C19-Norditerpenoid Alkaloids from Aconitum szechenyianum
Abstract
:1. Introduction
2. Results
3. Materials and Methods
3.1. General Information
3.2. Plant Material
3.3. Extraction and Isolation
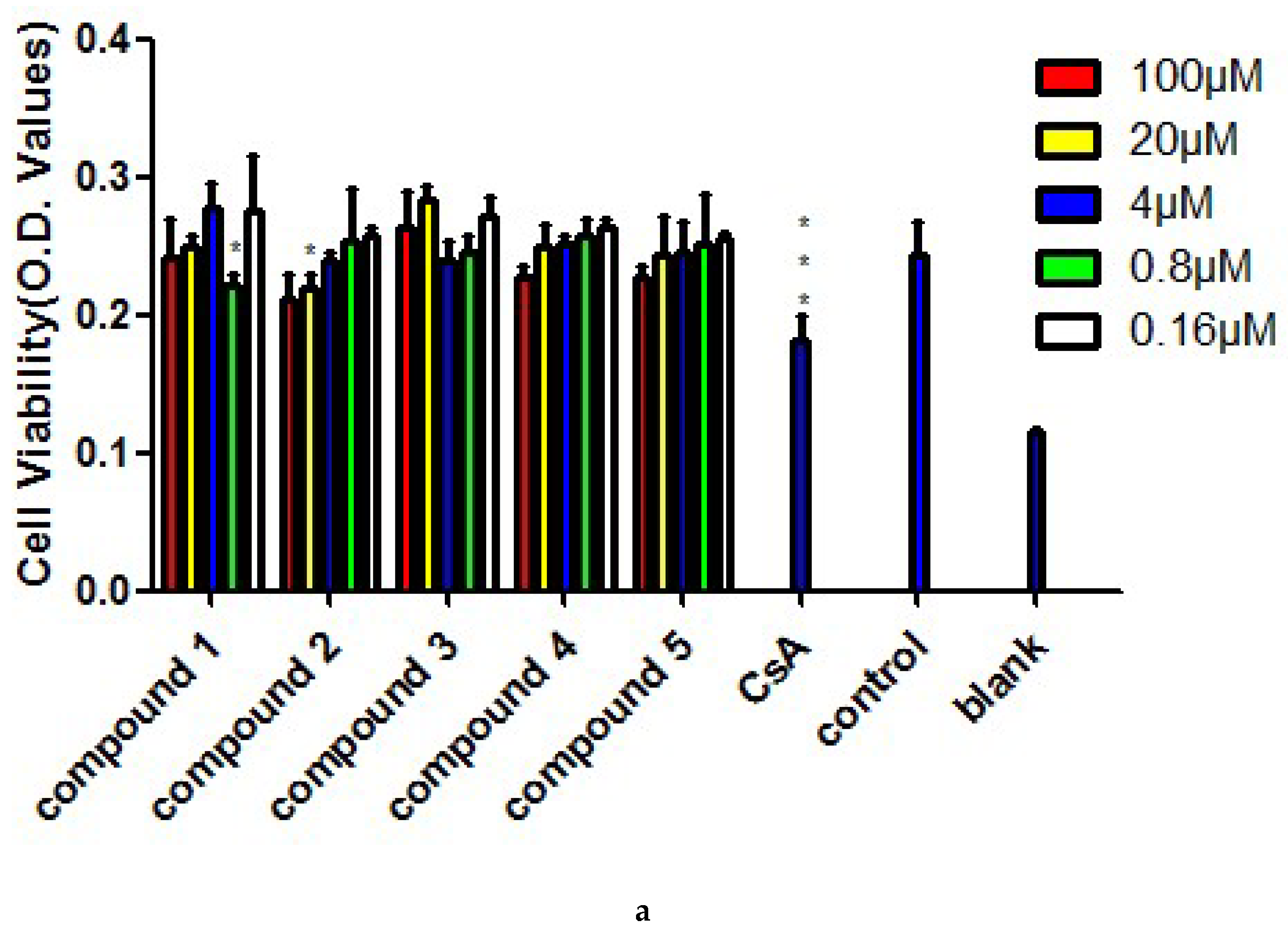
3.4. MTT Assay
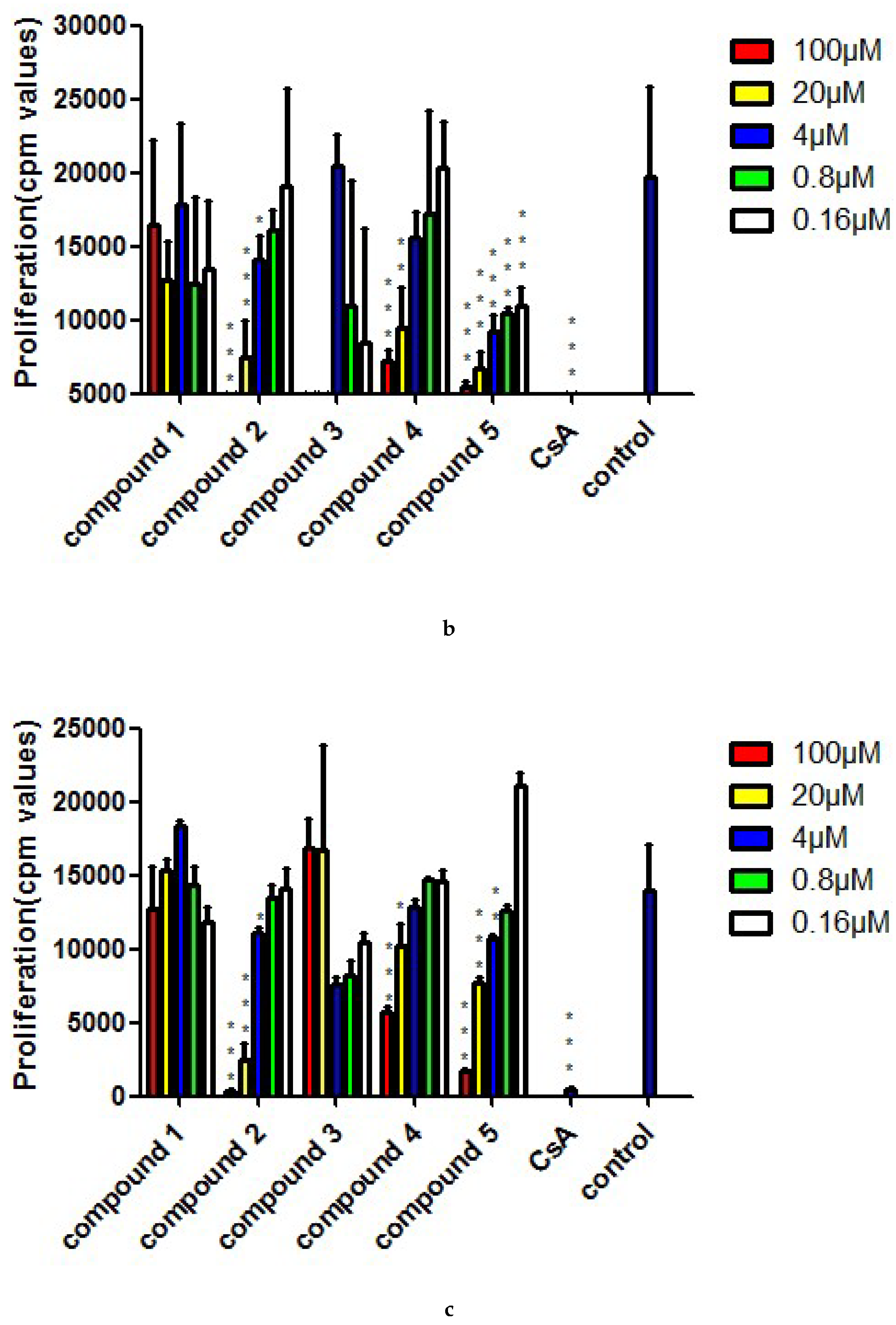
3.5. ConA- and LPS-Induced Assay
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Song, X.M.; Liu, H.J. Research and Application of “Qi-Medicines” in Taibai Mountains; People’s Medical Publishing House: Beijing, China, 2011. [Google Scholar]
- Fan, Z.C.; Zhang, Z.Q. Crystal and molecular structure of songorine from the aoot of Aconitum szechenyianum Gay. J. Chem. Crystallogr. 2008, 38, 635–639. [Google Scholar] [CrossRef]
- Wang, Y.J.; Zeng, C.J.; Yao, Z.; Zhang, J.; Zhang, Y.; Zhang, F. Diterpene alkaloids from roots and processed products of Aconitum pendulum. Chin. Tradit. Herb. Drugs 2010, 41, 347–351. [Google Scholar]
- Liu, L.M.; Wang, H.C.; Zhu, Y.L. Studies on Chinese drug Aconitum spp. XIX. The alkaloids of Aconitum pendulum and their chemical structure. Yao Xue Xue Bao 1983, 18, 39–44. [Google Scholar] [PubMed]
- Wang, Y.J.; Zhang, J.; Zeng, C.J.; Yao, Z.; Zhang, Y. Three new C19-diterpenoid alkaloids from Aconitum pendulum. Phytochem. Lett. 2011, 4, 166–169. [Google Scholar] [CrossRef]
- Wada, K.; Ohkoshi, E.; Morris-Natschke, S.L.; Bastow, K.F.; Lee, K.H. Cytotoxic esterified diterpenoid alkaloid derivatives with increased selectivity against a drug-resistant cancer cell line. Bioorg. Med. Chem. Lett. 2012, 22, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.F.; Zhang, Z.Q.; Kang, X.; Liu, J.L. LC-MS analysis for the components captured by ECV304 cell from extract of Aconitum szechenyianum Gay. Biomed. Chromatogr. 2009, 23, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Jiang, Y.; Liu, J.; Kang, Y.; Li, R.; Wang, J. The anti-tumor activity and mechanism of alkaloids from Aconitum szechenyianum Gay. Bioorg. Med. Chem. Lett. 2015, 26, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yue, Z.G.; Xie, P.; Zhang, L.; Li, Z.; Song, B.; Tang, Z.; Song, X.M. C19-Norditerpenoid Alkaloids from Aconitum szechenyianum and Their Effects on LPS-Activated NO Production. Molecules 2016, 21, 1175. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.K.; Zhao, T.F.; Lai, S. Alkaloids of cultivated Aconitum carmichaeli I. Chin. Pharm. J. 1995, 30, 716–719. [Google Scholar]
- Gao, L.M.; Wei, X.M.; Yang, L. Two New Norditerpenoid Alkaloids from Aconitum spicatum Stapf. Chin. Chem. Lett. 2005, 16, 475–478. [Google Scholar]
- Kang, X.L. Study on therapeutic effect of the effective parts of Aconitum flavum on Adjuvant arthritis in rats; Ningxia Medical university: Ningxia, China, 2013. [Google Scholar]
- Fu, X.Y.; Yu, L.; Kang, X.L.; Dong, L.; Zhang, Y.; Chen, J. Study on therapeutic effect and mechanism of the non-alkaloid fraction of Aconitum flavumn on Adjuvant arthritis in rats. Chin. Med Mater. 2014, 37, 312–315. [Google Scholar]
- Jiang, B.; Lin, S.; Zhu, C.; Wang, S.; Wang, Y.; Chen, M.; Zhang, J.; Hu, J.; Chen, N.; Yang, Y. Diterpenoid alkaloids from the lateral root of Aconitum carmichaelii. J. Nat. Prod. 2012, 75, 1145–1159. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.L.; Wang, F.P. New products from the reaction of acetyllycoctonine with N-bromosuccinimide (NBS). Chem. Pharm. Bull. 2004, 52, 1095–1097. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 4–5 are available from the authors. |
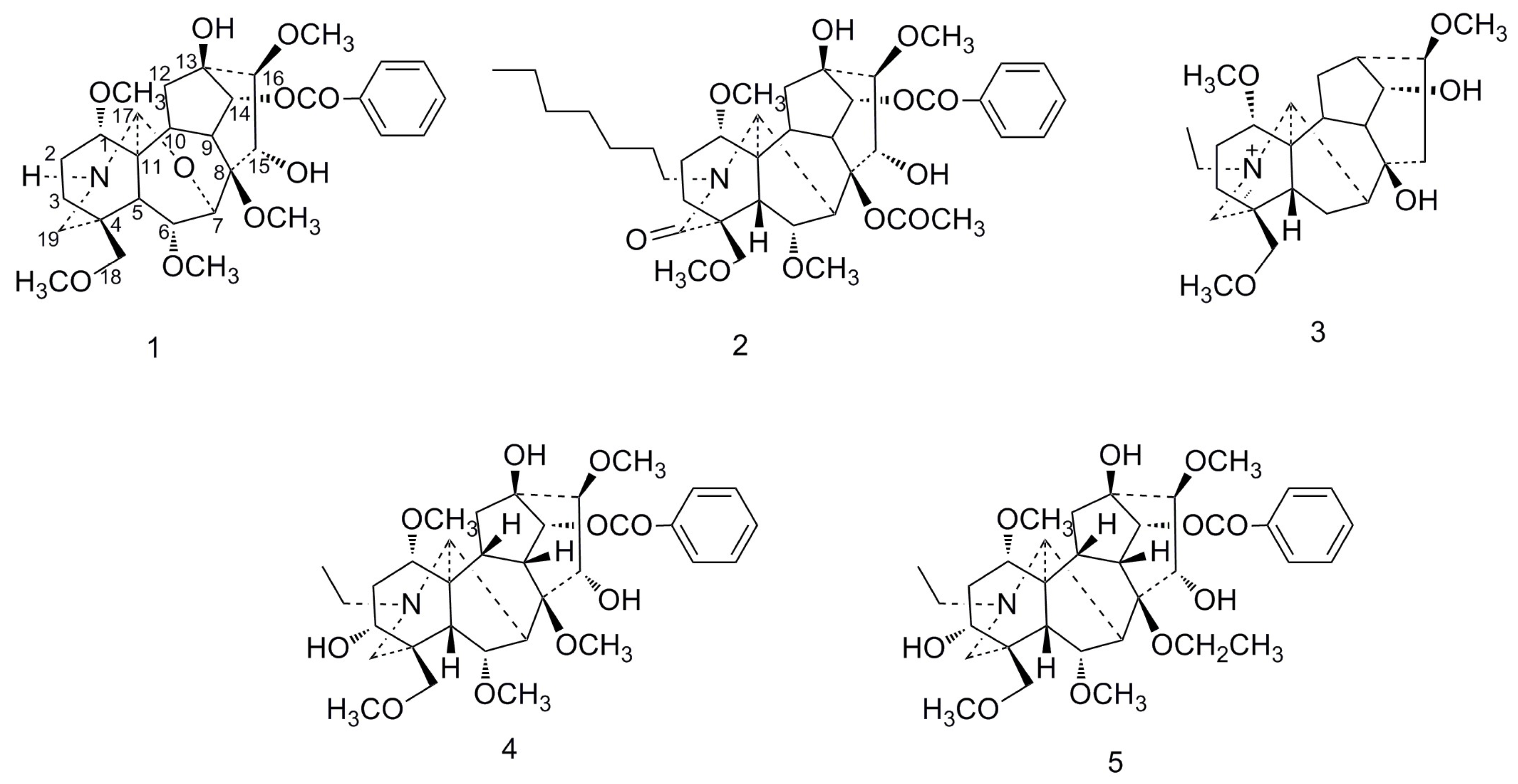
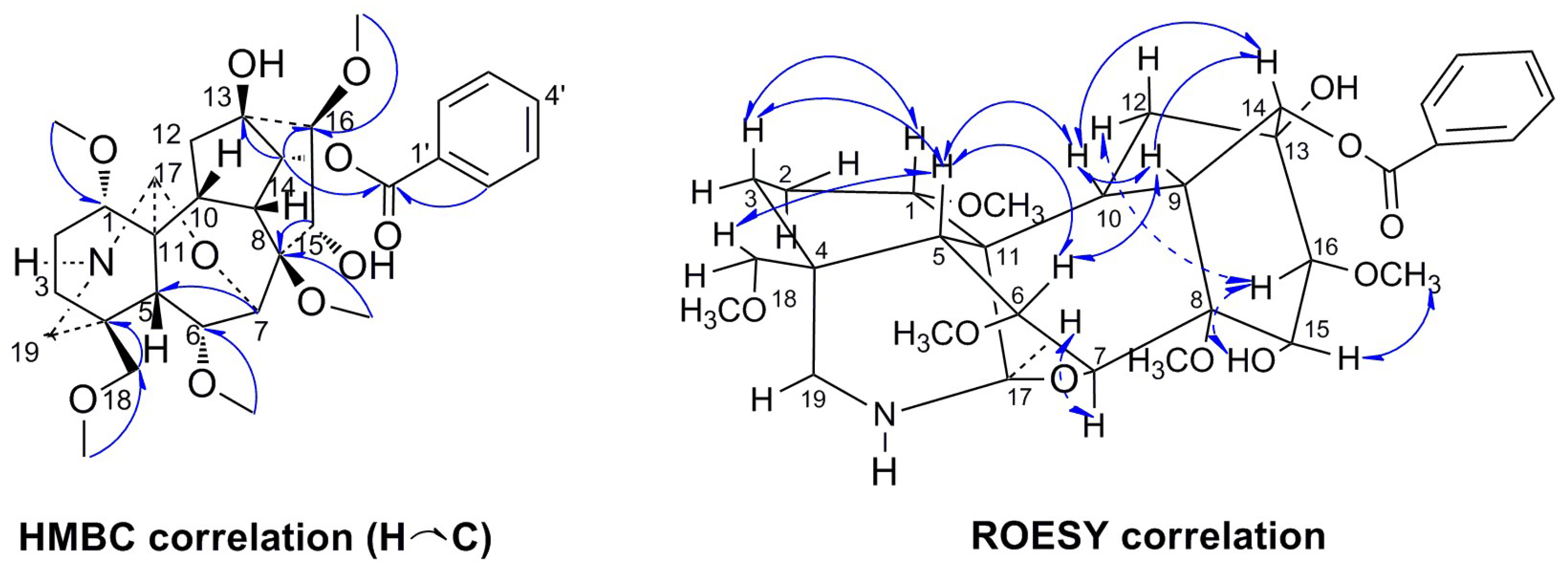
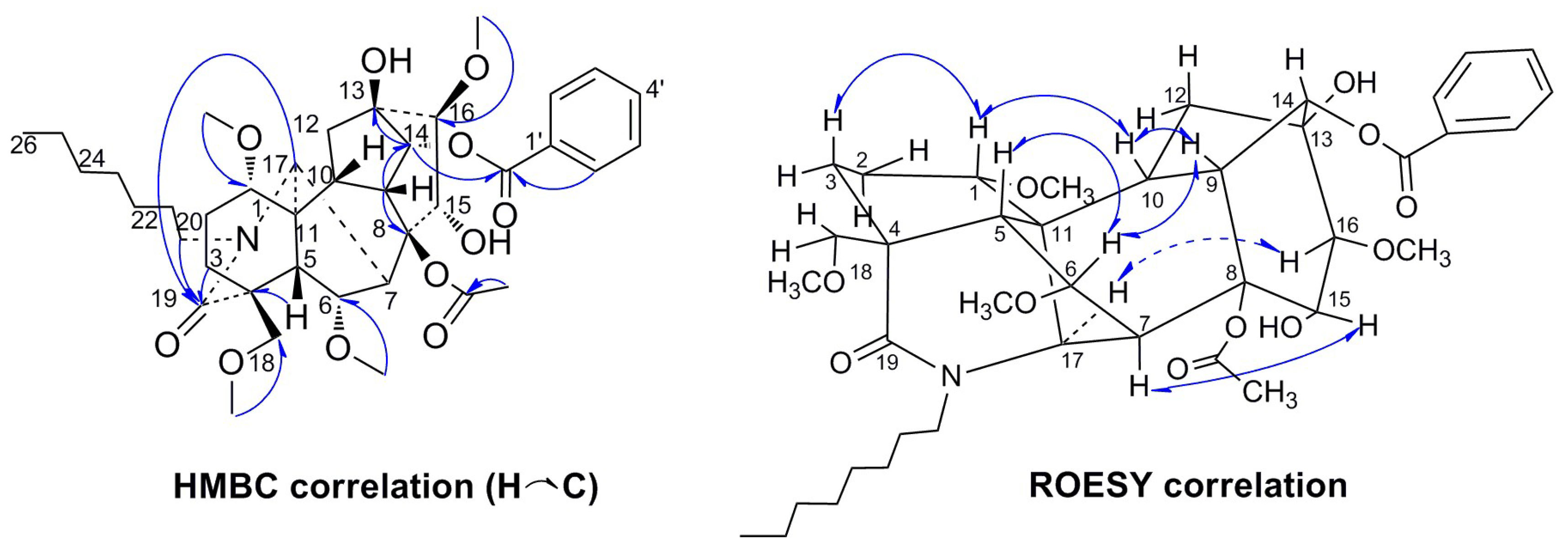
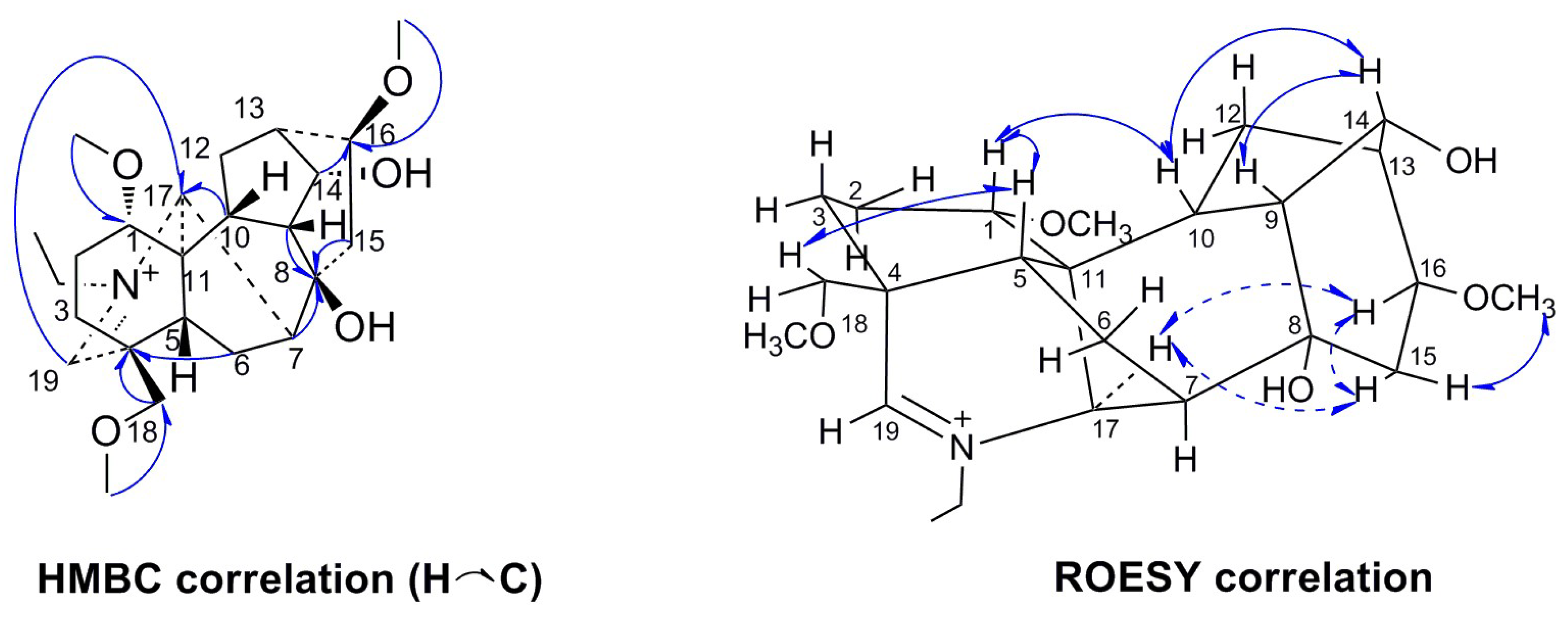
| NO. | 1 | 2 | 3 | 4 | 5 | |||
|---|---|---|---|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | δC | δC | |
| 1 | 80.3 | 3.45 (d, 7.6) | 82.4 | 3.07 (d, 8.3) | 80.6 | 3.40 (m) | 82.8 | 82.8 |
| 2 | 29.6 | 1.22 (m, H-2a) | 25.3 | 1.43 (m, H-2a) | 20.6 | 1.40 (m, H-2a) | 33.6 | 33.6 |
| 2.41 (m, H-2b) | 1.87 (m, H-2b) | 1.72 (m, H-2b) | ||||||
| 3 | 29.9 | 1.22 (m, H-3a) | 33.4 | 2.61 (m, H-3a) | 25.5 | 1.94 (m) | 72.0 | 71.9 |
| 1.40 (m, H-3b) | 2.71 (m, H-3b) | |||||||
| 4 | 43.5 | 37.8 | 48.6 | 43.3 | 43.2 | |||
| 5 | 42.3 | 2.41 (d, 6.7) | 47.6 | 2.29 (d, 6.7) | 38.4 | 2.46 (m) | 46.2 | 45.9 |
| 6 | 82.2 | 4.12 (d,6.7) | 83.3 | 4.04 (d, 6.7) | 24.6 | 1.82 (m, H-6a)2.20 (m, H-6b) | 83.6 | 83.7 |
| 7 | 64.4 | 3.32 (s) | 50.9 | 2.63 (s) | 45.1 | 2.22 (m) | 45.4 | 43.3 |
| 8 | 83.3 | 91.3 | 72.3 | 82.6 | 82.5 | |||
| 9 | 44.5 | 2.59 (t, 5.8) | 43.5 | 2.84 (t, 5.8) | 53.8 | 2.47 (m) | 42.7 | 45.4 |
| 10 | 40.8 | 2.26 (m) | 41.1 | 2.19 (m) | 38.3 | 2.01 (m) | 41.7 | 41.6 |
| 11 | 50.9 | 50.0 | 51.2 | 50.8 | 50.8 | |||
| 12 | 35.5 | 2.24 (m, H-12a) | 35.6 | 2.80 (m, H-12a) | 27.8 | 2.04 (m, H-12a)1.26 (m, H-12b) | 36.5 | 36.5 |
| 1.84 (m, H-12b) | 2.19 (m, H-12b) | |||||||
| 13 | 74.7 | 74.3 | 43.3 | 1.96 (m) | 75.0 | 75.0 | ||
| 14 | 78.8 | 4.84 (d, 5.8) | 78.8 | 4.89 (d, 5.8) | 75.0 | 4.21 (t, 4.9) | 79.7 | 79.8 |
| 15 | 76.4 | 4.65 (d, 5.4) | 79.0 | 4.49 (dd, 2.9, 5.4) | 39.5 | 2.27 (m, H-15a) | 78.0 | 78.7 |
| 2.40 (m, H-15b) | ||||||||
| 16 | 93.2 | 3.22 (d, 5.4) | 90.4 | 3.30 (d, 5.4) | 81.5 | 3.45 (m) | 93.6 | 93.6 |
| 17 | 70.0 | 4.29 (s) | 56.7 | 4.11 (s) | 68.1 | 3.79 (s) | 62.7 | 61.4 |
| 18 | 76.6 | 3.54(d, 8.2, H-18a) | 80.0 | 3.78(d,8.2,H-18a) | 73.4 | 3.71 (2H, m) | 77.2 | 77.2 |
| 3.46 (d,8.2, H-18b) | 3.06(d,8.2,H-18b) | |||||||
| 19 | 50.3 | 3.62(d,11.9,H-19a) | 173.3 | 179.2 | 9.19 (s) | 49.2 | 49.2 | |
| 3.72(d,11.9,H-19b) | ||||||||
| 20 | 45.5 | 3.04 (m, H-20a) | ||||||
| 3.92 (m, H-20b) | ||||||||
| 21 | 33.7 | 1.51 (m, H-21a) | ||||||
| 1.62 (m, H-21b) | ||||||||
| 22 | 25.0 | 1.59 (m, H-22a) | ||||||
| 1.68 (m, H-22b) | ||||||||
| 23 | 29.6 | 1.30 (m, H-23a) | ||||||
| 1.23 (m, H-23b) | ||||||||
| 24 | 31.9 | 1.30 (m, H-24a) | ||||||
| 2.26 (m, H-24b) | ||||||||
| 25 | 22.9 | 1.30 (2H, m) | ||||||
| 26 | 14.3 | 0.86 (3H, t,6.2) | ||||||
| 8-OAc | 172.5 | |||||||
| 21.6 | 1.35 (s) | |||||||
| 8-OCH2CH3 | 57.4 | |||||||
| 8-OCH2CH3 | 15.5 | |||||||
| 1-OCH3 | 55.4 | 3.37 (s) | 55.5 | 3.19 (s) | 56.7 | 3.16 (s) | 56.1 | 56.1 |
| 6-OCH3 | 59.3 | 3.29 (s) | 58.0 | 3.12 (s) | 59.4 | 58.8 | ||
| 8-OCH3 | 50.7 | 3.21 (s) | 50.1 | |||||
| 16-OCH3 | 62.2 | 3.80 (s) | 61.5 | 3.75 (s) | 56.9 | 3.36 (s) | 61.4 | 62.6 |
| 18-OCH3 | 59.0 | 3.29 (s) | 59.4 | 3.30 (s) | 59.8 | 3.38 (s) | 59.3 | 59.3 |
| N-CH2CH3 | 56.3 | 4.01 (dq, 13.9, 7.2) | 47.6 | 47.6 | ||||
| 4.42 (dq, 13.9, 7.2) | ||||||||
| N-CH2CH3 | 14.0 | 1.51 (t, 7.2) | 13.6 | 13.5 | ||||
| ArC=O | 166.4 | 166.2 | 166.5 | 166.4 | ||||
| ArC-1′ | 130.0 | 129.9 | 130.4 | 130.6 | ||||
| 3′, 5′ | 128.7 | 7.44 (t, 7.4) | 128.9 | 7.45 (t, 7.5) | 128.6 | 128.6 | ||
| 2′, 6′ | 130.0 | 8.00 (d, 7.4) | 129.8 | 8.01 (d, 7.5) | 129.9 | 129.9 | ||
| 4′ | 133.4 | 7.54 (t, 7.4) | 133.6 | 7.56 (t, 7.5) | 133.1 | 133.1 | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, B.; Jin, B.; Li, Y.; Wang, F.; Yang, Y.; Cui, Y.; Song, X.; Yue, Z.; Liu, J. C19-Norditerpenoid Alkaloids from Aconitum szechenyianum. Molecules 2018, 23, 1108. https://doi.org/10.3390/molecules23051108
Song B, Jin B, Li Y, Wang F, Yang Y, Cui Y, Song X, Yue Z, Liu J. C19-Norditerpenoid Alkaloids from Aconitum szechenyianum. Molecules. 2018; 23(5):1108. https://doi.org/10.3390/molecules23051108
Chicago/Turabian StyleSong, Bei, Bingliang Jin, Yuze Li, Fei Wang, Yifu Yang, Yuwen Cui, Xiaomei Song, Zhenggang Yue, and Jianli Liu. 2018. "C19-Norditerpenoid Alkaloids from Aconitum szechenyianum" Molecules 23, no. 5: 1108. https://doi.org/10.3390/molecules23051108




