Abstract
Lathyrus odoratus L., commonly known as sweet pea, is a plant with a distinctive aroma that can develop in various habitats. An analysis of the aromatic profile of the species was conducted using the HS-SPME (solid-phase microextraction headspace) technique. This study aimed to explore the composition of and variation in the floral scent emissions of L. odorathus. The floral scents from fresh flowers were collected over different months and analyzed using gas chromatography coupled with mass spectrometry on apolar and polar stationary phase columns. In the apolar column, the majority compounds included linalool (19.27–5.79%), α-trans-bergamotene (29.4–14.21%), and phenyl ethyl alcohol (30.01–1.56%), while on the polar column, the predominant compounds included myrcene (13.25%), (E,E)-α-farnesene (26.33–8.16%), α-trans-bergamotene (42.09–24.82%), and others. This investigation was complemented by enantioselective analysis using a chiral phase based in cyclodextrins, which revealed the presence of (1R)-(+)-α-pinene, (S)-(−)-limonene, (R)-(+)-germacrene D, and (R)-(E)-nerolidol as enantiomerically pure components and linalool as a racemic mixture. Notably, the principal component analysis (PCA) and heatmap revealed variations among the chemical compounds collected at different harvest times. This demonstrates that temporal factors indeed impact chemical compound production. Furthermore, research on the aromatic properties of flowers provides a theoretical basis for studying and improving the components of their scent.
1. Introduction
Aromatic plants, also known as herbs and spices, have been used for their preservative and medicinal properties since around 5000 BC []. Different cultures have been extracting unusual aromas, a source of great fascination, by means of techniques that have become popular over the years. The application of these aromas in as the food, cosmetics, and pharmaceutical industries is particularly noteworthy [].
The Fabaceae family has 770 genera and 19,500 species thanks to its wide distribution, making it part of the third-largest plant family in the world []. Its chemical composition includes mainly terpenes (mono and sesquiterpenes), fatty acids, and benzenoids []. Due to the large family and the variety of compounds, it has diverse properties for the treatment of ailments, pathologies, and syndromes [].
The genus Lathyrus comprises 160 species [] that have a remarkable ability to tolerate hostile environmental conditions such as drought, bogs, and low temperatures. Approximately twenty tree species are endemic of South América []. Lathyrus odoratus L., known as the sweet pea, is an annual climbing plant native to southern Europe and north Africa []. It is prized for its showy and fragrant flowers, which are very delicate to harvest and have a very short lifespan. In Ecuador, Lathyrus odoratus L. is an introduced species and can be considered a herb or vine. It inhabits the coastal or Andean region, can grow up to 3000 m above sea level, and can be found in the provinces of Guayas and Pichincha []. In this study, this species was identified for the first time in the south of Ecuador. According to the IUCN (International Union for Conservation Union) Red List of Threatened Species, the species is currently threatened with extinction [].
Various data on the volatile chemistry of this species are already available. Previous studies have reported the chemical composition of Lathyrus odoratus L. Essential oil, it consists of (E)-β-ocimene (22.9–46.5%) linalool (16.6–26.2%), geraniol (4.5–6.5%), nerol (3.3–10.1%), α-trans-bergamotene (1.3–6.8%), and β-sesquiphellandrene (0.2–1.2%) as the main compounds []. This plant has been used as an antidiuretic and to supply calcium to the body []. In other species, germacrene D (50.4%), germacrene B (18.7%), γ-elemene (9.5%), and myrcene (7.4%) are the main components in the oil of Lathyrus rotundifolius []. Additionally, involatile compounds isolated in L. odoratus were an unidentified carbohydrate L-1-O-methyl-myo-inositol and L-bornesitol []. Other volatile compounds in Lathyrus L. species were analyzed by SPME-GC-MS, and the main components of L. aphaca were tetradecane 14.3%, camphor 21.6–10.1%, and yomogi alcohol 26.1–16.5%; those of L. cicera camphor 18.7–2.0% and yomogi alcohol 20.3–3.0%; those of L. gorgonei yomogi camphor 17.1–9.0% and alcohol 24.5–13.1%; those of L. sativus camphor 9.0% and yomogi alcohol 11.4%; those of L. ochrus hexenal 7.0% and 2-methyl butanoic acid 7.2%; those of L. saxatilis tetradecane 5.4%, (Z)-3-hexenal 6.4%, and hexanal 7.7%; and those of L. blepharicarpos var. cyprius dodecane 5.1%, yomogi alcohol 5.9%, and (Z)-3-hexenal 8.6% [].
In this study, the main aromatic compounds present in Lathyrus odoratus L. were studied using the HS-SPME technique with the aim of discovering the components determining its fragrance, as well as taking advantage of the applicability of the technique of headspace solid-phase microextraction coupled with a gas chromatograph (HS-SPME-GC) for the extraction and analysis of volatile compounds and determining the compounds present at different times of collection. In the same way, we studied the enantiomeric composition of the volatile fraction and report it for the first time. We describe and apply the technique of enantioselective gas chromatography (GC) to assign the absolute configuration of chiral natural compounds. This configuration strongly influences the odor properties of their enantiomers [,]. Thus, for the first time, the present study focused on the variability of the chemical profile of Ecuadorian flowers in different months of the year to determine the best period for harvesting and achieving the highest level of desirable bioactive compounds for use in the pharmaceutical and food industries.
2. Results
2.1. Chemical Composition
The variability in the chemical composition of the volatile compounds from the flowers of Lathyrus odoratus L., analyzed using the DB5-ms column and ordered by retention index, is presented in Table 1. The major constituents in L. odoratus flowers were α-trans-bergamotene (14.21%, 29.35% and 28.68%), phenyl ethyl alcohol (24.67%, 7.58% and 7.16%), nerol (16.18%, 2.22% and 5.61%), linalool (5.79%, 18.6% and 19.27%), myrcene (3.48%, 1.41% and 1.20%), β-sesquiphellandrene (2.76%, 4.95% and 7.56%), and 2-phenyl ethyl acetate (2.35%, 0.35% and 0.38%) (Figure 1).

Table 1.
Volatile chemical composition of Lathyrus odoratus flowers in apolar column in different collection periods (2023).
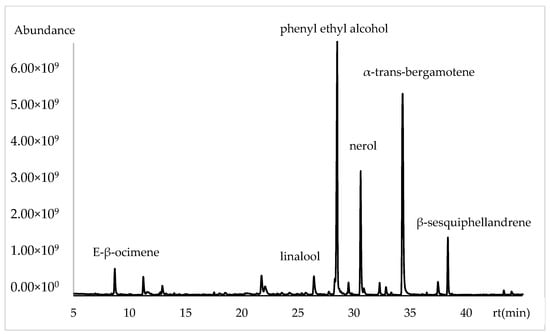
Figure 1.
Chromatogram of volatile chemical composition of Lathyrus odoratus flowers in apolar column.
The chemical composition of flowers using a polar phase based on polyethylene glycol (PEG) (Table 2 and Figure 2) showed variability in the chemical volatile majority compounds present during the studied months. The principal compounds were α-trans-bergamotene (42.09%, 43.48% and 24.82%), (E)-β-ocimene (12.14%, 7.21% and 2.89%), linalool (8.94%, 9.38% and 2.88%), β-sesquiphellandrene (7.03%, 6.53% and 0.33%), 7-epi-sesquithujene (5.21%, 4.30% and 2.03%), phenyl ethyl alcohol (8.16%, 1.76% and 30.01%) and finally (E)-nerolidol (1.43%, 1.21% and 5.30%).

Table 2.
Volatile chemical composition of Lathyrus odoratus flowers in polar column in different collection periods (2023).
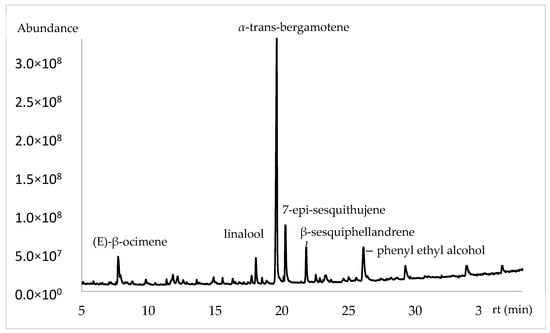
Figure 2.
Chromatogram of volatile chemical composition of Lathyrus odoratus flowers in polar column.
2.2. Enantiomeric Analysis
The enantioselective analysis was performed with a capillary column using 2,3-diethyl-6-tert-butyldimethylsilyl-β-cyclodextrin as a chiral selector. A total of six enantiomers were identified, along with their respective enantiomeric distribution and enantiomeric excess (e.e.). The enantiomers were (1R)-(+)-α-pinene, (S)-(−)-limonene, (R)-(+)-germacrene D and (R)-(E)-nerolidol were identified as enantiomerically pure components and the linalool as a racemic mixture. The complete enantioselective analysis is presented in Table 3 and Figure 3.

Table 3.
Enantioselective analysis of some chiral terpenes from Lathyrus odoratus L. flowers using β-cyclodextrin column.
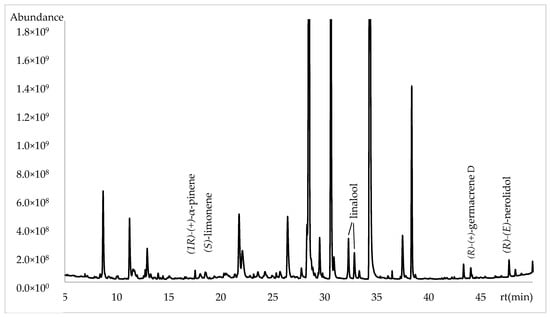
Figure 3.
Chromatogram of enantioselective analysis in Lathyrus odoratus flowers using β-cyclodextrin column.
2.3. Statistical Analysis
The principal component analysis (PCA), corresponding to the DB-5ms column, showed that component 1 explained 66.81% of the variance, while component 2 had 33.19% of the variance in the total analysis (Table 4).

Table 4.
PCA scores showing the main components of Lathyrus odorarus L. in different months of harvest using DB5-ms and HP-INNOWax columns.
The heatmap in Figure 4 shows two separate groups of the volatile compounds from the flowers of Lathyrus odoratus L. for DB5-ms column in relation to month. In this heatmap, the scale of color is relative to the value of the volatile compounds. The high similarity among volatile compounds in March and May is indicated by the dominance of the blue. On the other hand, where the similarity between compounds is lower, the similarity (e.g., July) is indicated in light blue. The results obtained on the basis of the tool used were able to better group all the compounds, thus identifying those that stand out more in the different months of collection, being the months of April and May, that present a greater variation in compounds. It should be emphasized that the compounds that predominate more in the month of May are α-trans-bergamotene, (E)-β-ocimene, and (Z)-β-farnesene and in April are nerol and phenyl ethyl alcohol.
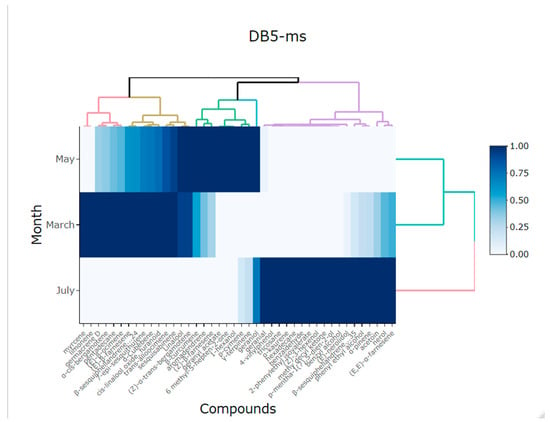
Figure 4.
Heatmap showing the main compounds of Lathyrus odorarus L. in different months of harvest using DB5-ms column.
PCA showed the variability between the two components corresponding to method 2 (HP-INNOWax): component 1 had a percentage of 75.97% and component 2 had 24.02%, giving a total of 99.99% of the explained variance (Table 4). The heatmap in Figure 5 shows two separate groups of volatile compounds from the flowers of Lathyrus odoratus L. for the HP-INNOWax column in relation to month. The high similarity among volatile compounds in March and May is indicated by the dominance of the blue in the heatmap. It was possible to identify the components that stood out in the different months of collection, with June being the month with the greatest variability of compounds, but it should be noted that the most predominant compounds were phenyl ethyl alcohol, geranial, α-trans-bergamotene, (Z)-β-farnesene, and (E,E)-α-farnesene.
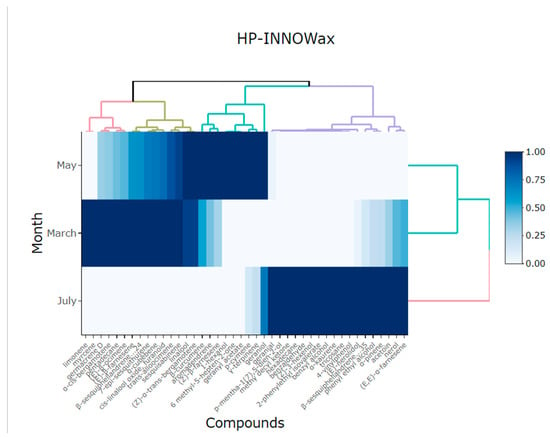
Figure 5.
Heatmap showing the main compounds of Lathyrus odorarus L. in different months of harvest using a HP-INNOWax column.
3. Discussion
Volatile compounds emitted from fresh Lathyrus odoratus flowers were obtained using HS-SPME-GC analysis on a DB-5MS column and 71 compounds were isolated. The analysis showed that the main volatile compounds in quantifiable amounts (>2%) were α-trans-bergamotene, (E)-β-ocimene, linalool, (E,E)-α-farnesene, 7-epi-sesquithujene and β-sesquiphellandrene. Volatile profiles similar to those we have recorded for flowering L. odoratus L. have been reported, such as (E)-β-ocimene, linalool, (E,E)-α-farnesene and β-sesquiphellandrene as the predominant volatiles []. Porter (1999) [] studied the floral volatiles of L. odoratus L. using thermal desorption–gas chromatography–mass spectrometry and the major components were (E)-β-ocimene (22.9–46.5%), linalool (16.6–26.2%), geraniol (4.5–6.5%), and nerol (3.3–10.1%), and in lower proportions α-trans-bergamotene (1.3–6.8%) and β-sesquiphellandrene (0.2–1.2%). In another study of L. odorathus flowers in four locations in the United Kingdom, the most abundant compounds were consistently found to be (E)-β-ocimene (22.9%, 27.8%, 35.3% and 46.5%) and linalool (16.6%, 20.7%, 23.6% and 26.2%) []. Bruce et al. (2002) evaluated the insecticidal properties of the species and identified three primary compounds responsible for this activity: linalool, phenylacetaldehyde, and benzyl alcohol []. Other studies on volatile compounds identified in different species, for example, HS-SPME of Lathyrus vernus L. collected in Turkey identified three major compounds: 1-octen-3-ol (49.8%), 2-hexenal (9.9%), and linalool (3.8%) []. A rather different composition was described for the volatile fraction of L. rotundifolius essential oil collected in Iran, and five major compounds were found: germacrene D (50.4%), germacrene B (18.7%), γ-elemene (9.5%), myrcene (7.4%), and β-sesquiphellandrene (2.6%) []. All of these studies demonstrate the variability in chemical composition between the same species collected in different locations, as well as between different species of the same genus. The major compound in our study was α-trans-bergamotene, which is used in applications in cosmetology and perfumery. In cosmetics, it is widely used due to its ability to refresh and flavor products, while in perfumery, it contributes a characteristic citrus aroma appreciated for its freshness and vitality, and this component exhibits antioxidant, anti-inflammatory, and antimicrobial properties, making it a beneficial ingredient for skin and hair care [].
According to Sexton et al. (2005) [], the synthesis of the floral aroma of the species L. odoratus L. develops in parts of the flower such as the standard petals and wings. The authors showed that the production of the characteristic aroma is due to the condensation of vapor, which contains an abundant quantity of terpenes surrounding the flowers. The increase in the emission of volatile compounds occurs in the final stages of flower opening, with the best sampling time being when the flowers are fully open.
The chemical composition of plants is subject to both quantitative and qualitative variations. Plant material collected at different times of the year may contain novel compounds with distinct bioactivities []. Seasonal variations in chemical composition may be influenced by phenological status and environmental conditions, which regulate biosynthesis []. Additionally, the location where the species is collected, including factors such as plant care, climatic conditions, soil nutrients, and pollinators, can differ from the geographical areas referenced in other studies. There is also limited information on the resulting compounds based on different collection times []. Research has extensively investigated the effect of seasonal changes on the production of secondary metabolites in plants, revealing variations in specific compounds produced during different seasons. These variations in phytochemical production significantly alter the chemical profile of plant materials, potentially affecting the quality of bioactive compounds. In addition, seasonal variations in chemical composition can influence the biological activity of the plant []. The flowers of L. odoratus L. contain anthocyanins with antioxidant, antiulcer and anti-inflammatory activities. Four major anthocyanins were identified in dark-pink flowers and the components of an alcoholic extract were analyzed. The total anthocyanins showed higher antimicrobial activity than the isolated compounds, being more effective against bacteria, yeasts and fungi [].
Regarding the HS-SPME-GC analysis, Lancioni et al. (2022) mentioned that the SPME technique has many advantages, one of the most important being the non-incorporation of solvents to obtain the compounds during the desorption phase, which helps reduce environmental pollution caused by the solvents used [].
A limitation of this research is the results obtained by GC-MS analysis in both columns, which reported different peak area percentages for the same compound. These results could be related to the following factors. (1) It is important to consider the effect of the column used on the selectivity of the separation, which may lead to variability in the elution of the compounds and co-elution of the peaks. (2) Different columns have different stationary phases, which affect the retention times of the compounds and thus the separation of the analytes []. (3) Columns with different polarities, such as apolar and polar, may separate structurally similar compounds differently, resulting in variations in the chromatographic profile []. (4) Take into account that for the polar stationary phase, the samples were stored. According to some studies, storage for one day can affect the emission of volatile compounds responsible for the aroma []. The synthesis and continuous emission of these compounds is interrupted due to lack of access to water and nutrients from the parent plant [], and can lead to a reduction in the intensity of the aroma and changes in the composition of the emitted volatile compounds. Therefore, the GC-MS results in our research are limited to the description of the compounds obtained by DB5-ms and HP-INNOWax.
To the best of our knowledge, this is the first enantioselective analysis of Lathyrus odoratus L. flowers. This understanding is crucial, as it allows us to determine their significance, which varies depending on the analyte being studied. Accurate knowledge of the enantiomeric ratios of aroma compounds is becoming increasingly important, particularly in the authentication of food products and essential oils, as well as in the development and creation of fragrances and perfumes []. Chiral discrimination is recognized as one of the key principles in biological activity and olfaction [].
4. Materials and Methods
4.1. Plant Material
Lathyrus odoratus L. flowers (Figure 6) were harvested in the morning, its were analyzed immediately in the apolar stationary phase and stored for 24 h for the polar stationary phase, from March to July 2023 in the Quisquinchir district, Saraguro Canton, Loja Province, at coordinates of 3°36′43.8″ S; 79°14′43.2″ W at an altitude of 2600 m a.s.l. A voucher specimen (14777) has been deposited in the HUTPL herbarium. This collection was carried out with the authorization of the Ministry of Environment, Water and Ecological Transition MAATE-ARSFC-2022. Our study was based on a short period of observation, less than 6 months, and it was always difficult to extrapolate and generalize these results, as Ecuador produces little seasonality throughout the year. There are only two defined seasons: wet or winter (October to May), and dry or summer (June to September). In our study, samples were collected in both seasons. The mean annual temperature is 12–13 °C with relatively little monthly variation (data from the local meteorological unit of INAMHI). The annual precipitation at the INAMHI station M142 in Saraguro (79°23′ W 3°62′ S 2525 m a.s.l.) is 827 mm, calculated from the last 25 years []. The soils of this area were formed on granodioritic plutonic rocks, partially sheared, and metamorphosed [].

Figure 6.
Lathyrus odoratus L. in the flowering period.
4.2. Extraction of Compounds by SPME
The SPME device and the fused silica fibers were purchased from Supelco (Bellafont, PA, USA). The fibers based in divinylbenzene/carboxene/polydimethylsiloxane (DVB/CAR/PDMS) (model 57328-U, Supelco, Bellefonte, PA, USA) of 10 mm length and conditioned prior to use at 270 °C for 1. The analytical conditions described for HS-SPME sampling were chosen after preliminary assays using different amounts of flowers and different extraction and desorption conditions (time, temperature, equilibrium time). Cut samples (5 g) were placed separately in glass vials (100 mL) and sealed hermetically using PTFE/silicone septa. The flowers were left at 40 °C with agitation (250 rpm) for 10 min to allow equilibration of volatiles in the headspace. After equilibration, the SPME needle was inserted and the fiber was exposed to the headspace for 40 min. The volatiles adsorbed were thermally desorbed in the hot injection port of a GC for 5 min at 250 °C with the purge valve off in splitless mode and deposited onto a capillary column [].
4.3. Chemical Profiling
4.3.1. GC-MS
Analytical gas chromatography was carried out using a Thermo Scientific model TRACE 1310 chromatograph (Waltham, MA, USA) equipped with a Thermo Scientific model ISQ 7000 mass spectrometer (Bartlesville, OK, USA) to analyze the volatile compounds. The carrier gas used was ultrapure helium (GC purity grade from Indura, Guayaquil, Ecuador) with a flow rate of 1 mL/min []. It was performed in splitless mode, with an injector temperature of 250 °C. The separation was achieved using two columns: a DB-5ms fused silica column (5% phenyl 95% polydimethylsiloxane, 30 m × 0.25 mm i.d., film thickness 0.25 µm) and an HP-INNOWax (polyethylene glycol, 30 m × 0.25 mm i.d., film thickness 0.25 µm) from J&W Scientific (Folsom, CA, USA). The column temperature was 40 °C for 5 min with a ramp of 3 °C/min to 150 °C, a second ramp of 5 °C/min to 180 °C, a third ramp of 7 °C/min to 230 °C, and finally held for 10 min. The ionization source and quadrupole temperatures were 230 °C and 150 °C, respectively, with a run time of 67 min. The spectra recordings represented a full scan with a mass range (30 and 350 amu) at a scan rate of 0.2 scan/s [].
The identification and determination of the constituents of each profile was tentatively made by comparing their mass spectral fragmentation patterns and linear retention indices (LRIs) relative to C9–C24 n-alkanes (Sigma-Aldrich, St. Louis, MO, USA, EE.UU.) with those reported in the literature, as well as those stored in an MS spectral literature database (NIST 2020), and an acceptable difference from literature data was ±25 units in LRI.
4.3.2. Enantioselective Analysis
The enantioselective analysis was carried out using an enantioselective MEGA-DEX-DAC Beta from Mega, MI, Italy, capillary column based on 2,3-diethyl-6-tert-butyldimethylsilyl-β-cyclodextrin. The column was 25 m long, 0.25 mm in internal diameter and with 0.25 μm phase thickness, installed in the same GC–MS instrument described for the qualitative analysis. Sample volumes, injector temperature, transfer line temperature, and MS parameters were the same as for the qualitative analyses, whereas the split ratio was 20:1. The GC method was as follows. Initial temperature was 60 °C for 2 min, followed by 2 °C/min to 220 °C, which was maintained for 2 min. A homologous series of n-alkanes (C9–C25) was also injected in order to calculate the linear retention indices. The enantiomers of the chiral components were identified by injection of enantiomerically pure standards (Sigma-Aldrich, St. Louis, MO, USA) [].
4.4. Statistical Analysis
Principal component analysis (PCA) was used to comprehend the similarity among the volatile compounds of essential oils in relation to the months for DB5-ms columns and HP-INNOWax []. A heatmap was generated using pheatmap and ggplot2 packages to visualize the similarity of chemical compositions of the volatile compounds from the flowers of Lathyrus odoratus L., using the DB5-ms column and HP-INNOWax ordered by retention time. Before conducting the heatmap analysis, the data were standardized to a common scale (0 to 1), where color intensity was used to represent the effect size. These results were obtained with the statistical software Rstudio version 1.1.453 [].
5. Conclusions
In this study, the combination of GC-SPME-MS and chemometric analysis provided a robust and reproducible method for the analysis of Lathyrus odoratus L. flowers. By integrating both polar and apolar phases in gas chromatography (GC), we identified a wide range of aromatic compounds. This study is the first to report the emission of chiral compounds from L. odoratus L. flowers, thereby improving our understanding of their chemical profile. Significant variations in chemical composition were observed at different flower harvests, leading to the identification of specific volatile compounds. In addition, the season of harvest was found to influence the chemical composition of L. odoratus. The observed seasonal variations provide valuable insights for selecting the optimal season to harvest components of interest, thereby enhancing the potential applications of L. odoratus L. in the food and pharmaceutical industries.
Author Contributions
Conceptualization, M.P. and J.C.; methodology, M.P.; software, Á.B.; formal analysis, J.C.; investigation, M.P., J.C. and Á.B.; writing—original draft preparation, M.P.; writing—review and editing, J.C. and Á.B.; supervision, J.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Universidad Técnica Particular de Loja, grant POA-VIN-056.
Data Availability Statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
We thank the Private Technical University of Loja (UTPL) for funding this Open Access publication.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Christaki, E.; Bonos, E.; Giannenas, I.; Florou-Paneri, P. Aromatic Plants as a Source of Bioactive Compounds. Agriculture 2012, 2, 228–243. [Google Scholar] [CrossRef]
- Drobnik, J.K.; Wełna, K. Cosmetic plants of the early 19th century. Rośliny kosmetyczne początku XIX wieku. Pol. J. Cosmetol. 2017, 20, 349–358. [Google Scholar]
- Jin, D.P.; Choi, I.S.; Choi, B.H. Plastid genome evolution in tribe Desmodieae (Fabaceae: Papilionoideae). PLoS ONE 2019, 14, e0218743. [Google Scholar] [CrossRef] [PubMed]
- Marinho, C.R.; Martucci, M.E.P.; Gobbo-Neto, L.; Teixeira, S.P. Chemical Composition and Secretion Biology of the Floral Bouquet in Legume Trees (Fabaceae). Bot. J. Linn. Soc. 2018, 187, 5–25. [Google Scholar] [CrossRef]
- Castañeda, R.; Gutiérrez, H.; Carrillo, É.; Sotelo, A. Leguminosas (Fabaceae) silvestres de uso medicinal del distrito de Lircay, provincia de Angaraes (Huancavelica, Perú). Bol. Latinoam Caribe Plantas Med. Aromát. 2017, 16, 136–149. [Google Scholar]
- Calvo, J.; Moreira-Muñoz, A. Nuevas adiciones a la flora introducida de Chile. Darwiniana 2018, 6, 179–185. [Google Scholar] [CrossRef]
- Chalup, L.; Grabiele, M.; Neffa, V.S.; Seijo, G. DNA content in South American endemic species of Lathyrus. J. Plant Res. 2014, 127, 469–480. [Google Scholar] [CrossRef]
- Bao, T.; Shadrack, K.; Yang, S.; Xue, X.; Li, S.; Wang, N.; Wang, Q.; Wang, L.; Gao, X.; Cronk, Q. Functional Characterization of Terpene Synthases Accounting for the Volatilized-Terpene Heterogeneity in Lathyrus Odoratus Cultivar Flowers. Plant Cell Physiol. 2020, 61, 1733–1749. [Google Scholar] [CrossRef]
- Jorgensen, P.M.; León-Yánez, S. Catalogue of the Vascular Plants of Ecuador; Missouri Botanical Garden: Saint Louis, MO, USA, 1999; Volume 75, ISBN 0-915297-60-06. [Google Scholar]
- IUCN. In The IUCN Red List of Threatened Species, version 2019-3. Available online: https://nc.iucnredlist.org/redlist/content/attachment_files/2020-3_RL_Stats_Table7.pdf (accessed on 19 November 2024).
- Porter, A.; Griffiths, D.; Robertson, G.; Sexton, R. Floral Volatiles of the Sweet Pea Lathyrus Odoratus. Phytochemistry 1999, 51, 211–214. [Google Scholar] [CrossRef]
- Inga Huilca, S.; Zavala Calahorrano, A. Uso de Plantas Medicinales En Las Mujeres de La Sierra Centro, Ecuador Durante El Postparto. Revista Vive 2021, 3, 198–212. [Google Scholar] [CrossRef]
- Tajbakhsh, M.; Khalilzadeh, M.A.; Dabiri, H.A. Volatile Constituents of Lathyrus Rotundifolius Willd. and Trifolium Mazanderanicum Rech. f. Two Papilionaceae Herbs Growing Wild in Iran. J. Essent. Oil Res. 2008, 20, 119–121. [Google Scholar] [CrossRef]
- Ichimura, K.; Kohata, K.; Mukasa, Y.; Yamaguchi, Y.; Goto, R.; Suto, K. Identification of L-bornesitol and changes in its content during flower bud development in sweet pea (Lathyrus odoratus L.). Biosc. Biotechnol. Biochem 1999, 63, 189–191. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Polatoğlu, K.; Arsal, S.; Demirci, B.; Başer, K.H.C. Unexpected Irregular Monoterpene “Yomogi Alcohol” in the Volatiles of the Lathyrus L. species (Leguminosae) of Cyprus. J. Oleo Sci. 2016, 65, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.; Pontes, K.V.; Nogueira, I.B.R. Enantiomers and Their Resolution. Encyclopedia 2022, 2, 151–188. [Google Scholar] [CrossRef]
- König, W.; Hochmuth, D. Enantioselective Gas Chromatography in Flavor and Fragrance Analysis: Strategies for the Identification of Known and Unknown Plant Volatiles. J. Chromatogr. Sci. 2004, 42, 423–439. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy; Allured Pub. Corp: Carol Stream, IL, USA, 2017; ISBN 9781932633214. [Google Scholar]
- Noorizadeh, H.; Farmany, A. Exploration of Linear and Nonlinear Modeling Techniques to Predict of Retention Index of Essential Oils. J. Chin. Chem. Soc. 2010, 57, 1268–1277. [Google Scholar] [CrossRef]
- Baser, K.H.C.; Demirci, B.; Özek, T.; Akalin, E.; Özhatay, N. Micro-distilled volatile compounds from Ferulago species growing in western Turkey. Pharm. Biol. 2002, 40, 466–471. [Google Scholar] [CrossRef]
- Valarezo, E.; Castillo, A.; Guaya, D.; Morocho, V.; Malagón, O. Chemical Composition of Essential Oils of Two Species of the Lamiaceae Family: Scutellaria Volubilis and Lepechinia Paniculata from Loja, Ecuador. J. Essent. Oil Res. 2012, 24, 31–37. [Google Scholar] [CrossRef]
- Başer, K.H.C.; Özek, T.; Demirci, B.; Duman, H. Composition of the Essential Oil of Prangos Heyniae H. Duman et M. F. Watson, a New Endemic from Turkey. Flavour. Frag. J. 2000, 15, 47–49. [Google Scholar] [CrossRef]
- Demirci, B.; Başer, K.H.C.; Yildiz, B.; Bahçecioǧlu, Z. Composition of the Essential Oils of Six Endemic Salvia Spp. from Turkey. Flavour. Fragr. J. 2003, 18, 116–121. [Google Scholar] [CrossRef]
- Charoensiddhi, S.; Anprung, P. Bioactive compounds and volatile compounds of Thai bael fruit (Aegle marmelos (L.) Correa) as a valuable source for functional food ingredients. Int. Food Res. J. 2008, 15, 287–295. [Google Scholar]
- Tabanca, N.; Demirci, F.; Demirci, B.; Wedge, D.E.; Baser, K.H.C. Composition, enantiomeric distribution, and antimicrobial activity of Tanacetum argenteum subsp. flabellifolium essential oil. J. Pharm. Biomed. Anal. 2007, 45, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Quijano, C.E.; Linares, D.; Pino, J.A. Changes in Volatile Compounds of Fermented Cereza Agria [Phyllanthus acidus (L.) Skeels] Fruit. Flavour. Fragr. J. 2007, 22, 392–394. [Google Scholar] [CrossRef]
- Blažević, I.; Mastelić, J. Free and bound volatiles of rocket (Eruca sativa Mill.). Flavour. Fragr. J. 2008, 23, 278–285. [Google Scholar] [CrossRef]
- Paolini, J.; Muselli, A.; Bernardini, A.F.; Bighelli, A.; Casanova, J.; Costa, J. Thymol Derivatives from Essential Oil of Doronicum corsicum L. Flavour. Fragr. J. 2007, 22, 479–487. [Google Scholar] [CrossRef]
- Bouhlel, C.; Dolhem, G.; Fernandez, X.; Antoniotti, S. Model Study of the Enzymatic Modification of Natural Extracts: Peroxidase-Based Removal of Eugenol from Rose Essential Oil. J. Agric. Food Chem. 2012, 60, 1052–1058. [Google Scholar] [CrossRef]
- Calvopiña, K.; Malagón, O.; Capetti, F.; Sgorbini, B.; Verdugo, V.; Gilardoni, G. A New Sesquiterpene Essential Oil from the Native Andean Species Jungia Rugosa Less (Asteraceae): Chemical Analysis, Enantiomeric Evaluation, and Cholinergic Activity. Plants 2021, 10, 2102. [Google Scholar] [CrossRef] [PubMed]
- Patiño-Bayona, W.R.; Plazas, E.; Bustos-Cortes, J.J.; Prieto-Rodríguez, J.A.; Patiño-Ladino, O.J. Essential Oils of Three Hypericum Species from Colombia: Chemical Composition, Insecticidal and Repellent Activity against Sitophilus Zeamais Motsch. (Coleoptera: Curculionidae). Rec. Nat. Prod. 2021, 15, 111–121. [Google Scholar] [CrossRef]
- Mancini, E.; Arnold, N.A.; De Martino, L.; De Feo, V.; Formisano, C.; Rigano, D.; Senatore, F. Chemical Composition and Phytotoxic Effects of Essential Oils of Salvia Hierosolymitana Boiss. and Salvia Multicaulis Vahl. Var. Simplicifolia Boiss. Growing Wild in Lebanon. Molecules 2009, 14, 4725–4736. [Google Scholar] [CrossRef]
- Thakeow, P.; Angeli, S.; Weißbecker, B.; Schütz, S. Respuestas Antenales y Conductuales de Cis Boleti al Olor Fúngico de Trametes Gibbosa. Chem. Senses 2008, 33, 379–387. [Google Scholar] [CrossRef]
- Malagón, O.; Bravo, C.; Vidari, G.; Cumbicus, N.; Gilardoni, G. Essential Oil and Non-Volatile Metabolites from Kaunia longipetiolata (Sch.Bip. ex Rusby) R. M. King and H. Rob., an Andean Plant Native to Southern Ecuador. Plants 2022, 11, 2972. [Google Scholar] [CrossRef] [PubMed]
- Couladis, M.; Tzakou, O.; Stojanovic, D.; Mimica-Dukic, N.; Jancic, R. The Essential Oil Composition of Salvia argentea L. Flavour. Fragr. J. 2001, 16, 227–229. [Google Scholar] [CrossRef]
- Lee, J.-G.; Lee, C.-G.; Kwag, J.-J.; Buglass, A.J.; Lee, G.-H. Determination of optimum conditions for the analysis of volatile components in pine needles by double-shot pyrolysis-gas chromatography-mass spectrometry. J. Chromatogr. A 2005, 1089, 227–234. [Google Scholar] [CrossRef]
- Alavez-Rosas, D.; Nguyen, L.M.; Keefover-Ring, K. Retention indices for naturally-occurring chiral and achiral compounds on common gas chromatography chiral stationary phases. Results Chem. 2022, 4, 100659. [Google Scholar] [CrossRef]
- Maldonado, Y.E.; Malagón, O.; Cumbicus, N.; Gilardoni, G. A new leaf essential oil from the Andean species Gynoxys szyszylowiczii Hieron. of southern Ecuador: Chemical and enantioselective analyses. Sci. Rep. 2024, 14, 16360. [Google Scholar] [CrossRef]
- Thoming, G.; Norli, H.R. Olfactory cues from different plant species in host selection by female pea moths. J. Agric. Food Chem. 2015, 63, 2127–2136. [Google Scholar] [CrossRef]
- Bruce, T.J.; Cork, A.; Hall, D.R.; Dunkelblum, E. Laboratory and Field Evaluation of Floral Odours from African Marigold, Tagetes Erecta, and Sweet Pea, Lathyrus Odoratus, as Kairomones for the Cotton Bollworm Helicoverpa Armigera. IOBC wprs Bull. 2002, 25, 315–322. [Google Scholar]
- Yilmaz Iskender, N.; Yayli, N.; Yasar, A.; Çoskunçelebi, K.; Yayli, N. Volatile Constituents of the Flower, Leaf and Stem of Lathyrus vernus (L.) Grown in Turkey. Asian J. Chem. 2009, 21, 6290–6294. [Google Scholar]
- Xing, C.; Qin, C.; Li, X.; Zhang, F.; Linhardt, R.J.; Sun, P.; Zhang, A. Chemical Composition and Biological Activities of Essential Oil Isolated by HS-SPME and UAHD from Fruits of Bergamot. LWT 2019, 104, 38–44. [Google Scholar] [CrossRef]
- Sexton, R.; Stopford, A.P.; Moodie, W.T.; Porter, A.E.A. Aroma Production from Cut Sweet Pea Flowers (Lathyrus odoratus): The Role of Ethylene. Physiol. Plant 2005, 124, 381–389. [Google Scholar] [CrossRef]
- Eloff, J.N. It is possible to use the herbarium specimens to screen for antibacterial activity components in some plants. J. Ethnopharmacol. 1999, 67, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Masotti, V.; Juteau, F.; Bessiere, J.M.; Viano, J. Seasonal and phonological variation of the essential oil from the narrow endemic species Artemisia molinieri and its biological activities. J. Agric. Food Chem. 2003, 51, 7115–7121. [Google Scholar] [CrossRef] [PubMed]
- Gómez, C.A.; Herrera, A.O.; Flórez, V.J. Consideraciones Sobre Factores que Influyen en la Longevidad Poscosecha de Flores de Corte. Consideraciones Sobre Producción, Manejo y Poscosecha de Flores de Corte con Énfasis en Rosa y Clavel; Editorial Universidad Nacional de Colombia: Bogotá, Colombia, 2017; pp. 191–212. [Google Scholar]
- Lemos, M.F.; Lemos, M.F.; Pacheco, H.P.; Endringer, D.C.; Scherer, R. Seasonality modifies rosemary’s composition and biological activity. Ind. Crops Prod. 2015, 70, 41–47. [Google Scholar] [CrossRef]
- Mohamed, S.M. Chemical and biological evaluation of Lathyrus odoratus L. flowers. Planta Med. 2008, 74, PB11. [Google Scholar] [CrossRef]
- Lancioni, C.; Castells, C.; Candal, R.; Tascon, M. Headspace Solid-Phase Microextraction: Fundamentals and Recent Advances. Adv. Sample Prep. 2022, 3, 100035. [Google Scholar] [CrossRef]
- Sawikowska, A.; Piasecka, A.; Kachlicki, P.; Krajewski, P. Separation of Chromatographic Co-Eluted Compounds by Clustering and by Functional Data Analysis. Metabolites 2021, 11, 214. [Google Scholar] [CrossRef]
- Bizzo, H.R.; Brilhante, N.S.; Nolvachai, Y.; Marriott, P.J. Use and abuse of retention indices in gas chromatography. J. Chromatograp. A 2023, 1708, 464376. [Google Scholar] [CrossRef]
- Barman, M.; Mitra, A. Floral maturation and changing air temperatures influence scent volatiles biosynthesis and emission in Jasminum auriculatum Vahl. Environ. Exp. Bot. 2021, 181, 104296. [Google Scholar] [CrossRef]
- Midzi, J.; Jeffery, D.W.; Baumann, U.; Rogiers, S.; Tyerman, S.D.; Pagay, V. Stress-Induced Volatile Emissions and Signalling in Inter-Plant Communication. Plants 2022, 11, 2566. [Google Scholar] [CrossRef]
- Bernreuther, A.; Ulrich, E.; Bernhard, K. Enantiomers: Why they are important and how to resolve them. In Techniques for Analyzing Food Aroma; CRC Press: Boca Raton, FL, USA, 2020; pp. 143–207. [Google Scholar]
- Mosandl, A. Enantioselective capillary gas chromatography and stable isotope ratio mass spectrometry in the authenticity control of flavors and essential oils. Food Rev. Int. 1995, 11, 597–664. [Google Scholar] [CrossRef]
- Hernández, O.H.Á.; Peralta, T.E.M. Completamiento de series de precipitación en la región sur de Ecuador y caracterización de su pluviometría y aridez. Rev. Climatol. 2017, 17, 17–27. [Google Scholar]
- Hungerbühler, D.; Steinmann, M.; Winkler, W.; Seward, D.; Egüez, A.; Peterson, D.E.; Helg, U.; Hammer, C. Neogene stratigraphy and Andean geodynamics of southern Ecuador. Earth-Sci. Rev. 2002, 57, 75–124. [Google Scholar] [CrossRef]
- Aparicio-Ruiz, R.; Casadei, E.; Ortiz-Romero, C.; García-González, D.L.; Servili, M.; Selvaggini, R.; Toschi, T.G. Method for the analysis of volatile compounds in virgin olive oil by SPME-GC-MS or SPME-GC-FID. MethodsX 2023, 10, 101972. [Google Scholar] [CrossRef] [PubMed]
- Calva, J.; Silva, M.; Morocho, V. Composition and Anti-Acetylcholinesterase Properties of the Essential Oil of the Ecuadorian Endemic Species Eugenia Valvata McVaugh. Molecules 2023, 28, 8112. [Google Scholar] [CrossRef]
- Calva, J.; Cartuche, L.; González, S.; Montesinos, J.V.; Morocho, V. Chemical Composition, Enantiomeric Analysis and Anticholinesterase Activity of Lepechinia Betonicifolia Essential Oil from Ecuador. Pharm. Biol. 2022, 60, 206–211. [Google Scholar] [CrossRef]
- Zhou, Y.; Abbas, F.; Wang, Z.; Yu, Y.; Yue, Y.; Li, X.; Yu, R.; Fan, Y. HS–SPME–GC–MS and Electronic Nose Reveal Differences in the Volatile Profiles of Hedychium Flowers. Molecules 2021, 26, 5425. [Google Scholar] [CrossRef]
- Pons-Morera, C.; Darós, L.; Gil-Pechuán, I. Diagrama de Afinidad Aplicado a Mejorar Los Servicios Tecnológicos de La Universidad Politécnica de Valencia. Work. Pap. Oper. Manag. 2012, 3, 46–60. [Google Scholar] [CrossRef][Green Version]
- RStudio Team. RStudio: Integrated Development for R; RStudio Team: Boston, MA, USA, 2016. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).