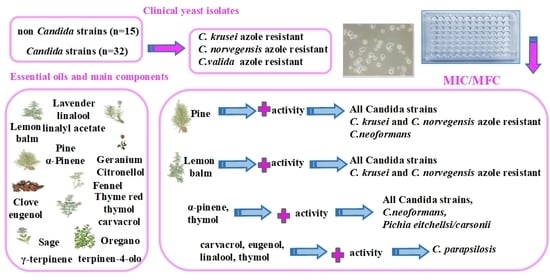
The Inhibition of Non-albicans Candida Species and Uncommon Yeast Pathogens by Selected Essential Oils and Their Major Compounds
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Essential Oils and Main Components
4.2. Antifungal Drugs
4.3. Yeast Isolates
4.4. Yeast Inocula
4.5. Antifungal Susceptibility Testing
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Enoch, D.A.; Yang, H.; Aliyu, S.H.; Micallef, C. The changing epidemiology of invasive fungal infections. Methods Mol. Biol. 2017, 1508, 17–65. [Google Scholar]
- Mandras, N.; Roana, J.; Scalas, D.; Fucale, G.; Allizond, V.; Banche, G.; Barbui, A.; Li Vigni, N.; Newell, V.A.; Cuffini, A.M.; et al. In vitro antifungal activity of fluconazole and voriconazole against non-Candida yeasts and yeast-like fungi clinical isolates. New Microbiol. 2015, 38, 583–587. [Google Scholar]
- Scalas, D.; Mandras, N.; Roana, J.; Tardugno, R.; Cuffini, A.M.; Ghisetti, V.; Benvenuti, S.; Tullio, V. Use of Pinus sylvestris L. (Pinaceae), Origanum vulgare L. (Lamiaceae), and Thymus vulgaris L. (Lamiaceae) essential oils and their main components to enhance itraconazole activity against azole susceptible/not-susceptible Cryptococcus neoformans strains. BMC Complement. Altern. Med. 2018, 18, 143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajkowska, K.; Kunicka-Styczyńska, A.; Maroszyńska, M. Selected essential oils as antifungal agents against antibiotic-resistant Candida spp.: In vitro study on clinical and food-borne isolates. Microb. Drug Resist. 2017, 23, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Černáková, L.; Light, C.; Salehi, B.; Rogel-Castillo, C.; Victoriano, M.; Martorell, M.; Sharifi-Rad, J.; Martins, N.; Rodrigues, C.F. Novel therapies for biofilm-based Candida spp. Infections. Adv. Exp. Med. Biol. 2019, 1214, 93–123. [Google Scholar] [PubMed]
- Arendrup, M.C.; Boekhout, T.; Akova, M.; Meis, J.F.; Cornely, O.A.; Lortholary, O. European society of clinical microbiology and infectious diseases fungal infection study group; european confederation of medical mycology. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of rare invasive yeast infections. Clin. Microbiol. Infect. 2014, 20, 76–98. [Google Scholar] [CrossRef] [Green Version]
- Pfaller, M.A.; Rhomberg, P.R.; Messer, S.A.; Jones, R.N.; Castanheira, M. Isavuconazole, micafungin, and 8 comparator antifungal agents’ susceptibility profiles for common and uncommon opportunistic fungi collected in 2013: Temporal analysis of antifungal drug resistance using CLSI species-specific clinical breakpoints and proposed epidemiological cutoff values. Diagn. Microbiol. Infect. Dis. 2015, 82, 303–313. [Google Scholar]
- Wiederhold, N.P. Antifungal resistance: Current trends and future strategies to combat. Infect. Drug Resist. 2017, 10, 249–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kordalewska, M.; Perlin, D.S. Identification of drug resistant Candida auris. Front. Microbiol. 2019, 10, 1918. [Google Scholar] [CrossRef]
- D’agostino, M.; Tesse, N.; Frippiat, J.P.; Machouart, M.; Debourgogne, A. Essential oils and their natural active compounds presenting antifungal properties. Molecules 2019, 15, 3713. [Google Scholar] [CrossRef] [Green Version]
- Babushok, V.I.; Linstrom, P.J.; Zenkevichb, I.G. Retention indices for frequently reported compounds of plant essential oils. J. Phys. Chem. Ref. Data 2011, 40, 043101. [Google Scholar] [CrossRef] [Green Version]
- de Groot, A.; Schmidt, E. Essential oils part V: Peppermint Oil, lavender oil, and lemongrass oil. Dermatitis 2016, 27, 325–332. [Google Scholar] [CrossRef]
- Sakkas, H.; Papadopoulou, C. Antimicrobial activity of basil, oregano, and thyme essential oils. J. Microbiol. Biotechnol. 2017, 28, 429–438. [Google Scholar] [CrossRef] [Green Version]
- Rivas da Silva, A.C.; Lopes, P.M.; Barros de Azevedo, M.M.; Costa, D.C.; Alviano, C.S.; Alviano, D.S. Biological activities of α-pinene and β-pinene enantiomers. Molecules 2012, 17, 6305–6316. [Google Scholar] [CrossRef] [Green Version]
- Motiejūnaite, O.; Peciulyte, D. Fungicidal properties of Pinus sylvestris L. for improvement of air quality. Medicina 2004, 40, 787–794. [Google Scholar] [PubMed]
- Zengin, H.; Baysal, A.H. Antibacterial and antioxidant activity of essential oil terpenes against pathogenic and spoilage-forming bacteria and cell structure-activity relationships evaluated by SEM microscopy. Molecules 2014, 19, 17773–17798. [Google Scholar] [CrossRef] [Green Version]
- Salehi, B.; Upadhyay, S.; Erdogan Orhan, I.; Kumar Jugran, A.; Jayaweera, S.L.D.; Dias, D.A.; Sharopov, F.; Taheri, Y.; Martins, N.; Baghalpour, N.; et al. Therapeutic potential of α- and β-pinene: A miracle gift of nature. Biomolecules 2019, 14, 738. [Google Scholar] [CrossRef] [Green Version]
- Abdellatif, F.; Boudjella, H.; Zitouni, A.; Hassani, A. Chemical composition and antimicrobial activity of the essential oil from leaves of Algerian Melissa officinalis L. EXCLI J. 2014, 13, 772–781. [Google Scholar] [PubMed]
- Songkro, S.; Hayook, N.; Jaisawang, J.; Maneenuan, D.; Chuchome, T.; Kaewnopparat, N. Investigation of inclusion complexes of citronella oil, citronellal and citronellol with β-cyclodextrin for mosquito repellent . J. Incl. Phenom. Macrocycl. Chem. 2012, 72, 339–355. [Google Scholar] [CrossRef]
- Zhou, H.E.; Tao, N.G.; Jia, L. Antifungal activity of citral octanal and α-terpineol against Geotrichum citri-aurantii. Food Control 2014, 37, 277–283. [Google Scholar] [CrossRef]
- Marchese, A.; Orhan, I.E.; Daglia, M.; Barbieri, R.; Di Lorenzo, A.; Nabavi, S.F.; Gortzi, O.; Izadi, M.; Nabavi, S.M. Antibacterial and antifungal activities of thymol: A brief review of the literature. Food Chem. 2016, 1, 402–414. [Google Scholar] [CrossRef] [PubMed]
- Tariq, S.; Wani, S.; Rasool, W.; Shafi, K.; Bhat, M.A.; Prabhakar, A.; Shalla, A.H.; Rather, M.A. A comprehensive review of the antibacterial, antifungal and antiviral potential of essential oils and their chemical constituents against drug-resistant microbial pathogens. Microb. Pathog. 2019, 134, 103580. [Google Scholar] [CrossRef] [PubMed]
- El Ouadi, Y.; Manssouri, M.; Bouyanzer, A.; Majidi, L.; Bendaif, H.; Elmsellem, H.; Shariati, M.A.; Melhaoui, A.; Hammouti, B. Essential oil composition and antifungal activity of Melissa officinalis originating from north-Est Morocco, against postharvest phytopathogenic fungi in apples. Microb. Pathog. 2017, 107, 321–326. [Google Scholar] [CrossRef]
- Wińska, K.; Mączka, W.; Łyczko, J.; Grabarczyk, M.; Czubaszek, A.; Szumny, A. Essential oils as antimicrobial agents-myth or real alternative? Molecules 2019, 24, 2130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tullio, V.; Roana, J.; Scalas, D.; Mandras, N. Evaluation of the antifungal activity of Mentha X piperita (Lamiaceae) of Pancalieri (Turin, Italy) essential oil and its synergistic interaction with Azoles. Molecules 2019, 29, 3148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shellie, R.; Mondello, L.; Marriott, P.; Dugo, G. Characterisation of lavender essential oils by using gas chromatography-mass spectrometry with correlation of linear retention indices and comparison with comprehensive two-dimensional gas chromatography. J. Chromatogr. A. 2002, 13, 225–234. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, 3th ed.; CLSI: Wayne, PA, USA, 2008; Volume 28. [Google Scholar]
- Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; CLSI: Wayne, PA, USA, 2012. [Google Scholar]
- Mandras, N.; Nostro, A.; Roana, J.; Scalas, D.; Banche, G.; Ghisetti, V.; Del Re, S.; Fucale, G.; Cuffini, A.M.; Tullio, V. Liquid and vapour-phase antifungal activities of essential oils against Candida albicans and non-albicans Candida. BMC Complement. Altern. Med. 2016, 30, 330. [Google Scholar] [CrossRef]
| Components | LRI a | Foeniculum vulgare % b | Lavandula vera % b | Melissa officinalis % b | Pinus sylvestris % b | Salvia officinalis % b | Thymus vulgaris % b | Eugenia caryophyllata % b | Origanum vulgare % b | Pelargonium asperum % b |
|---|---|---|---|---|---|---|---|---|---|---|
| Anethole | 1285 | 72.1 | - | - | - | - | - | - | - | - |
| Fenchone | 1089 | 14.2 | - | - | - | - | - | - | - | - |
| α-Pinene | 933 | 3.7 | - | - | 55.7 | - | 11.5 | - | - | - |
| Eugenol | 1358 | - | - | - | - | - | - | 82.75 | - | - |
| Methyl chavicol | 1196 | 3.7 | - | - | - | - | - | - | - | - |
| Citronellal | 1154 | - | - | 25.2 | - | - | - | - | - | 25.5 |
| β-Pinene | 979 | - | - | - | 10 | - | - | - | - | - |
| Limonene | 1029 | 3 | - | 12.4 | 9.7 | - | 13.2 | - | - | - |
| Geranial | 1270 | - | - | 8.8 | - | - | - | - | - | - |
| β-Caryophyllene | 1425 | - | 2.9 | - | - | - | - | 7.9 | - | - |
| Eugenyl acetate | 1526 | - | - | - | - | - | - | 7.1 | - | - |
| p-Cymene | 1025 | - | - | - | - | - | 16.2 | - | 12.4 | - |
| 1,8-Cineole | 1032 | - | - | - | - | 7.7 | - | - | - | - |
| γ-Terpinene | 1059 | - | - | - | - | - | 4 | - | 7.6 | - |
| Linalool | 1099 | - | 41.9 | - | - | - | - | - | - | 10.9 |
| α-Terpineol | 1192 | - | - | 6.3 | - | - | - | - | - | - |
| Geraniol | 1255 | - | - | 6.3 | - | - | - | - | - | 16.2 |
| Neral | 1220 | - | - | 5.4 | - | - | - | - | - | - |
| Camphor | 1144 | - | - | - | - | 22.6 | - | - | - | - |
| trans-Sabinene hydrate | 1094 | - | - | - | - | 29.4 | - | - | - | - |
| Linalyl acetate | 1254 | - | 32.7 | - | - | - | - | - | - | - |
| Lavandulyl acetate | 1289 | - | 3.2 | - | - | - | - | - | - | - |
| Terpinen-4-ol | 1179 | - | 2 | - | - | - | - | - | - | - |
| Carvacrol | 1305 | - | - | - | - | - | 7.8 | - | 62.6 | - |
| Thymol | 1295 | - | - | - | - | - | 26.52 | - | - | - |
| Citronellyl formate | 1352 | - | - | - | - | - | - | - | - | 5.1 |
| Total % b | 96.7 | 82.7 | 64.4 | 75.4 | 59.7 | 79.22 | 97.75 | 82.6 | 57.7 |
| C. krusei (n = 16) | C. parapsilosis (n = 4) | C. valida (n = 6) | C. lusitaniae (n = 2) | C. norvegensis (n = 4) | All Candida spp. (n = 32) | ||
|---|---|---|---|---|---|---|---|
| Eugenia caryophyllata (Clove) | MIC a | 0.06 | 0.12 | 0.12 | 0.06 | 0.06 | |
| MIC range | 0.06–0.12 | 0.06–0.25 | 0.06–0.12 | 0.06–0.12 | 0.06–0.12 | 0.06–0.25 | |
| MFC a | 0.12 | 0.25 | 0.25 | 0.12 | 0.06 | ||
| Foeniculum vulgare (Fennel) | MIC a | 0.25 | 0.25 | 0.5 | 0.25 | 0.25 | |
| MIC range | 0.12–0.25 | 0.25 | 0.25–1 | −/− | 0.25–0.5 | 0.12–1 | |
| MFC a | 0.5 | 0.25 | 1 | 0.25 | 0.25 | ||
| Pelargonium asperum (Geranium) | MIC a | 0.12 | 0.12 | 0.12 | 0.12 | 0.25 | |
| MIC range | 0.06–0.12 | 0.06–0.12 | 0.12 | −/− | 0.12–0.25 | 0.06–0.25 | |
| MFC a | 0.12 | 0.12 | 0.12 | 0.12 | 0.25 | ||
| Lavandula vera (Lavender) | MIC a | 0.25 | 0.25 | 0.5 | 0.25 | 0.25 | |
| MIC range | 0.12–0.25 | 0.25 | 0.25–0.5 | −/− | 0.12–0.25 | 0.12–0.5 | |
| MFC a | 0.25 | 0.5 | 0.5 | 0.25 | 0.25 | ||
| Melissa officinalis (Lemon balm) | MIC a | 0.06 | 0.12 | 0.12 | 0.06 | 0.03 | |
| MIC range | 0.03–0.12 | 0.12 | 0.03–0.12 | −/− | 0.03–0.06 | 0.03–0.12 | |
| MFC a | 0.06 | 0.25 | 0.25 | 0.12 | 0.06 | ||
| Origanum vulgare (Oregano) | MIC a | 0.12 | 0.12 | 0.12 | 0.12 | 0.25 | |
| MIC range | 0.12–0.25 | 0.12–0.25 | 0.12–0.25 | −/− | 0.12–0.25 | 0.12–0.25 | |
| MFC a | 0.25 | 0.25 | 0.25 | 0.12 | 0.25 | ||
| Pinus sylvestris (Pine) | MIC a | 0.03 | 0.12 | 0.06 | 0.03 | 0.03 | |
| MIC range | 0.03–0.06 | −/− | 0.03–0.06 | −/− | 0.03–0.06 | 0.03–0.12 | |
| MFC a | 0.03 | 0.12 | 0.06 | 0.03 | 0.06 | ||
| Salvia officinalis (Sage) | MIC a | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | |
| MIC range | 0.25–0.5 | 0.5–1 | 0.25–1 | −/− | 0.25–0.5 | 0.25–1 | |
| MFC a | 0.5 | 1 | 1 | 0.5 | 0.5 | ||
| Thymus vulgaris (Thyme red) | MIC a | 0.12 | 0.5 | 0.06 | 0.12 | 0.06 | |
| MIC range | 0.06–0.12 | 0.12–0.5 | 0.06–0.12 | −/− | 0.06–0.12 | 0.06–0.5 | |
| MFC a | 0.12 | 0.5 | 0.12 | 0.12 | 0.06 | ||
| α-pinene | MIC a | 0.002 | 0.008 | 0.004 | 0.002 | 0.004 | |
| MIC range | 0.002–0.004 | 0.008–0.016 | −/− | −/− | 0.004–0.008 | 0.002–0.016 | |
| MFC a | 0.016 | 0.016 | 0.008 | 0.002 | 0.008 | ||
| Carvacrol | MIC a | 0.12 | 0.06 | 0.12 | 0.12 | 0.12 | |
| MIC range | 0.12–0.25 | 0.06–0.12 | 0.12–0.25 | −/− | −/− | 0.06–0.25 | |
| MFC a | 0.25 | 0.12 | 0.25 | 0.12 | 0.12 | ||
| Citronellal | MIC a | 0.12 | 0.5 | 0.06 | 0.25 | 0.25 | |
| MIC range | 0.12–0.25 | −/− | −/− | −/− | −/− | 0.06–0.5 | |
| MFC a | 0.25 | 0.5 | 0.06 | 0.25 | 0.25 | ||
| Eugenol | MIC a | 0.12 | 0.06 | 0.06 | 0.12 | 0.12 | |
| MIC range | 0.12–0.25 | −/− | 0.06–0.12 | −/− | 0.12–0.25 | 0.06–0.25 | |
| MFC a | 0.25 | 0.12 | 0.12 | 0.12 | 0.12 | ||
| γ-terpinene | MIC a | >1 | 1 | 1 | >1 | >1 | |
| MIC range | −/− | −/− | −/− | −/− | −/− | 1->1 | |
| MFC a | >1 | >1 | >1 | >1 | >1 | ||
| Linalool | MIC a | 0.06 | 0.06 | 0.06 | 0.12 | 0.12 | |
| MIC range | 0.06–0.25 | 0.06–0.12 | 0.06–0.25 | −/− | 0.12–0.25 | 0.06–0.25 | |
| MFC a | 0.12 | 0.12 | 0.12 | 0.25 | 0.25 | ||
| Linalylacetate | MIC a | 1 | 1 | 0.5 | 0.25 | 1 | |
| MIC range | −/− | −/− | 0.5–1 | −/− | −/− | 0.25–1 | |
| MFC a | 1 | 1 | 0.5 | 0.25 | 1 | ||
| Terpinen-4-ol | MIC a | 0.12 | 0.25 | 0.12 | 0.25 | 0.25 | |
| MIC range | 0.12–0.5 | 0.25–0.5 | 0.12–0.25 | −/− | 0.25–0.5 | 0.12–0.5 | |
| MFC a | 0.25 | 0.25 | 0.25 | 0.25 | 0.5 | ||
| Thymol | MIC a | 0.06 | 0.06 | 0.12 | 0.06 | 0.12 | |
| MIC range | 0.06–0.25 | 0.06–0.12 | 0.12–0.25 | −/− | 0.12–0.25 | 0.06–0.25 | |
| MFC a | 0.12 | 0.12 | 0.25 | 0.25 | 0.25 | ||
| Fluconazole | MIC a | 128 | 0.5 | 0.12 | 0.12 | 128 | |
| MIC range | 4–256 b | 0.5–4 | 0.12–1 | −/− | 32–128 | 0.12–256 | |
| MFC a | >256 | 4 | 0.12 | 0.5 | >256 | ||
| Voriconazole | MIC a | 0.06 | 0.06 | 0.25 | 012 | 0.25 | |
| MIC range | 0.06–1 | 0.06–1 | 0.06–2 | −/− | 0.25–1 | 0.06–2 | |
| MFC a | 0.25 | 0.12 | 1 | 0.12 | 1 |
| Cryptococcus neoformans (n = 7) | Pichia etchellsii/carsonii (n = 2) | Kloechera japonica (n = 1) | Saccharomyces cerevisiae (n = 4) | Sporobolomyces salmonicolor (n = 1) | Non-Candida spp. (n = 15) | ||
|---|---|---|---|---|---|---|---|
| Eugenia caryophyllata (Clove) | MIC a | 0.06 | 0.015 | 0.12 | 0.06 | 0.25 | |
| MIC range | 0.03–0.06 | −/− | −/− | 0.06–0.12 | −/− | 0.015–0.25 | |
| MFC a | 0.06 | 0.015 | 0.12 | 0.25 | 0.25 | ||
| Foeniculum vulgare (Fennel) | MIC a | 0.12 | 0.12 | 1 | 0.25 | 0.5 | |
| MIC range | 0.12–1 | −/− | −/− | 0.25–1 | −/− | 0.12–1 | |
| MFC a | 0.25 | 0.5 | >1 | 1 | >1 | ||
| Pelargonium asperum (Geranium) | MIC a | 0.12 | 0.25 | 0.25 | 0.12 | 0.25 | |
| MIC range | 0.03–0.12 | −/− | −/− | 0.12–025 | −/− | 0.03–0.25 | |
| MFC a | 0.12 | 0.25 | 0.25 | 0.25 | 0.25 | ||
| Lavandula vera (Lavender) | MIC a | 0.12 | 0.12 | 1 | 0.5 | 1 | |
| MIC range | −/− | −/− | −/− | 0.12–0.5 | −/− | 0.12–1 | |
| MFC a | 0.12 | 0.12 | 1 | 0.5 | 1 | ||
| Melissa officinalis (Lemon balm) | MIC a | 0.12 | 0.06 | 0.5 | 0.06 | 0.25 | |
| MIC range | 0.06–0.12 | −/− | −/− | 0.06–0.12 | −/− | 0.06–0.5 | |
| MFC a | 0.12 | 0.06 | 0.5 | 0.12 | 0.25 | ||
| Origanum vulgare (Oregano) | MIC a | 0.03 | 0.03 | 0.06 | 0.12 | 0.06 | |
| MIC range | 0.03–0.06 | −/− | −/− | −/− | −/− | 0.03–0.12 | |
| MFC a | 0.06 | 0.03 | 0.06 | 0.12 | 0.06 | ||
| Pinus sylvestris (Pine) | MIC a | 0.015 | 0.004 | 0.12 | 0.03 | 0.12 | |
| MIC range | 0.015–0.06 | −/− | −/− | 0.015–0.06 | −/− | 0.004–0.12 | |
| MFC a | 0.015 | 0.015 | 0.12 | 0.06 | 0.12 | ||
| Salvia officinalis (Sage) | MIC a | 0.12 | 0.12 | >1 | 1 | >1 | |
| MIC range | 0.12–0.5 | −/− | −/− | 0.25–1 | −/− | 0.12–1 | |
| MFC a | 0.12 | 0.25 | >1 | 1 | >1 | ||
| Thymus vulgaris (Thyme red) | MIC a | 0.06 | 0.06 | 0.12 | 0.12 | 0.12 | |
| MIC range | 0.06–0.12 | −/− | −/− | 0.03–0.12 | −/− | 0.03–0.12 | |
| MFC a | 0.12 | 0.06 | 0.12 | 0.12 | 0.12 | ||
| α-pinene | MIC a | 0.06 | 0.03 | 0.03 | 0.06 | 0.03 | |
| MIC range | 0.06–0.12 | −/− | −/− | 0.06–0.12 | −/− | 0.03–0.12 | |
| MFC a | 0.06 | 0.03 | 0.03 | 0.06 | 0.03 | ||
| Carvacrol | MIC a | 0.06 | 0.12 | 0.03 | 0.03 | 0.06 | |
| MIC range | 0.06–0.12 | −/− | −/− | −/− | 0.06–0.12 | 0.03–0.12 | |
| MFC a | 0.12 | 0.12 | 0.03 | 0.03 | 0.06 | ||
| Citronellal | MIC a | 0.5 | >1 | 1 | 1 | >1 | |
| MIC range | 0.25–0.5 | −/− | −/− | −/− | −/− | 0.25− >1 | |
| MFC a | 0.5 | >1 | >1 | >1 | >1 | ||
| Eugenol | MIC a | 0.06 | 0.06 | 0.06 | 0.12 | 0.12 | |
| MIC range | 0.03–0.12 | −/− | −/− | 0.12–0.25 | 0.12–0.25 | 0.03–0.25 | |
| MFC a | 0.06 | 0.12 | 0.12 | 0.25 | 0.25 | ||
| γ-terpinene | MIC a | >1 | >1 | 1 | 1 | >1 | |
| MIC range | −/− | −/− | −/− | −/− | −/− | 1− >1 | |
| MFC a | >1 | >1 | >1 | >1 | >1 | ||
| Linalool | MIC a | 0.5 | 0.12 | 0.06 | 0.12 | 0.25 | |
| MIC range | 0.5–1 | −/− | −/− | 0.12–0.25 | −/− | 0.06–1 | |
| MFC a | 0.5 | 0.12 | 0.12 | 0.25 | 0.25 | ||
| Linalylacetate | MIC a | 0.12 | >1 | >1 | >1 | >1 | |
| MIC range | 0.06–0.12 | −/− | −/− | −/− | −/− | 0.06– >1 | |
| MFC a | 0.12 | >1 | >1 | >1 | >1 | ||
| Terpinen-4-ol | MIC a | 0.06 | 0.25 | 0.25 | 0.5 | 0.25 | |
| MIC range | 0.03–0.12 | −/− | −/− | 0.25–0.5 | −/− | 0.03–0.5 | |
| MFC a | 0.12 | 0.25 | 0.5 | 0.5 | 0.5 | ||
| Thymol | MIC a | 0.004 | 0.03 | 0.06 | 0.12 | 0.06 | |
| MIC range | 0.002–0.008 | −/− | −/− | 0.06–0.12 | −/− | 0.002–0.12 | |
| MFC a | 0.004 | 0.06 | 0.06 | 0.25 | 0.06 | ||
| Fluconazole | MIC a | 0.25 | 0.12 | 0.25 | 0.5 | 0.25 | |
| MIC range | 0.25– >64 | 0.12–0.25 | −/− | 0.5–2 | −/− | 0.12– >64 | |
| MFC a | 4 | 0.5 | 0.5 | 2 | 0.5 | ||
| Voriconazole | MIC a | 0.015 | 0.06 | 0.12 | 0.25 | 0.25 | |
| MIC range | 0.015–32 | −/− | −/− | 0.25–1 | −/− | 0.015–32 | |
| MFC a | 0.06 | 0.06 | 0.12 | 1 | 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandras, N.; Roana, J.; Scalas, D.; Del Re, S.; Cavallo, L.; Ghisetti, V.; Tullio, V. The Inhibition of Non-albicans Candida Species and Uncommon Yeast Pathogens by Selected Essential Oils and Their Major Compounds. Molecules 2021, 26, 4937. https://doi.org/10.3390/molecules26164937
Mandras N, Roana J, Scalas D, Del Re S, Cavallo L, Ghisetti V, Tullio V. The Inhibition of Non-albicans Candida Species and Uncommon Yeast Pathogens by Selected Essential Oils and Their Major Compounds. Molecules. 2021; 26(16):4937. https://doi.org/10.3390/molecules26164937
Chicago/Turabian StyleMandras, Narcisa, Janira Roana, Daniela Scalas, Simonetta Del Re, Lorenza Cavallo, Valeria Ghisetti, and Vivian Tullio. 2021. "The Inhibition of Non-albicans Candida Species and Uncommon Yeast Pathogens by Selected Essential Oils and Their Major Compounds" Molecules 26, no. 16: 4937. https://doi.org/10.3390/molecules26164937