2.1. Total Phenolic and Flavonoid Contents
The phenolic content in the blue highland barley was examined (
Table 1). It was observed that the 12 blue highland barley samples showed differences in the contents of free, bound and total phenolics (
p < 0.05). The total phenolic content was 336.21–453.94 mg of gallic acid eq./100 g DW, which was higher than that of corn (1.21–1.68 fold), wheat (2.71–3.60 fold), oat (3.11–4.20 fold) and rice (3.71–5.04 fold) [
20]. Among them, Beiqing 2 had the highest content of total phenolics (453.94 ± 11.38 mg GAE/100 g DW), while Dulihuang had the lowest content (336.29 ± 5.42 mg GAE/100 g DW). The free and bound phenolic contents (170.11–240.75 mg of gallic acid eq./100 g DW) were lower than the free (410.1–551.2 mg of gallic acid eq./100 g DW) and bound phenolic contents (376.1–861.2 mg of gallic acid eq./100 g DW) in blue hulled barley reported by Abdel-Aal [
12], and also lower than the content of free phenolics (407.52 mg GAE/100 g DW) in highland barley reported by Liu, H.L [
16]. The difference in phenolic content was influenced by the varieties, cultivation environment, extracted solvents and extracted fractions. In our experiment, 80% acetone was used to extract free phenolics from highland barley, acid hydrolysis was used to extract the bound phenolic content. The extracted material was the whole grain of highland barley, while an 80% methanol and alkali process was used to extract the free and bound phenolic content from barley [
12]. The polyphenols were extracted from highland barley bran by 60% acetone [
16]. However, the content was higher than the content of free and bound phenolic in maize, wheat, oats and rice [
20], thus suggesting that blue highland barley can be used as a good dietary source of phenolics. In about 46.7% of the tested highland barley varieties from Lhasa, Tibet and the Haibei region of Qinghai Province (Beiqing 8, Beijing 9, Zangqing 320, Zangqing 690 and Xunhua Lianglan), the free phenolic content was significantly higher than the bound phenolic content, and 53.3% of the highland barley varieties showed a higher bound phenolic content than free phenolic content. This result is different from previous reports which showed that 95% phenolic acid in the hulled barley exists in a combining form [
12,
24]. This signifies that the gene type and growth environment of blue highland barley are the critical points to influencing the free and bound phenolic contents in its grains. This is consistent with the report results of Griffiths and Welch [
25].
In the present study, the free and bound phenolic contents accounted for 45.56–54.30% and 45.71–53.38% in the blue highland barley, respectively, with average values of 50.09% and 49.91%. Previous studies have shown that free and bound phenolic contents respectively exert their functional roles in the upper gastrointestinal tract and colon [
20]. Therefore, according to the proportion of free and bound phenolic contents in the total phenolic compounds, it may be concluded that the consumption of blue-grained highland barley alone could be beneficial to the health of the human digestive tract and colon, and that its function is superior to that of other cereals.
In the blue highland barley samples, the free, bound and total flavonoid contents in the blue highland barley varieties were tested, and there were significant differences among the different varieties (
p < 0.05) (
Table 1). The content of total flavonoids was 37.91–47.98 mg CE/100 g, which was equivalent to the content in maize, wheat, oat and rice [
20]. Among the blue grain highland barley varieties which were tested, Beiqing 2 (47.98 ± 2.00 mg CE /100 g DW) had the highest content of total flavonoids, and the content of Mennong 1 (37.93 ± 0.77 mg CE/100 g DW) was the lowest. In the present study, the content (20.61–25.59 mg CE/100 g) of free flavonoid in the tested varieties was significantly (
p < 0.05) higher than the other bound flavonoid contents (14.91–22.38 mg/100 g). This is different from the existence form of flavonoid in common buckwheat [
26], wheat, rice, corn and oat [
20]. In addition, the numbers of flavonoids existing in a bound form in common buckwheat, wheat, rice, corn and oat are higher than those in a free form. However, similar to the existence form of flavone in tartary buckwheat [
27] and black rice [
28], the content of flavone existing in a free form is higher than that in a bound form. The contents of free and bound flavonoid respectively accounted for 50.71–65.27% and 34.71–49.33% in the content of the total flavonoids, with respective average values of 55.90% and 44.10%.
Among these, the content of free flavonoids in Zangqing 690 accounted for 65.27% of the total flavonoids, which is significantly higher than the other varieties tested (p < 0.05), thus signifying that edible Zangqing 690 can play a better protection role in the upper respiratory tract. Therefore, the comprehensive and accurate evaluation of the content of free and bound phenolic compounds in blue highland barley is of great value to guiding its development and utilization.
The 12 varieties of blue highland barley were divided according to the different locations. The average contents of free phenol, combined phenol, total phenol, free flavones, combined flavones and total flavones are shown in
Table 2. It can be seen from the table that there is a significant difference (
p < 0.05) between the average contents of the free, bound and total phenolics of the highland barley in the different locations, and highland barley samples showed differences in the contents of free, bound and total flavonoids (
p < 0.05) which indicates that planting environment has a significant impact on the contents of the phenolics and flavonoids of highland barley. The contents of the free phenolic of the blue highland barley planted in Menyuan (Qinghai) and Lhasa (Tibet) have no significant difference, and were higher than that of the blue highland barley planted in Hezuo (Gansu). In addition, the contents (216.68 mg/100 g DW) of the bound and total phenolics of the blue highland barley planted in Qinghai are higher than those planted in Lhasa (Tibet) and Hezuo (Gansu), which is related to the fact that the content of phenolic can be improved by promoting the synthesis of plant phenylalanine ammonia lyase at low temperature [
29]. Moreover, the average content of blue highland barley varieties in the three locations is higher than those of Croatia [
30] (148.01–159.00 mg GAE/100 g DW) and Tunisian barley [
31] (40.01–123.00 mg GAE/100 DW). In addition, the contents of free, bound and total flavonoids of the blue highland barley planted in Menyuan (Qinghai) and Lhasa (Tibet) show no significant difference, among which the content of free flavones of the blue highland barley planted in Lhasa (Tibet) (255.2 mg/100 g DW) is the highest. The content of bound (19.68 mg/100 g DW) and total flavonoids (42.39 mg/100 g DW) of the blue highland barley planted in Hezuo (Gansu) are the highest. This indicates that the content of phenolic of the blue highland barley planted in Menyuan (Qinghai) is higher, as is the content of flavonoid of the blue highland barley planted in Hezuo (Gansu). Aside from the influence of genotype of highland barley, the content may be related to the climate, temperature and other conditions of the planting environment of blue highland barley. Narwal [
13] and other scholars have pointed out that the free phenolics of India barley are more greatly influenced by genotype, while the contents of bound and total phenolics have been affected more by the planting environment. Therefore, the content of blue highland barley is simultaneously influenced by both the genotype and environment of the varieties.
2.2. Composition and Content of Phenolic Compounds in Blue Highland Barley
In this study, the phenolic acids such as phloroglucinol, gallic acid, protocatechuic acid, chlorogenic acid, 2,4-dihydroxybenzoic acid, vanillic acid, syringic acid,
p-coumaric acid, ferulic acid, salicylic acid, benzoic acid,
o-coumaric acid, and 3,4-dimethoxybenzoic acid, and flavonoids such as catechin, naringin, hesperidin, myricetin, quercetin, naringenin, kaempferol and rutin were detected in the free and bound phenolic extracts of blue highland barley, using a high-performance liquid chromatography (HPLC) system (
Figure 1,
Table 3). All of the monomer phenolic substances detected had a large amplitude of variation in content and high variable coefficient, which shows that there was a significant difference among the varieties. Gallic acid, benzoic acid, syringic acid and
p-coumaric are the main phenolic acids of blue highland barley, their combined sum accounting for 87.56% of the total phenolic acids, which was similar to the value shown in a previous report, in which syringic acid and
p-coumaric acid were determined to be the main phenolic acids of barley [
16,
17,
32]. However, unlike other current reports, the present study showed that gallic acid and benzoic acid were the main free phenolic acids in blue grain highland barley. Phloroglucinol, 2,4-dihydroxybenzoic acid, vanillic acid, ferulic acid, salicylic acid and
o-coumaric acid have lower relative average contents in the blue highland barley tested; this is inconsistent with the report results of Abdel-Aal [
12] and Zielinski [
33], which showed that ferulic acid is the main phenolic acid in barley, wheat and rye. In all of the phenolic acids detected, the contents of protocatechuic acid, chlorogenic acid, 2,4-dihydroxybenzoic acid and vanillic acid in the free phenolic extract of the blue highland barley were respectively 19.0, 2.62, 1.21 and 1.39 times those in the bound phenolic extract, which means that these four types of phenolic acids mainly exist in the free phenolic content of blue highland barley. This is inconsistent with the report results which showed that protocatechuic acid, chlorogenic acid, 2,4-dihydroxybenzoic acid and vanillic acid mainly exist in a bound state in cereals [
5]. For the remaining phenolic acids tested, their contents were significantly higher in the bound extracts than in the free ones. Among them, the contents of gallic acid, syringic acid,
p-coumaric, benzoic acid and 3,4-dimethoxybenzoic acid in the bound extracts were 13.81–102.80 times those of the free content, and these are the main contributors to the content of the bound total phenolic acids.
o-Coumaric acid was not detected in the free phenolics extracts in any of the blue highland barley tested, and the detected content of salicylic acid was (1.01–20.55 μg/g DW), while the remaining phenolic acid contents were not detected in the free phenolic extracts of some of the highland barley varieties tested. The following items were detected in the bound phenolic extracts of all of the highland barley varieties tested:
p-coumaric (14.61–583.54 μg/g DW), ferulic acid (5.61–13.88 μg/g), salicylic acid (7.41–28.38 μg/g DW), benzoic acid (8.81–528.56 μg/g DW),
o-coumaric acid (15.11–60.26 μg/g DW) and 3,4-dimethoxybenzoic acid (18.51–110.85 μg/g DW). The contents of the remaining phenolic acids were not detected in the bound phenolic extracts of a portion of the highland barley varieties tested. This means that the abovementioned monomer phenolic acids were the prevalent characteristic phenolic acids in the blue highland barley. In addition, there were differences in the phenolic acid contents of the tested main monomers among the different varieties of blue grain highland barley. Among them, the content of gallic acid was rich in Walan (1366.69 ± 2.34 μg/g DW, Beiqing 8 (1173.02 ± 1.32 μg/g DW), Dulihuang (1031.00 ± 1.57μg/g DW) and Beiqing 9 (459.84 ± 1.49 μg/g DW). Chlorogenic acid only had a high content in Ganqing 4 (98.36 ± 1.68 μg/g DW). The existing research has proven that chlorogenic acid was a kind of plant allelochemical found in plants, which could inhibit the growth of weeds and did less harm to soil [
34]. Therefore, Ganqing 4 highland barley had the potential to both protect the environment and develop botanical pesticides. The content of syringic acid was rich in Xunhualianglan (916.20 ± 6.02 μg/g DW), Zangqing 320 (657.04 ± 1.24 μg/g DW), Walan (591.99 ± 0.98 μg/g DW) and Beiqing 2 (573.71 ± 1.45 μg/g DW). The
p-coumaric acid was rich in Ganqing 4 (583.54 ± μg/g DW), Walan (200.34 ± 0.98 μg/g DW), Dulihuang (133.94 ± 4.22 μg/g DW), Zangqing 320 (129.00 ± 1.04 μg/g DW) and Beiqing 9 (100.48 ± 2.05 μg/g DW). The benzoic acid was rich in Beiqing 2 (521.74 ± 1.82 μg/g DW), Xunhualianglan (442.88 ± 2.02 μg/g DW) and Dulihuang (432.17 ± 0.98 μg/g DW). The Zangqing 320, Zangqing 690 and Mennong 1 varieties contained 109.27, 106.16 and 112.99 μg/g of 3,4-dimethoxybenzoic acid, respectively.
The main monomer phenolic acids detected in Beiqing 4 were fewer in number. It was thus indicated that genotype had a significant effect on the composition and content of phenolic acids. The contents of catechin, naringin, hesperidin, myricetin, quercetin, naringenin, kaempferol and rutin were detected in the free and bound extracts of the blue highland barley tested. Among these, naringenin had the highest average content (137.09 ± 172.60 μg/g DW), which is measured for the first time in existing highland barley. This was followed by hesperidin (104.07 ± 81.23 μg/g DW), then by rutin, catechin, quercetin and kaempferol (56.81–69.18 μg/g DW), while naringin (16.71 ± 17.09 μg/g DW) and (15.05 ± 21.81 μg/g DW) had the lowest average contents. This means that naringenin and hesperidin are the main flavonoids in blue highland barley, which is inconsistent with the report results which showed that myricetin is the main flavonoid in colored barley, and naringin and hesperidin have extremely low contents in colored barley [
10]. This difference is related to the gene type, planting environment and extraction method of the tested varieties. Among them, genotype had great influence on the content of flavonoids in different blue highland barley. In this study, four kinds of flavonoids of Menyuanlianglan were found in the blue highland barley, including catechin (101.33 ± 1.04 μg/g DW), naringenin (502.48 ± 0.28 μg/g DW), kaempferol (192.27 ± 0.86 μg/g DW) and rutin (141.44 ± 0.56 μg/g DW). The kaempferol (76.89 ± 0.08 μg/g DW) and rutin (118.86 ± 0.10 μg/g DW) were rich in Zangqing 690, catechin (95.44 ± 0.52 μg/g DW) and hesperidin (187.40 ± 0.06 μg/g DW) were rich in Mennong 1, catechin (96.87 ± 0.10 μg/g DW) and quercetin (87.30 ± 0.12 μg/g DW) were rich in Xunhualianglan. Rutin (92.09 ± 0.04 μg/g DW), quercetin (60.41 ± 0.05 μg/g DW), catechin (105.38 ± 0.28 μg/g DW), naringenin (507.70 ± 0.36 μg/g DW) and hesperidin (152.63 ± 0.21 μg/g DW) were respectively rich in Beiqing 2, Beiqing 4, Beiqing 8, Ganqing 4 and Beiqing 9. The content of the tested main flavonoid monomer in Zangqing 320 was low in comparison. Myricetin and quercetin were not detected in the free extracts of the tested blue-grained highland barley. The average content of catechinic acid in the free-state extracts was slightly higher than that in the bound state, but the difference was not significant. However, the average contents of naringin, hesperidin, myricetin, quercetin, naringenin, kaempferol and rutin in the bound extract were about 5.21–51.02 times those of the corresponding free extracts, which means that they mainly exist in the blue highland barley in a bound state. These results are inconsistent with the existence form of flavonoid measured by using the chemical method. A possible reason for this is that the chemical method uses catechin as the standard item to calculate the content of flavonoid in extract, while the measurement using the HPLC system result shows that the content of catechin in the free extracts is slightly higher than in the bound content, which leads to the result that the content of flavonoid in the free phenolic extract measured by using the chemical method is higher than the bound content.
Table 4 compares the free and combined individual phenolic compounds of the blue highland barley planted in different areas. It is shown that for the blue highland barley planted in different locations, the contents of free, bound and total phenolic compounds have significant differences (
p < 0.05). There are 11 kinds of free phenolic acids and 12 kinds of bound phenolic acid which were detected from the blue highland barley planted in Menyuan (Qinghai) and Hezuo (Gansu), and eight kinds of free phenolic acids and nine kinds of bound phenolic acid detected from the blue highland barley planted in Lhasa (Tibet). The average contents of free, bound and total phenolic acids are the highest (201.54 ± 8.94, 1498.69 ± 102.58, 1623.69 ± 100.91 ug/g DW) in the blue highland barley planted in Menyuan (Qinghai), followed by those in Hezuo (Gansu) (102.47 ± 9.35, 1289.05 ± 228.76, 1392.52 ± 227.57 ug/g DW), with the lowest those planted in Lhasa (Tibet) (87.79 ± 10.25, 914.00 ± 125.77, 1001.79 ± 124.80 ug/g DW). For the blue highland barley planted in Menyuan (Qinghai), Lhasa (Tibet) and Hezuo (Gansu), respectively six, four and four kinds of free flavonoids detected, and eights kinds of flavonoid monomers were detected from the bound extract of the blue highland barley planted in the three locations. For the free flavonoids, the content of the blue highland barley planted in Menyuan (Qinghai) is the highest (76.54 ± 15.24 ug/g DW), while the content of bound and total flavonoids of the blue highland barley planted in Hezuo (Gansu) is the highest (559.78 ± 66.52 ug/g DW, 594.24 ± 69.70 ug/g DW). These results are consistent with the contents of free, bound and total phenolics detected by chemical methods. Therefore, it is indicated that the blue highland barley planted in Menyuan (Qinghai) includes more abundant types of phenolic compounds and higher content of phenolic acids, while that planted in Hezuo (Gansu) includes a higher content of flavonoids, which provides a reliable basis for the selection of the materials for different purposes. The contents of gallic acid, protocatechuic acid, 2,4-dihydroxybenzoic acid and catechin in the blue highland barley planted in Menyuan (Qinghai) are relatively high, those of phloroglucinol, chlorogenic acid,
p-coumaric, naringin, hesperidin and myricetin in that planted in Hezuo (Gansu) are rich, and the contents of syringic acid, benzoic acid and rutin in that planted in Lhasa (Tibet) are rich.
Therefore, it can be indicated that the planting environment exhibits a significant impact on the monomer composition and content of polyphenol in blue highland barley. Consequently, we must give full consideration to environmental factors while selecting the raw materials of blue highland barley according to specific needs.
2.3. Antioxidant Properties
Among the three antioxidant systems, the polyphenols in blue highland barley show a relatively higher capacity to scavenge DPPH
• (1336.51–1640.01 mg/100 g DW) and ABTS
•+ (817.41–1291.78 mg/100 g DW). It also has a relatively stronger ferric reducing antioxidant power (FRAP) (693.41–1041.02 mg/100 g DW) (
Figure 1,
Figure 2 and
Figure 3), which is significantly higher than the antioxidant activity of Canadian, Egyptian [
35] and Tunisian [
14] barley of the same unit, as well as rice [
36] and carrot [
37] with different colors. Therefore, blue highland barley can be used as a potential and good dietary source for scavenging free radicals.
According to
Figure 2, the capacity of bound phenolic extracts in blue highland barley to scavenge the DPPH
• radical is much stronger than the free phenolic extracts, but the free phenolic extract has a higher total DPPH
• radical scavenging capacity and FRAP than the bound phenolic extract. Among these, the average contribution of the bound phenolic extract to the total DPPH
• radical scavenging capacity is greater than 86%, and the average contribution of free phenolic extract to total ABTS
•+ radical scavenging capacity is greater than 79%. Therefore, it can be concluded that the bound phenolic extract of blue highland barley is the main contributor to the scavenging of DPPH
• radical, and free phenolic extract is the main contributor to the scavenging of ABTS
•+ radical. Free phenolic extract (53%) and bound phenolic extract (47%) have equivalent contribution to the iron ion reducing capacity. In addition, different varieties of blue highland barley have significant differences in the scavenging capacity of DPPH
• and ABTS
•+ and FRAP capacity (
p < 0.05). Among them, Xunhualianglan has the highest value of scavenging the DPPH
• and ABTS
•+ radical, and Beiqing 8 has the highest total FRAP. This kind of difference is related to the composition and content of different forms of phenolic substances in different varieties of highland barley.
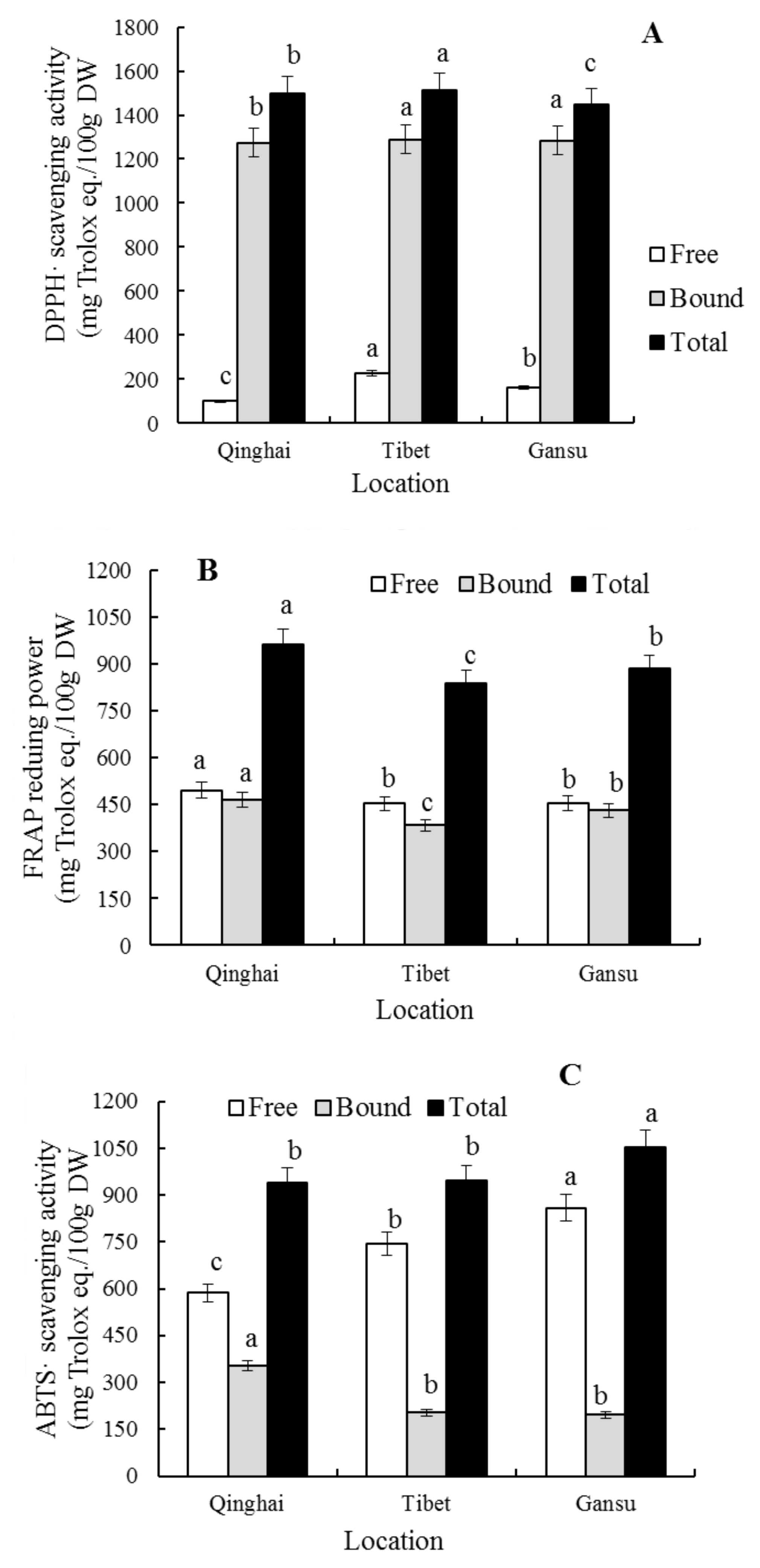
The capabilities of the free and bound polyphenol extraction of blue highland barley planted in the different areas in removing DPPH
• (1336.51–1640.01 mg/100 g) and ABTS
•+ (817.41–1291.78 mg/100 g) and reducing FRAP iron ion are significantly different (
p < 0.05) (
Figure 3). The free (227.24 ± 29.70 mg/100 g) and bound phenolic (1288.67 ± 62.20 mg/100 g) of the blue highland barley planted in Lhasa (Tibet) have a strong ability to remove DPPH
•, while the free (495.10 ± 34.28 mg/100 g) and bound phenolics (465.84 ± 28.56 mg/100 g) of that planted in Menyuan (Qinghai) have a strong ability to reduce FRAP iron ion, and the bound phenolics (353.75 ± 42.35 mg/100 g) of that planted in Menyuan (Qinghai) and the free phenolics (859.21 ± 61.54 mg/100 g) of that planted in Hezuo (Gansu) have a strong ability to remove ABTS
•+. Polyphenols are the main contributors to the antioxidant activity of blue highland barley, and the different planting environment affects the compositions and contents of different polyphenols of blue highland barley, and leads to differences in the antioxidant activity. Therefore, it can be concluded that planting environment plays an important role in the strength of the antioxidant activity of highland barley, which is consistent with the results reported by Abdel-Aal, E.M. [
15].
2.4. Correlations between Phenolic Compounds and Antioxidant Activities
In order to clarify the relationship between the contents of the free and bound phenolic compounds and their antioxidant activities in blue highland barley, correlation analysis was carried out, and the analysis results are shown in
Table 5 and
Table 6. The free phenolic content showed an extremely significant positive correlation with the DPPH
• and ABTS
•+ radical scavenging capacity and the FRAP
(p < 0.01). The content of free total phenolic compounds showed a significant positive correlation with the FRAP capacity. The hydroxybenzoic acid and protocatechuic acid in the mon
o-phenolic content of free extracts show an extremely significant (
p < 0.01 and
p < 0.05, respectively) positive correlation with the ABTS
•+ radical scavenging capacity, and the hydroxybenzoic acid shows a significant positive correlation with the DPPH
• radical scavenging capacity. This signifies that hydroxybenzoic acid and protocatechuic acid are the main contributors to the DPPH
• and ABTS
•+ radical scavenging capacity. However, the content of gallic acid shows a significant negative correlation with the ABTS
•+ radical scavenging capacity, which is consistent with the research results of Zhao [
21], which showed that naringenin had a significant negative correlation with the FRAP capacity.
The content of bound phenolics has an extremely significant positive correlation with the ABTS
•+ radical scavenging capacity and FRAP capacity, while the bound flavonoid content has a significant positive correlation with the ABTS
•+ radical scavenging capacity. This means that the bound phenolic extract of the blue highland barley contains a greater number of phenolic substances scavenging the ABTS
•+ radical. Chlorogenic acid, vanillic acid, ferulic acid and quercetin have a significant positive correlation with the ABTS
•+ radical scavenging capacity, which signifies that they are the main contributors to the ABTS
•+ radical scavenging capacity. This is similar to the results of Zhao, H.F. [
21], which showed that ferulic acid and vanillic acid were the main contributors to the ABTS
•+ radical scavenging capacity of the barley extract. In addition, we also observed that ferulic acid had a significant positive correlation with the FRAP capacity. This means that ferulic acid has a very strong antioxidant activity, despite the fact that its content in the blue highland barley is not high, and it is the main antioxidant substance in the bound phenolic extract of blue highland barley. This is consistent with the research results of Abdel-Aal, EM [
12]. Quercetin had a significant positive correlation with the DPPH
• radical scavenging capacity, which signifies that quercetin is the main contributor to the DPPH
• radical scavenging capacity of bound phenolic extract of the blue highland barley. 3,4-Dimethoxybenzoic acid had a significant negative correlation with the FRAP capacity, and hesperidin had a significant negative correlation with the ABTS
•+ free radical scavenging capacity. This signifies that different kinds of monomer phenolics exhibit selectivity for different antioxidant activity evaluation methods.
In addition, we observed that there was an extremely significant positive correlation between the free phenolic content and free flavonoid content under the test condition (p < 0.01), which means that the higher the content of free phenolic in the blue highland barley was, the higher the contained content of free flavonoid would be. The content of catechin had a significant positive correlation with the content of free phenolic, naringenin had a significant negative correlation with the content of free phenolic, and the contents of ferulic acid, quercetin and rutin had significant positive correlations with the bound phenolic. Therefore, we can evaluate the contents of catechin and naringenin in the blue highland barley according to the content of the free phenolic, and evaluate the content of ferulic acid, quercetin and rutin according to the content of the bound phenol. All of these provide a simple method by which to select the special variety of highland barley. In addition, the content of protocatechuic acid had a significant positive correlation with the content of free type total phenolic compounds, which signifies that the higher the content of protocatechuic acid in the blue highland barley was, the higher the content of its free total phenolic acid would be. In accordance with the positive correlation of the content of phloroglucinol, pcoumaric acid and myricetin with the content of the bound total phenolic acid, the higher the contents of phloroglucinol, p-coumaric acid and myricetin were, the higher the content of bound total phenolic acid of the blue highland barley would be. However, in future research, a larger sample number must be used to investigate or further verify the correlation between monomeric phenolics and total phenolic acids in blue highland barley.