a more complete picture of neutralizing antibody responses than HAI assays, and may also be an important correlate of protection. MNTs were not performed here due to limitations in sample availability. This study was performed as a cluster randomized trial. Therefore, cluster effects should, thus, also be considered when interpreting this data. For example, shedding of LAIV between vaccinated and not-yet-vaccinated individuals within the LAIV cluster could influence the kinetics and magnitude of antibody boosting. However, if this were to occur, it did not result in more robust community protection afforded by LAIV in this trial [
14]. Furthermore, most children would have received the same vaccine formulation in the previous two influenza seasons.
Taken together, the results of this study provide new insights into the nature of the humoral immune response elicited by IIV and LAIV in children. While vaccine efficacy against all strains of influenza virus were equivalent in this cohort, the immune responses elicited by each vaccine were distinct, as were their respective correlates of protection. By most measures, IIV demonstrated superior immunogenicity compared to LAIV. The data presented here reinforce the notion that the HAI titer suggestive of antibody-mediated protection may be greater than 1:40 in children—and that distinct correlates of protection are required for IIV and LAIV.