Methylmalonic Acid and Homocysteine as Indicators of Vitamin B12 Deficiency in Patients with Gastric Cancer after Gastrectomy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Analytical Procedures
2.3. Statistical Analysis
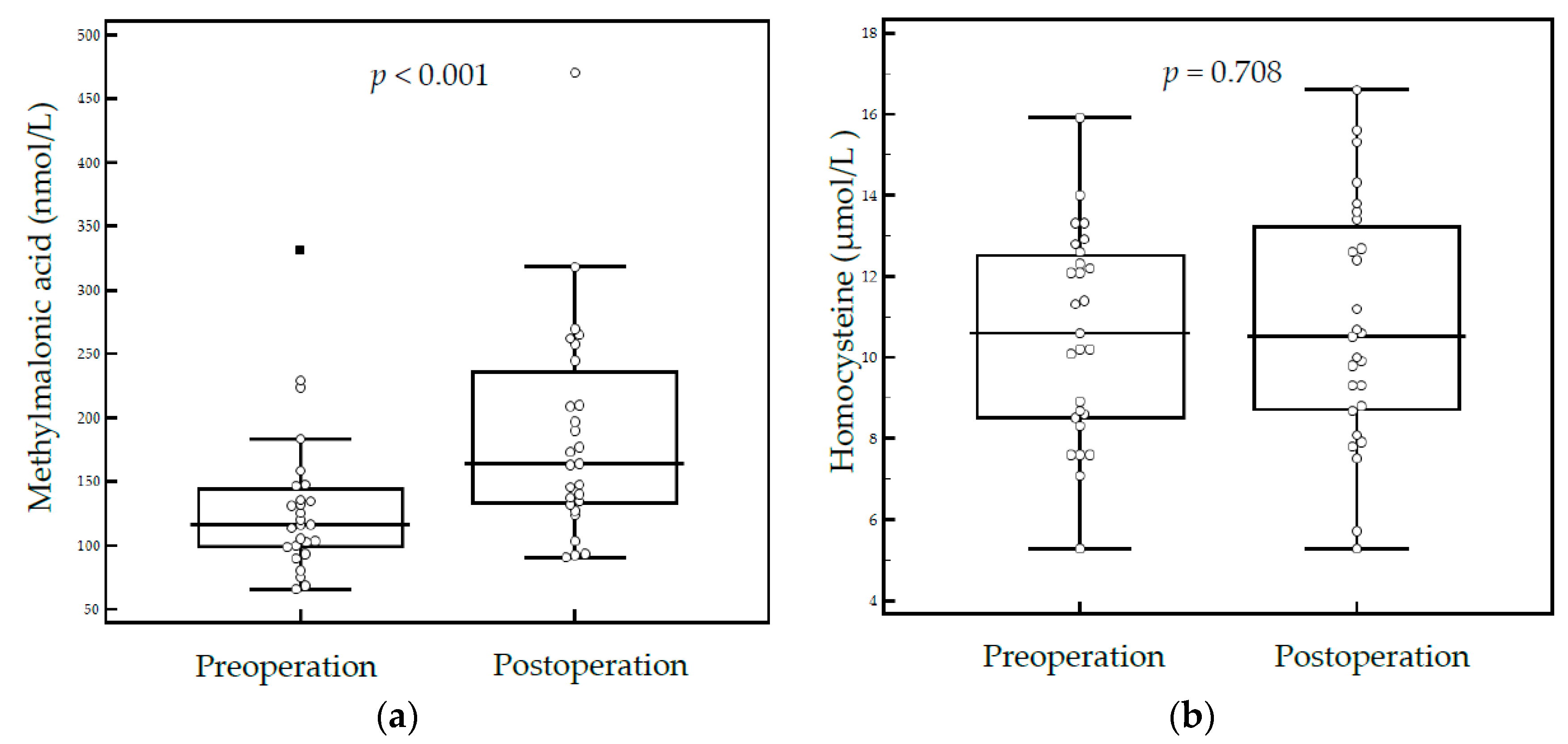
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Oh, R.; Brown, D.L. Vitamin B12 deficiency. Am. Fam. Physician 2003, 67, 979–986. [Google Scholar] [PubMed]
- Allen, L.H. How common is vitamin B-12 deficiency? Am. J. Clin. Nutr. 2009, 89, 693S–696S. [Google Scholar] [CrossRef] [PubMed]
- Beyan, C.; Beyan, E.; Kaptan, K.; Ifran, A.; Uzar, A.I. Post-gastrectomy anemia: Evaluation of 72 cases with post-gastrectomy anemia. Hematology 2007, 12, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Devalia, V.; Hamilton, M.S.; Molloy, A.M.; British Committee for Standards in Haematology. Guidelines for the diagnosis and treatment of cobalamin and folate disorders. Br. J. Haematol. 2014, 166, 496–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, C.M.; Won, Y.J.; Jung, K.W.; Kong, H.J.; Cho, H.; Lee, J.K.; Lee, D.H.; Lee, K.H.; Community of Population-Based Regional Cancer Registries. Cancer statistics in Korea: Incidence, mortality, survival, and prevalence in 2013. Cancer Res. Treat. 2016, 48, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.I.; Hyung, W.J.; Song, K.J.; Choi, S.H.; Kim, C.B.; Noh, S.H. Oral vitamin B12 replacement: An effective treatment for vitamin B12 deficiency after total gastrectomy in gastric cancer patients. Ann. Surg. Oncol. 2011, 18, 3711–3717. [Google Scholar] [CrossRef] [PubMed]
- Bailey, R.L.; Carmel, R.; Green, R.; Pfeiffer, C.M.; Cogswell, M.E.; Osterloh, J.D.; Sempos, C.T.; Yetley, E.A. Monitoring of vitamin B-12 nutritional status in the united states by using plasma methylmalonic acid and serum vitamin B-12. Am. J. Clin. Nutr. 2011, 94, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Hoey, L.; Strain, J.J.; McNulty, H. Studies of biomarker responses to intervention with vitamin B-12: A systematic review of randomized controlled trials. Am. J. Clin. Nutr. 2009, 89, 1981S–1996S. [Google Scholar] [CrossRef] [PubMed]
- Palacios, G.; Sola, R.; Barrios, L.; Pietrzik, K.; Castillo, M.J.; Gonzalez-Gross, M. Algorithm for the early diagnosis of vitamin B12 deficiency in elderly people. Nutr. Hosp. 2013, 28, 1447–1452. [Google Scholar] [PubMed]
- Valente, E.; Scott, J.M.; Ueland, P.M.; Cunningham, C.; Casey, M.; Molloy, A.M. Diagnostic accuracy of holotranscobalamin, methylmalonic acid, serum cobalamin, and other indicators of tissue vitamin B12 status in the elderly. Clin. Chem. 2011, 57, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Schneede, J.; Dagnelie, P.C.; van Staveren, W.A.; Vollset, S.E.; Refsum, H.; Ueland, P.M. Methylmalonic acid and homocysteine in plasma as indicators of functional cobalamin deficiency in infants on macrobiotic diets. Pediatr. Res. 1994, 36, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Choi, R.; Choi, S.; Lim, Y.; Cho, Y.Y.; Kim, H.J.; Kim, S.W.; Chung, J.H.; Oh, S.Y.; Lee, S.Y. A prospective study on serum methylmalonic acid and homocysteine in pregnant women. Nutrients 2016, 8, 797. [Google Scholar] [CrossRef] [PubMed]
- Schroder, T.H.; Quay, T.A.; Lamers, Y. Methylmalonic acid quantified in dried blood spots provides a precise, valid, and stable measure of functional vitamin B-12 status in healthy women. J. Nutr. 2014, 144, 1658–1663. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.W.; Garrod, M.G.; Rockwood, A.L.; Kushnir, M.M.; Allen, L.H.; Haan, M.N.; Green, R. Measurement of total vitamin B12 and holotranscobalamin, singly and in combination, in screening for metabolic vitamin B12 deficiency. Clin. Chem. 2006, 52, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Hunt, A.; Harrington, D.; Robinson, S. Vitamin B12 deficiency. BMJ 2014, 349, g5226. [Google Scholar] [CrossRef] [PubMed]
- Hempen, C.; Wanschers, H.; van der Sluijs Veer, G. A fast liquid chromatographic tandem mass spectrometric method for the simultaneous determination of total homocysteine and methylmalonic acid. Anal. Bioanal. Chem. 2008, 391, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Lildballe, D.L.; Fedosov, S.; Sherliker, P.; Hin, H.; Clarke, R.; Nexo, E. Association of cognitive impairment with combinations of vitamin B12-related parameters. Clin. Chem. 2011, 57, 1436–1443. [Google Scholar] [CrossRef] [PubMed]
- Yetley, E.A.; Pfeiffer, C.M.; Phinney, K.W.; Bailey, R.L.; Blackmore, S.; Bock, J.L.; Brody, L.C.; Carmel, R.; Curtin, L.R.; Durazo-Arvizu, R.A.; et al. Biomarkers of vitamin B-12 status in nhanes: A roundtable summary. Am. J. Clin. Nutr. 2011, 94, 313S–321S. [Google Scholar] [CrossRef]
- Klee, G.G. Cobalamin and folate evaluation: Measurement of methylmalonic acid and homocysteine vs vitamin B12 and folate. Clin. Chem. 2000, 46, 1277–1283. [Google Scholar]
- Carmel, R. Diagnosis and management of clinical and subclinical cobalamin deficiencies: Why controversies persist in the age of sensitive metabolic testing. Biochimie 2013, 95, 1047–1055. [Google Scholar] [CrossRef]
- Bilici, A.; Sonkaya, A.; Ercan, S.; Ustaalioglu, B.B.; Seker, M.; Aliustaoglu, M.; Orcun, A.; Gumus, M. The changing of serum vitamin B12 and homocysteine levels after gastrectomy in patients with gastric cancer: Do they associate with clinicopathological factors? Tumour Biol. 2015, 36, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Kim, H.I.; Hyung, W.J.; Song, K.J.; Lee, J.H.; Kim, Y.M.; Noh, S.H. Vitamin B12 deficiency after gastrectomy for gastric cancer: An analysis of clinical patterns and risk factors. Ann. Surg. 2013, 258, 970–975. [Google Scholar] [CrossRef] [PubMed]
- Sakuta, H.; Suzuki, T.; Yasuda, H.; Wakiyama, H.; Hase, K. Plasma vitamin B12, folate and homocysteine levels in gastrectomized men. Clin. Nutr. 2005, 24, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.H.; Kim, S.W.; Kim, W.C.; Kim, J.S.; Cho, Y.K.; Park, J.M.; Lee, I.S.; Choi, M.G.; Song, K.Y.; Jeon, H.M.; et al. Anemia after gastrectomy for early gastric cancer: Long-term follow-up observational study. World J. Gastroenterol. 2012, 18, 6114–6119. [Google Scholar] [CrossRef] [PubMed]
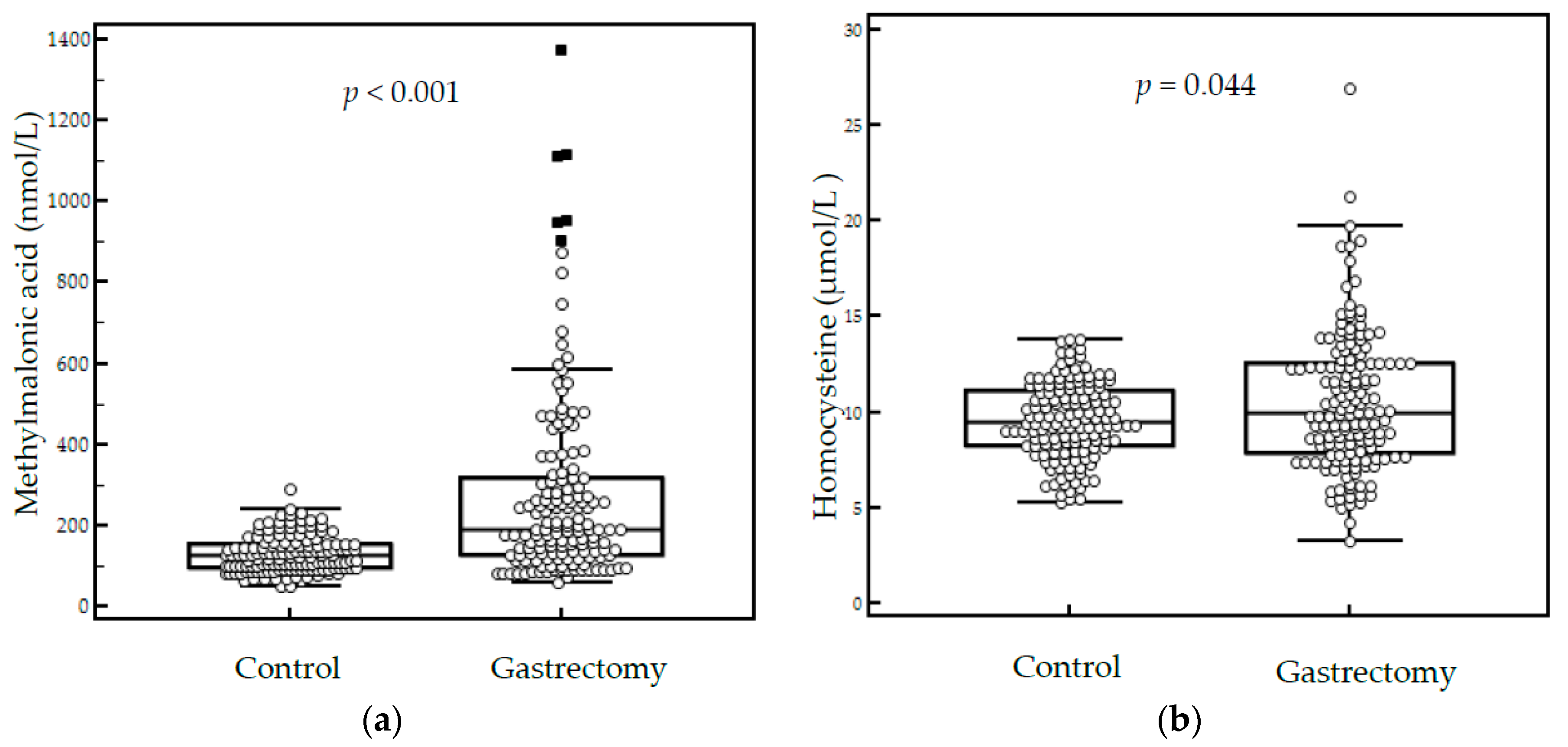
| Parameter | Controls (n = 142) | Gastrectomy Patients (n = 151) | p-Value |
|---|---|---|---|
| Age (years) | 52 (33–75) | 56 (32–78) | 0.001 a |
| Sex | 0.525 b | ||
| Male (n) | 87 | 87 | |
| Female (n) | 55 | 64 | |
| Hemoglobin (g/dL) | 14.5 (1.3) | 12.4 (1.9) | <0.001 c |
| MCV (fL) | 92.5 (85.3–102.9) | 90.3 (63.3–111.0) | <0.001 a |
| Creatinine (mg/dL) | 0.84 (0.55–0.97) | 0.84 (0.55–1.42) | 0.159 a |
| Total protein (g/dL) | 7.0 (6.3–7.9) | 7.0 (3.8–8.2) | 0.568 a |
| Albumin (g/dL) | 4.6 (4.1–5.1) | 4.3 (2.5–5.0) | <0.001 a |
| Total cholesterol (mg/dL) | 195.9 (32.8) | 168.3 (33.1) | <0.001 c |
| AST (U/L) | 23 (12–81) | 24 (13–123) | 0.099 a |
| ALT (U/L) | 21 (7–97) | 20 (7–93) | 0.115 a |
| MMA (nmol/L) | 125.7 (48.4–291.5) | 191.3 (60.2–1374.6) | <0.001 a |
| Homocysteine (μmol/L) | 9.5 (5.3–13.8) | 10.0 (3.3–26.9) | 0.044 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-M.; Oh, J.; Chun, M.-R.; Lee, S.-Y. Methylmalonic Acid and Homocysteine as Indicators of Vitamin B12 Deficiency in Patients with Gastric Cancer after Gastrectomy. Nutrients 2019, 11, 450. https://doi.org/10.3390/nu11020450
Lee S-M, Oh J, Chun M-R, Lee S-Y. Methylmalonic Acid and Homocysteine as Indicators of Vitamin B12 Deficiency in Patients with Gastric Cancer after Gastrectomy. Nutrients. 2019; 11(2):450. https://doi.org/10.3390/nu11020450
Chicago/Turabian StyleLee, Sae-Mi, Jongwon Oh, Mi-Ryung Chun, and Soo-Youn Lee. 2019. "Methylmalonic Acid and Homocysteine as Indicators of Vitamin B12 Deficiency in Patients with Gastric Cancer after Gastrectomy" Nutrients 11, no. 2: 450. https://doi.org/10.3390/nu11020450





