MnO2/rGO/CNTs Framework as a Sulfur Host for High-Performance Li-S Batteries
Abstract
:1. Introduction
2. Results and Discussions
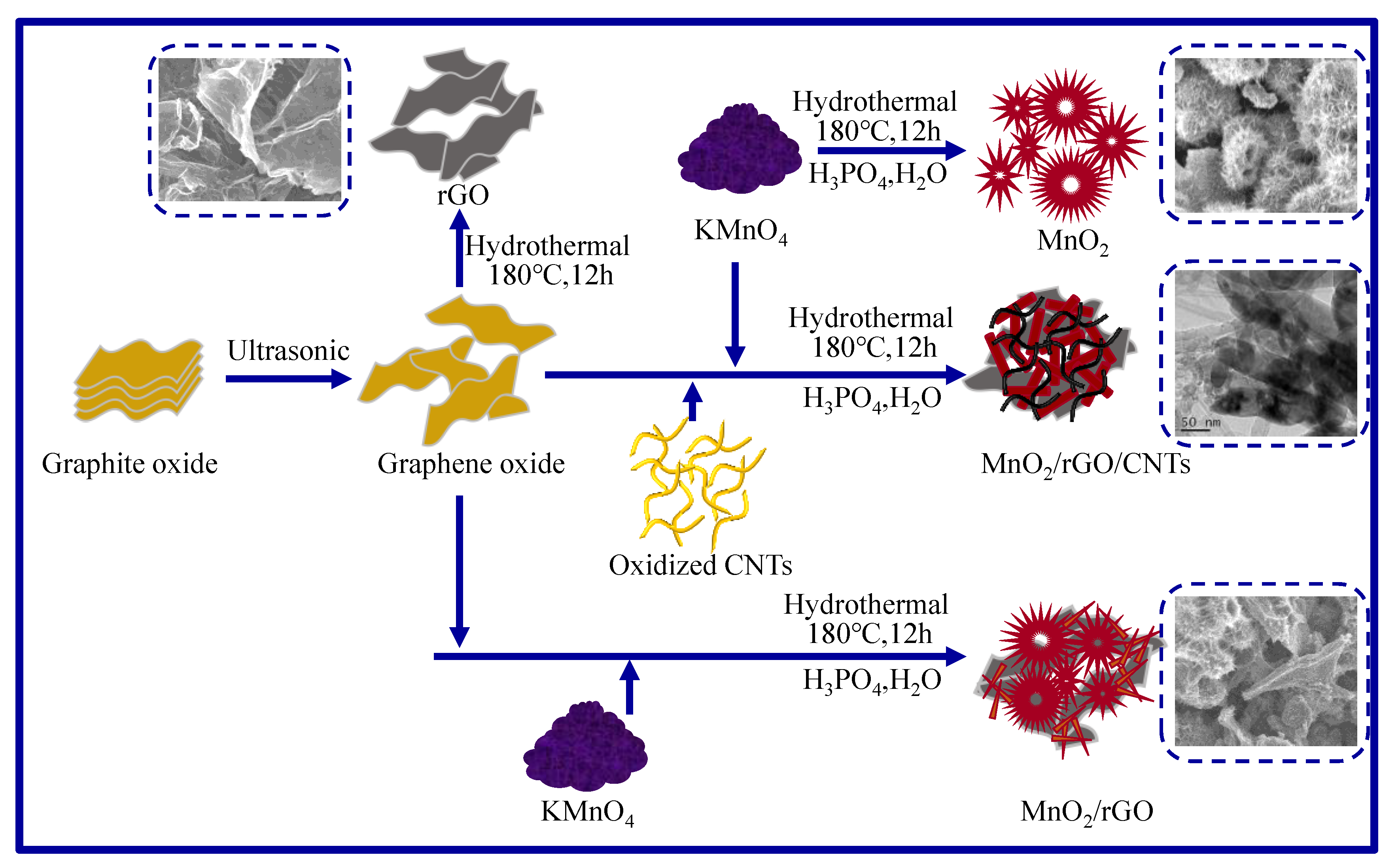
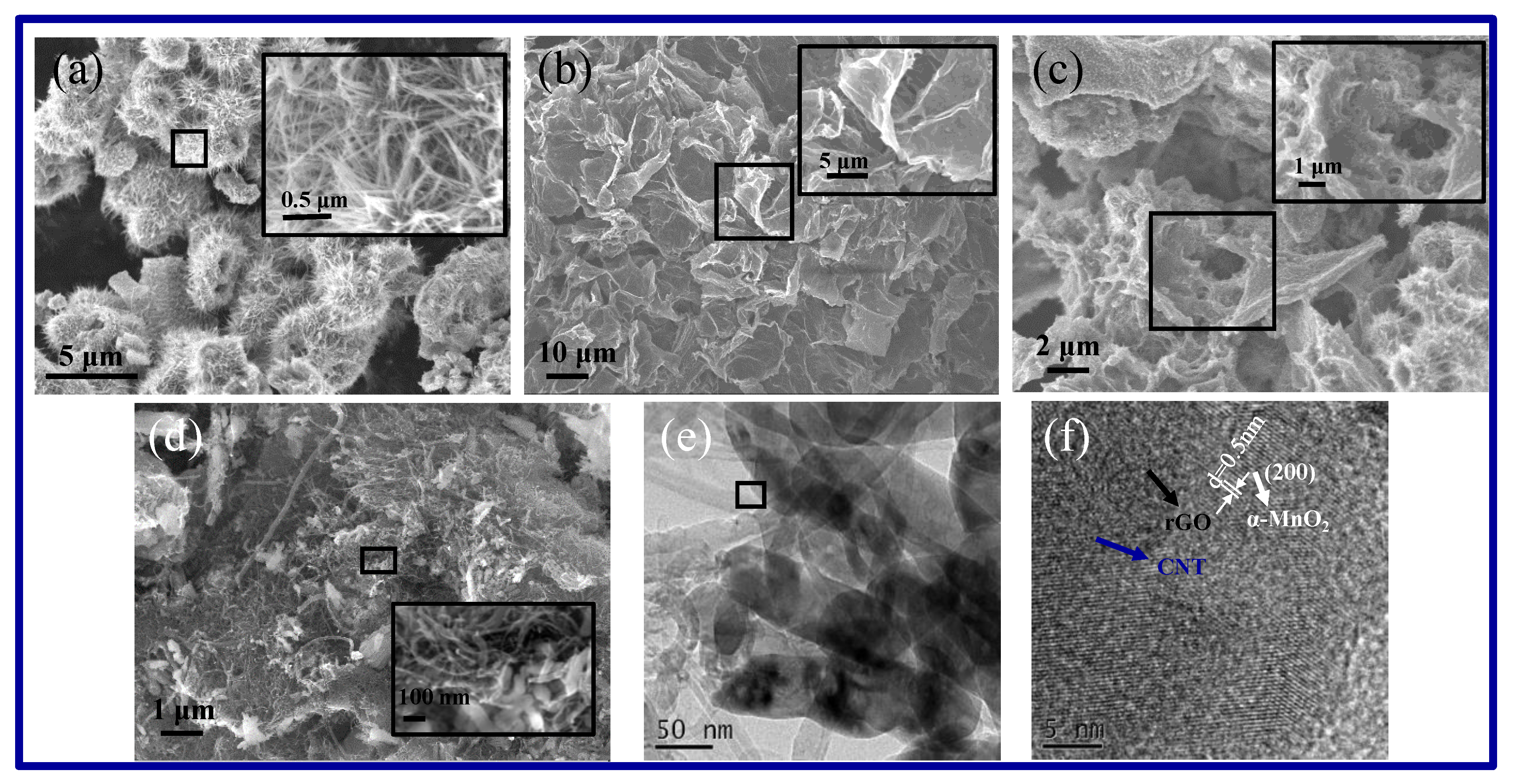
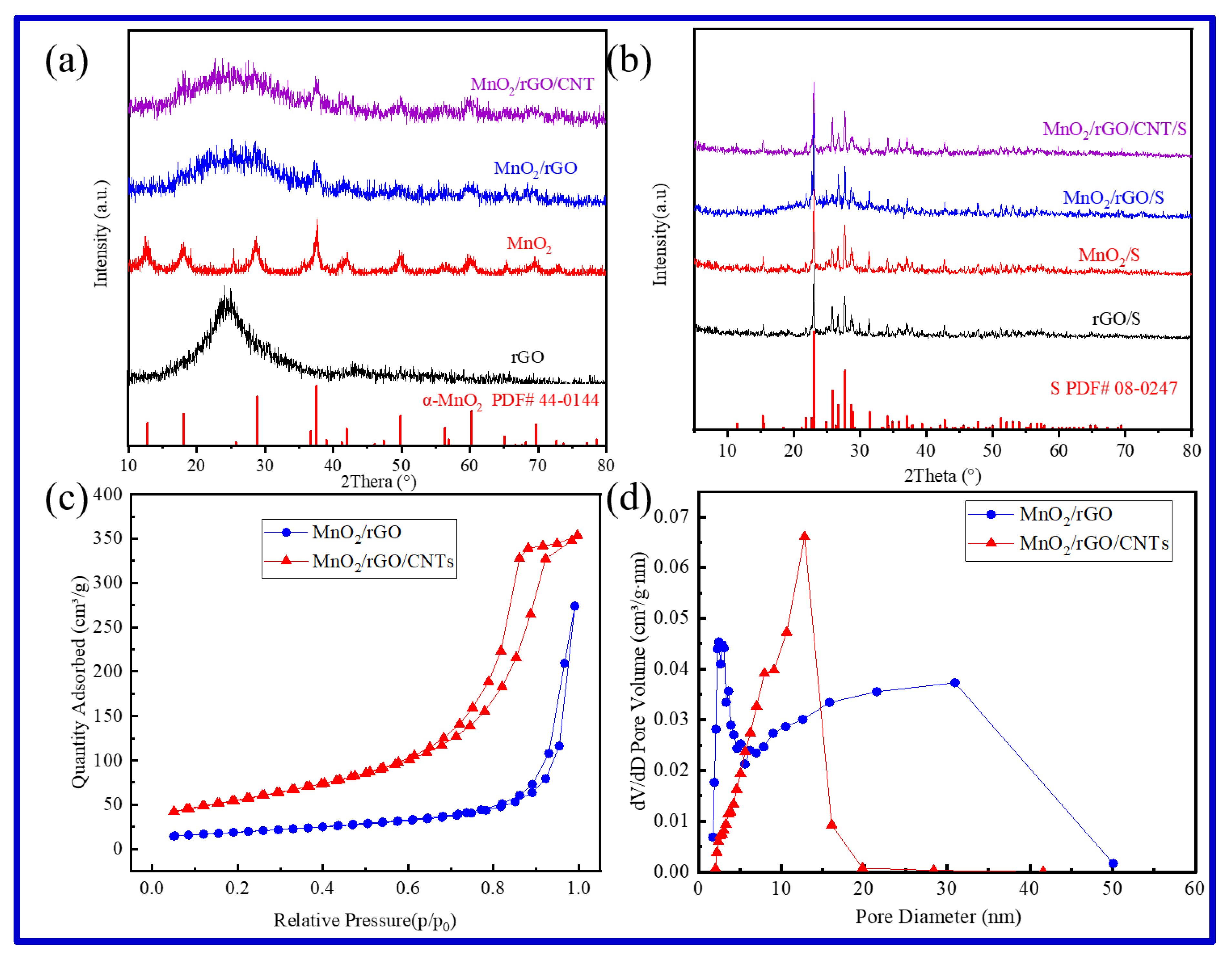
2.1. Microstructure and Composition
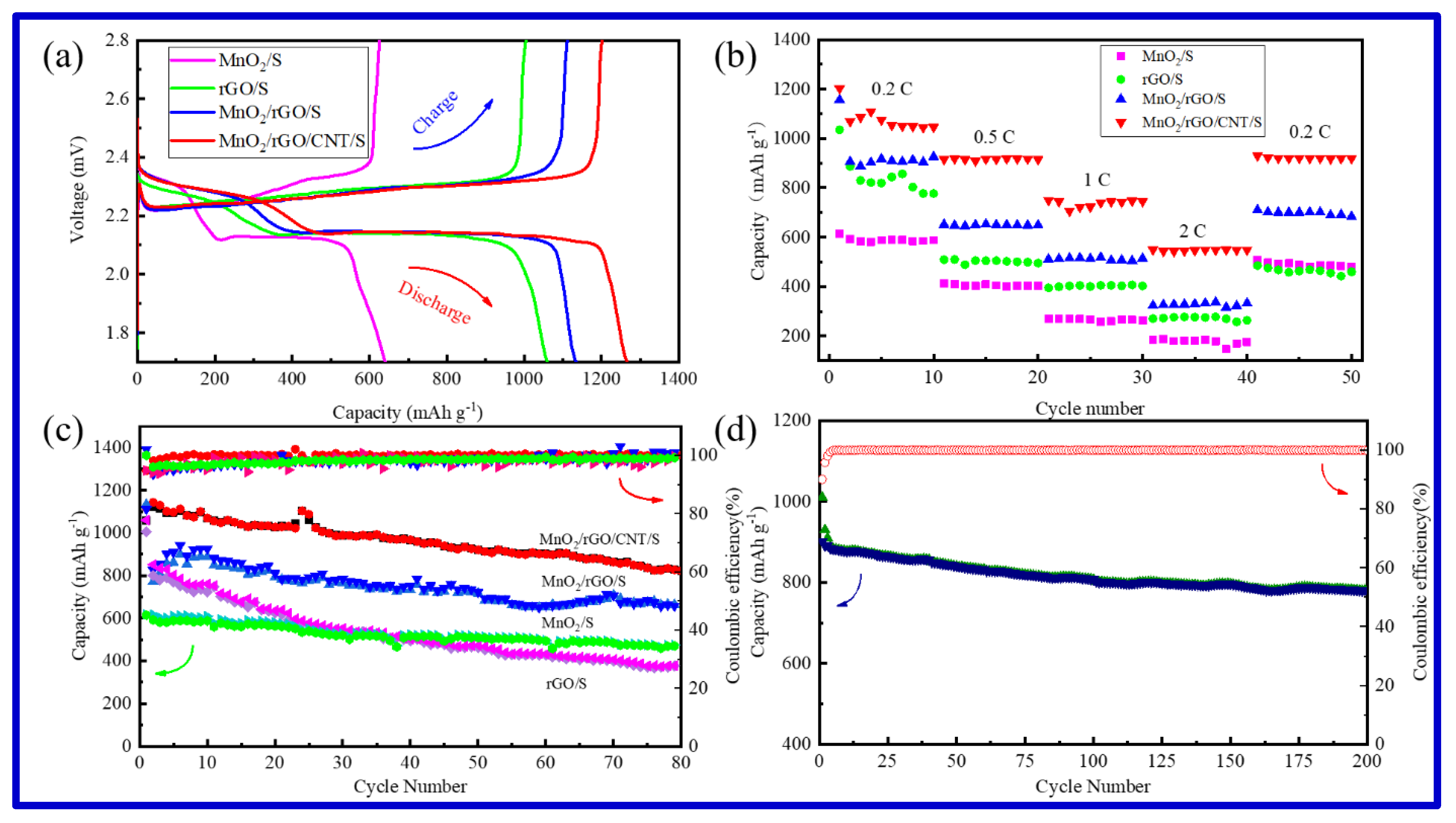
2.2. Electrochemical Performance
3. Materials and Methods
3.1. Preparation of Materials
3.2. Materials Characterization
3.3. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hong, X.; Wang, R.; Liu, Y.; Fu, J.; Liang, J.; Dou, S. Recent advances in chemical adsorption and catalytic conversion materials for Li–S batteries. J. Energy Chem. 2020, 42, 144–168. [Google Scholar] [CrossRef] [Green Version]
- Petnikota, S.; Rotte, N.K.; Reddy, M.V.; Srikanth, V.V.S.S.; Chowdari, B.V.R. MgO-decorated few-layered graphene as an anode for Li-ion batteries. ACS Appl. Mater. Interfaces 2015, 7, 2301–2309. [Google Scholar] [CrossRef]
- Raić, M.; Mikac, L.; Marić, I.; Štefanić, G.; Škrabić, M.; Gotić, M.; Ivanda, M. Nanostructured silicon as potential anode material for Li-ion batteries. Molecules 2020, 25, 891. [Google Scholar] [CrossRef] [Green Version]
- Kulkarni, P.; Nataraj, S.K.; Balakrishna, R.G.; Nagaraju, D.H.; Reddy, M.V. Nanostructured binary and ternary metal sulfides: Synthesis methods and their application in energy conversion and storage devices. J. Mater. Chem. A 2017, 5, 22040–22094. [Google Scholar] [CrossRef]
- Appadurai, T.; Subramaniyam, C.M.; Kuppusamy, R.; Karazhanov, S.; Subramanian, B. Electrochemical Performance of Nitrogen-Doped TiO2 Nanotubes as Electrode Material for Supercapacitor and Li-Ion Battery. Molecules 2019, 24, 2952. [Google Scholar] [CrossRef] [Green Version]
- Rana, M.; Ahad, S.A.; Li, M.; Luo, B.; Wang, L.; Gentle, I.; Knibbe, R. Review on areal capacities and long-term cycling performances of lithium sulfur battery at high sulfur loading. Energy Storage Mater. 2019, 18, 289–310. [Google Scholar] [CrossRef]
- Tsao, C.H.; Hsu, C.H.; Zhou, J.D.; Chin, C.W.; Kuo, P.L.; Chang, C.H. Vulcanized polymeric cathode material featuring a polyaniline skeleton for high-rate rechargeability and long-cycle stability lithium-sulfur batteries. Electrochim. Acta 2018, 276, 111–117. [Google Scholar] [CrossRef]
- Tao, X.; Wang, J.; Liu, C.; Wang, H.; Yao, H.; Zheng, G.; Seh, Z.W.; Cai, Q.; Li, W.; Zhou, G.; et al. Balancing surface adsorption and diffusion of lithium-polysulfides on nonconductive oxides for lithium-sulfur battery design. Nat. Commun. 2016, 7, 11203. [Google Scholar] [CrossRef]
- Fu, A.; Wang, C.; Pei, F.; Cui, J.; Fang, X.; Zheng, N. Recent Advances in Hollow Porous Carbon Materials for Lithium-Sulfur Batteries. Small 2019, 15, e1804786. [Google Scholar] [CrossRef]
- Fang, R.; Chen, K.; Yin, L.; Sun, Z.; Li, F.; Cheng, H.-M. The Regulating Role of Carbon Nanotubes and Graphene in Lithium-Ion and Lithium-Sulfur Batteries. Adv. Mater. 2019, 31, 31. [Google Scholar]
- Garapati, M.S.; Piriya, A.V.S.; Sundara, R. Synergy between partially exfoliated carbon nanotubes-sulfur cathode and nitrogen rich dual function interlayer for high performance lithium sulfur battery. Carbon N. Y. 2019, 147, 364–376. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, Y.; Long, L.; Fu, X.; Sui, G.; Yang, X. An optimal carbon fiber interlayer integrated with bio-based gel polymer electrolyte enabling trapping-diffusion-conversion of polysulfides in lithium-sulfur batteries. Chem. Eng. J. 2019, 370, 1068–1076. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Niu, Z.; Chen, J. Advanced nanostructured carbon-based materials for rechargeable lithium-sulfur batteries. Carbon N. Y. 2019, 141, 400–416. [Google Scholar] [CrossRef]
- Zha, C.; Wu, D.; Zhang, T.; Wu, J.; Chen, H. A facile and effective sulfur loading method: Direct drop of liquid Li2S8 on carbon coated TiO2 nanowire arrays as cathode towards commercializing lithium-sulfur battery. Energy Storage Mater. 2019, 17, 118–125. [Google Scholar] [CrossRef]
- Liu, T.; Sun, X.; Sun, S.; Niu, Q.; Liu, H.; Song, W.; Cao, F.; Li, X.; Ohsaka, T.; Wu, J. A robust and low-cost biomass carbon fiber@SiO2 interlayer for reliable lithium-sulfur batteries. Electrochim. Acta 2019, 295, 684–692. [Google Scholar] [CrossRef]
- Guo, Y.; Li, J.; Pitcheri, R.; Zhu, J.; Wen, P.; Qiu, Y. Electrospun Ti4O7/C conductive nanofibers as interlayer for lithium-sulfur batteries with ultra long cycle life and high-rate capability. Chem. Eng. J. 2019, 355, 390–398. [Google Scholar] [CrossRef]
- Zhu, F.; Liu, J.; Zhao, H.; Li, J.; Li, Q.; Xi, Y.; Liu, M.; Wang, C. Preparation and Performance of Porous Polyetherimide/Al2O3 Separator for Enhanced Lithium-Sulfur Batteries. Chemelectrochem 2019, 6, 2883–2890. [Google Scholar] [CrossRef]
- Sun, W.; Sun, X.; Peng, Q.; Wang, H.; Ge, Y.; Akhtar, N.; Huang, Y.; Wang, K. Nano-MgO/AB decorated separator to suppress shuttle effect of lithium-sulfur battery. Nanoscale Adv. 2019, 1, 1589–1597. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.; Zhang, S.; Zhang, T.; Cao, S.; Ye, H.; Yao, Q.; Zheng, G.W.; Lee, J.Y. A Cathode-Integrated Sulfur-Deficient Co9S8 Catalytic Interlayer for the Reutilization of “Lost” Polysulfides in Lithium-Sulfur Batteries. ACS Nano 2019, 13, 7073–7082. [Google Scholar] [CrossRef]
- Chen, M.; Yin, X.; Reddy, M.V.; Adams, S. All-solid-state MoS2/Li6PS5Br/In-Li batteries as a novel type of Li/S battery. J. Mater. Chem. A 2015, 3, 10698–10702. [Google Scholar] [CrossRef]
- Chen, H.; Dong, W.D.; Xia, F.J.; Zhang, Y.J.; Yan, M.; Song, J.P.; Zou, W.; Liu, Y.; Hu, Z.Y.; Liu, J.; et al. Hollow nitrogen-doped carbon/sulfur@MnO2 nanocomposite with structural and chemical dual-encapsulation for lithium-sulfur battery. Chem. Eng. J. 2020, 381, 122746. [Google Scholar] [CrossRef]
- Tu, S.; Zhao, X.; Cheng, M.; Sun, P.; He, Y.; Xu, Y. Uniform Mesoporous MnO2 Nanospheres as a Surface Chemical Adsorption and Physical Confinement Polysulfide Mediator for Lithium-Sulfur Batteries. ACS Appl. Mater. Interfaces 2019, 11, 10624–10630. [Google Scholar] [CrossRef]
- Nithyadharseni, P.; Reddy, M.V.; Fanny, H.; Adams, S.; Chowdari, B.V.R. Facile one pot synthesis and Li-cycling properties of MnO2. RSC Adv. 2015, 5, 60552–60561. [Google Scholar] [CrossRef]
- Reddy, M.V.; Subba Rao, G.V.; Chowdari, B.V.R. Metal oxides and oxysalts as anode materials for Li ion batteries. Chem. Rev. 2013, 113, 5364–5457. [Google Scholar] [CrossRef]
- Tan, S.; Yang, Z.; Yuan, H.; Zhang, J.; Yang, Y.; Liu, H. MnO2-decorated graphene aerogel with dual-polymer interpenetrating network as an efficient hybrid host for Li-S batteries. J. Alloys Compd. 2019, 791, 483–489. [Google Scholar] [CrossRef]
- Xiaoman, L.; Qinglin, Z.; Weimin, G.; Qinghua, L. The catalytic activity of manganese dioxide supported on graphene promoting the electrochemical performance of lithium-sulfur batteries. J. Electroanal. Chem. 2019, 840, 144–152. [Google Scholar] [CrossRef]
- Luna-Lama, F.; Hernández-Rentero, C.; Caballero, A.; Morales, J. Biomass-derived carbon/γ-MnO2 nanorods/S composites prepared by facile procedures with improved performance for Li/S batteries. Electrochim. Acta 2018, 292, 522–531. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Yang, Z.; Zhang, H.; Tan, H.; Yu, J.; Wu, J. Mesoporous β-MnO2/sulfur composite as cathode material for Li-S batteries. Electrochim. Acta 2013, 106, 307–311. [Google Scholar] [CrossRef]
- Cao, Y.L.; Yang, H.X.; Ai, X.P.; Xiao, L.F. The mechanism of oxygen reduction on MnO2-catalyzed air cathode in alkaline solution. J. Electroanal. Chem. 2003, 557, 127–134. [Google Scholar] [CrossRef]
- Kalubarme, R.S.; Cho, M.S.; Yun, K.S.; Kim, T.S.; Park, C.J. Catalytic characteristics of MnO2 nanostructures for the O-2 reduction process. Nanotechnology 2011, 22, 395402. [Google Scholar] [CrossRef]
- Liu, X.; Huang, J.Q.; Zhang, Q.; Mai, L. Nanostructured Metal Oxides and Sulfides for Lithium–Sulfur Batteries. Adv. Mater. 2017, 29, 1601759. [Google Scholar] [CrossRef]
- Xiao-Miao, F.; Zhen-Zhen, Y.; Ning-Na, C. Synthesis of Hollow Urchin-Like MnO2 via a Facile Hydrothermal Method and Its Application in Supercapacitors. Chinese J. Inorg. Chem. 2014, 30, 2509–2515. [Google Scholar]
- Zeng, H.C. Ostwald ripening: A synthetic approach for hollow nanomaterials. Curr. Nanosci. 2007, 3, 177–181. [Google Scholar] [CrossRef]
- Su, T.; Zhao, B.; Fan, B.; Li, H.; Zhang, R. Enhanced microwave absorption properties of novel hierarchical core-shell delta/alpha MnO2 composites. J. Solid State Chem. 2019, 273, 192–198. [Google Scholar] [CrossRef]
- Cao, K.; Liu, H.; Li, Y.; Wang, Y.; Jiao, L. Encapsulating sulfur in δ-MnO2 at room temperature for Li-S battery cathode. Energy Storage Mater. 2017, 9, 78–84. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, J.; Lou, X.W. Hollow Carbon Nanofibers Filled with MnO2 Nanosheets as Efficient Sulfur Hosts for Lithium-Sulfur Batteries. Angew. Chemie Int. Ed. 2015, 54, 12886–12890. [Google Scholar] [CrossRef]
- Dang, R.; Ma, X.; Liu, J.; Chen, M.; Zhang, Y.; Luo, J. Mesoporous MnO2 fibers as an efficient bifunctional absorber for high-performance lithium-sulfur batteries. Int. J. Hydrogen Energy 2018, 43, 18754–18758. [Google Scholar] [CrossRef]
- Chen, M.; Lu, Q.; Jiang, S.; Huang, C.; Wang, X.; Wu, B.; Xiang, K.; Wu, Y. MnO2 nanosheets grown on the internal/external surface of N-doped hollow porous carbon nanospheres as the sulfur host of advanced lithium-sulfur batteries. Chem. Eng. J. 2018, 335, 831–842. [Google Scholar] [CrossRef]
- Huang, X.; Shi, K.; Yang, J.; Mao, G.; Chen, J. MnO2-GO double-shelled sulfur (S@MnO2@GO) as a cathode for Li-S batteries with improved rate capability and cyclic performance. J. Power Sources 2017, 356, 72–79. [Google Scholar] [CrossRef]
- Ling, B.; Chen, A.; Liu, W.; Liu, K.; Hu, H.; Zhang, J. Simply and rapidly synthesized composites of MnO2 nanosheets anchoring on carbon nanotubes as efficient sulfur hosts for Li-S batteries. Mater. Lett. 2018, 218, 321–324. [Google Scholar] [CrossRef]
- Kim, A.-Y.; Kim, M.K.; Kim, J.Y.; Wen, Y.; Gu, L.; Dao, V.-D.; Choi, H.-S.; Byun, D.; Lee, J.K. Ordered SnO nanoparticles in MWCNT as a functional host material for high-rate lithium-sulfur battery cathode. Nano Res. 2017, 10, 2083–2095. [Google Scholar] [CrossRef]
- Xu, C.; Wu, Y.; Zhao, X.; Wang, X.; Du, G.; Zhang, J.; Tu, J. Sulfur/three-dimensional graphene composite for high performance lithium-sulfur batteries. J. Power Sources 2015, 275, 22–25. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, H.; Diao, P.; Chang, W.; Hong, G.; Li, Y.; Gong, M.; Xie, L.; Zhou, J.; Wang, J.; et al. Oxygen Reduction Electrocatalyst Based on Strongly Coupled Cobalt Oxide Nanocrystals and Carbon Nanotubes. J. Am. Chem. Soc. 2012, 134, 15849–15857. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
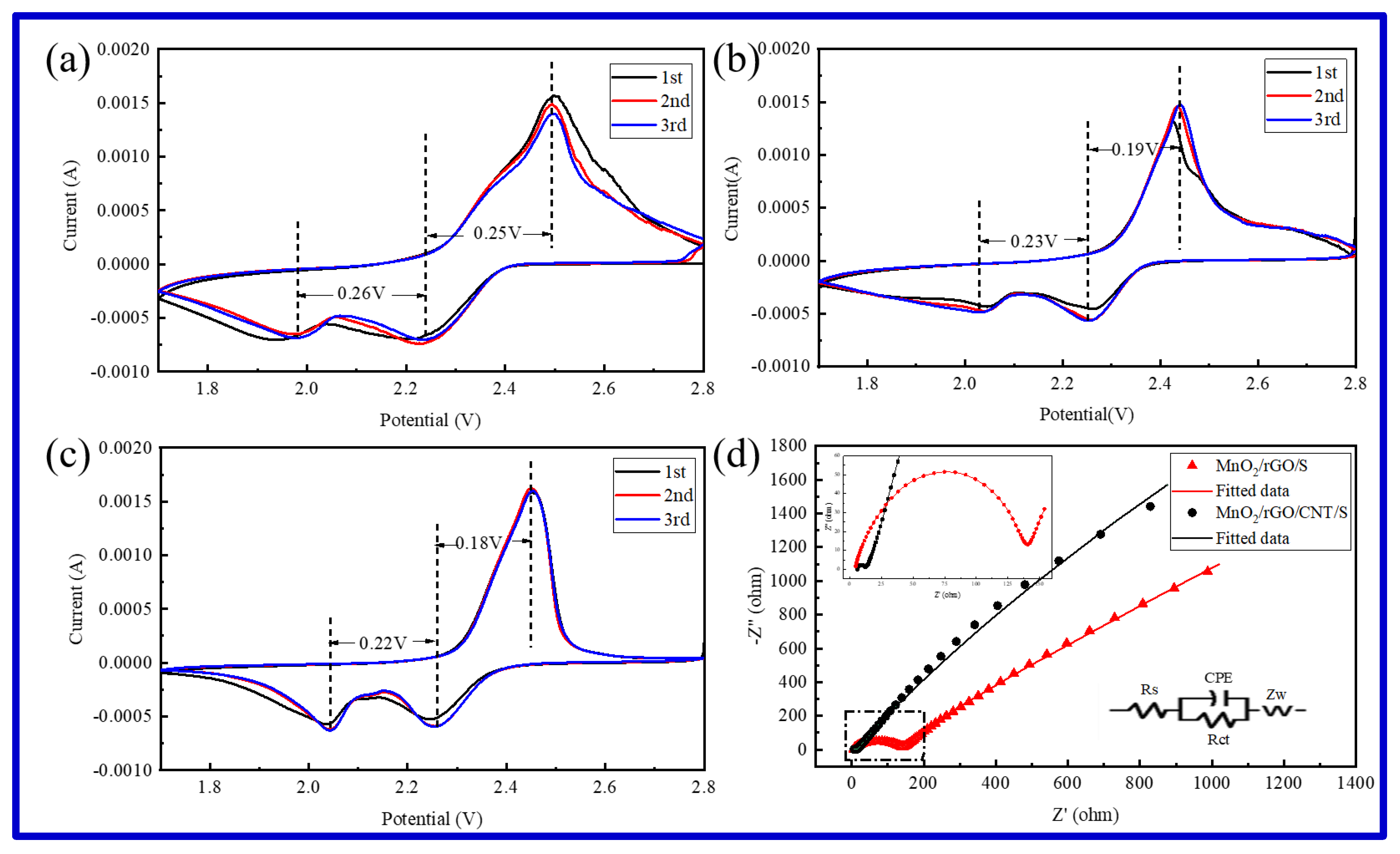
| Sample | Rs(Ω) | Rct(Ω) |
|---|---|---|
| MnO2/rGO/S | 5.6 | 106.1 |
| MnO2/rGO/CNTs/S | 2.9 | 22.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, W.; Meng, L.; Hong, X.; Liu, S.; Shen, D.; Xia, Y.; Yang, S. MnO2/rGO/CNTs Framework as a Sulfur Host for High-Performance Li-S Batteries. Molecules 2020, 25, 1989. https://doi.org/10.3390/molecules25081989
Dong W, Meng L, Hong X, Liu S, Shen D, Xia Y, Yang S. MnO2/rGO/CNTs Framework as a Sulfur Host for High-Performance Li-S Batteries. Molecules. 2020; 25(8):1989. https://doi.org/10.3390/molecules25081989
Chicago/Turabian StyleDong, Wei, Lingqiang Meng, Xiaodong Hong, Sizhe Liu, Ding Shen, Yingkai Xia, and Shaobin Yang. 2020. "MnO2/rGO/CNTs Framework as a Sulfur Host for High-Performance Li-S Batteries" Molecules 25, no. 8: 1989. https://doi.org/10.3390/molecules25081989