The Effect of Intraoperative Ferric Carboxymaltose in Joint Arthroplasty Patients: A Randomized Trial
Abstract
:1. Introduction
2. Methods
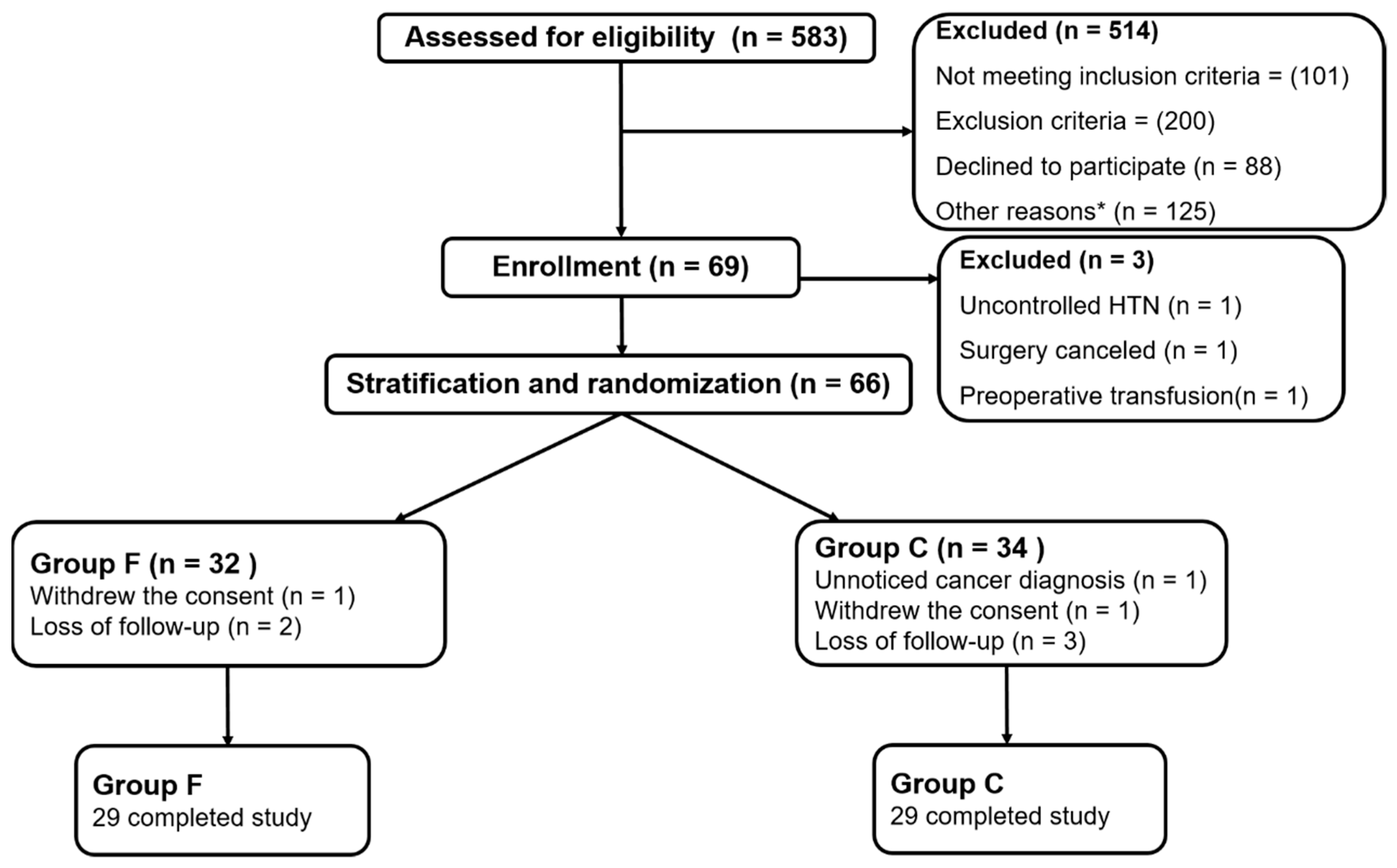
2.1. Study Population and Randomization
2.2. Data Collected and Outcomes Measures
2.3. Anesthetic Management
2.4. Statistical Analysis and Sample Size
3. Results
3.1. Baseline and Clinical Characteristics
3.2. Primary and Secondary Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Larocque, B.J.; Gilbert, K.; Brien, W.F. Prospective validation of a point score system for predicting blood transfusion following hip or knee replacement. Transfusion 1998, 38, 932–937. [Google Scholar] [CrossRef] [PubMed]
- Slover, J.; Lavery, J.A.; Schwarzkopf, R.; Iorio, R.; Bosco, J.; Gold, H.T. Incidence and risk factors for blood transfusion in total joint arthroplasty: Analysis of a statewide database. J. Arthroplast. 2017, 32, 2684–2687. [Google Scholar] [CrossRef] [PubMed]
- Nadkarni, G.N.; Patel, A.A.; Ahuja, Y.; Annapureddy, N.; Agarwal, S.K.; Simoes, P.K.; Konstantinidis, I.; Kamat, S.; Archdeacon, M.; Thakar, C.V. Incidence, risk factors, and outcome trends of acute kidney injury in elective total hip and knee arthroplasty. Am. J. Orthop. (Belle Mead NJ) 2016, 45, E12–E19. [Google Scholar] [PubMed]
- Vamvakas, E.C.; Blajchman, M.A. Transfusion-related mortality: The ongoing risks of allogeneic blood transfusion and the available strategies for their prevention. Blood 2009, 113, 3406–3417. [Google Scholar] [CrossRef]
- Kotze, A.; Carter, L.A.; Scally, A.J. Effect of a patient blood management programme on preoperative anaemia, transfusion rate, and outcome after primary hip or knee arthroplasty: A quality improvement cycle. Br. J. Anaesth. 2012, 108, 943–952. [Google Scholar] [CrossRef]
- Petis, S.M.; Lanting, B.A.; Vasarhelyi, E.M.; Naudie, D.D.R.; Ralley, F.E.; Howard, J.L. Is there a role for preoperative iron supplementation in patients preparing for a total hip or total knee arthroplasty? J. Arthroplast. 2017, 32, 2688–2693. [Google Scholar] [CrossRef]
- Cuenca, J.; Garcia-Erce, J.A.; Martinez, F.; Cardona, R.; Perez-Serrano, L.; Munoz, M. Preoperative haematinics and transfusion protocol reduce the need for transfusion after total knee replacement. Int. J. Surg. 2007, 5, 89–94. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Porras, J.R.; Colado, E.; Conde, M.P.; Lopez, T.; Nieto, M.J.; Corral, M. An individualized pre-operative blood saving protocol can increase pre-operative haemoglobin levels and reduce the need for transfusion in elective total hip or knee arthroplasty. Transfus. Med. 2009, 19, 35–42. [Google Scholar] [CrossRef]
- Rineau, E.; Chaudet, A.; Chassier, C.; Bizot, P.; Lasocki, S. Implementing a blood management protocol during the entire perioperative period allows a reduction in transfusion rate in major orthopedic surgery: A before-after study. Transfusion 2016, 56, 673–681. [Google Scholar] [CrossRef]
- Alsaleh, K.; Alotaibi, G.S.; Almodaimegh, H.S.; Aleem, A.A.; Kouroukis, C.T. The use of preoperative erythropoiesis-stimulating agents (ESAs) in patients who underwent knee or hip arthroplasty: A meta-analysis of randomized clinical trials. J. Arthroplast. 2013, 28, 1463–1472. [Google Scholar] [CrossRef]
- Munoz, M.; Gomez-Ramirez, S.; Cuenca, J.; Garcia-Erce, J.A.; Iglesias-Aparicio, D.; Haman-Alcober, S.; Ariza, D.; Naveira, E. Very-short-term perioperative intravenous iron administration and postoperative outcome in major orthopedic surgery: A pooled analysis of observational data from 2547 patients. Transfusion 2014, 54, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Na, H.S.; Shin, S.Y.; Hwang, J.Y.; Jeon, Y.T.; Kim, C.S.; Do, S.H. Effects of intravenous iron combined with low-dose recombinant human erythropoietin on transfusion requirements in iron-deficient patients undergoing bilateral total knee replacement arthroplasty (CME). Transfusion 2011, 51, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Theusinger, O.M.; Leyvraz, P.F.; Schanz, U.; Seifert, B.; Spahn, D.R. Treatment of iron deficiency anemia in orthopedic surgery with intravenous iron: Efficacy and limits: A prospective study. Anesthesiology 2007, 107, 923–927. [Google Scholar] [CrossRef] [PubMed]
- Cuenca, J.; Garcia-Erce, J.A.; Martinez, F.; Perez-Serrano, L.; Herrera, A.; Munoz, M. Perioperative intravenous iron, with or without erythropoietin, plus restrictive transfusion protocol reduce the need for allogeneic blood after knee replacement surgery. Transfusion 2006, 46, 1112–1119. [Google Scholar] [CrossRef]
- Munoz, M.; Gomez-Ramirez, S.; Garcia-Erce, J.A. Intravenous iron in inflammatory bowel disease. World J. Gastroenterol. 2009, 15, 4666–4674. [Google Scholar] [CrossRef]
- Garcia-Erce, J.A.; Cuenca, J.; Martinez, F.; Cardona, R.; Perez-Serrano, L.; Munoz, M. Perioperative intravenous iron preserves iron stores and may hasten the recovery from post-operative anaemia after knee replacement surgery. Transfus. Med. 2006, 16, 335–341. [Google Scholar] [CrossRef]
- Koskenkorva-Frank, T.S.; Weiss, G.; Koppenol, W.H.; Burckhardt, S. The complex interplay of iron metabolism, reactive oxygen species, and reactive nitrogen species: Insights into the potential of various iron therapies to induce oxidative and nitrosative stress. Free Radic. Biol. Med. 2013, 65, 1174–1194. [Google Scholar] [CrossRef]
- Muñoz, M.; García-Erce, J.; Cuenca, J.; Bisbe, E.; Naveira, E. On the role of iron therapy for reducing allogeneic blood transfusion in orthopaedic surgery. Blood Transfus. 2012, 10, 8–22. [Google Scholar] [CrossRef]
- Frew, N.; Alexander, D.; Hood, J.; Acornley, A. Impact of a blood management protocol on transfusion rates and outcomes following total hip and knee arthroplasty. Ann. R. Coll. Surg. Engl. 2016, 98, 380–386. [Google Scholar] [CrossRef] [Green Version]
- Kei, T.; Mistry, N.; Curley, G.; Pavenski, K.; Shehata, N.; Tanzini, R.M.; Gauthier, M.F.; Thorpe, K.; Schweizer, T.A.; Ward, S.; et al. Efficacy and safety of erythropoietin and iron therapy to reduce red blood cell transfusion in surgical patients: A systematic review and meta-analysis. Can. J. Anesth./J. Can. D’anesth. 2019, 66, 716–731. [Google Scholar] [CrossRef]
- Zauber, N.P.; Zauber, A.G.; Gordon, F.J.; Tillis, A.C.; Leeds, H.C.; Berman, E.; Kudryk, A.B. Iron supplementation after femoral head replacement for patients with normal iron stores. JAMA 1992, 267, 525–527. [Google Scholar] [CrossRef] [PubMed]
- Biesma, D.H.; Van de Wiel, A.; Beguin, Y.; Kraaijenhagen, R.J.; Marx, J.J. Post-operative erythropoiesis is limited by the inflammatory effect of surgery on iron metabolism. Eur. J. Clin. Investig. 1995, 25, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Khalafallah, A.A.; Hyppa, A.; Chuang, A.; Hanna, F.; Wilson, E.; Kwok, C.; Yan, C.; Gray, Z.; Mathew, R.; Falloon, P.; et al. A prospective randomised controlled trial of a single intravenous infusion of ferric carboxymaltose vs. single intravenous iron polymaltose or daily oral ferrous sulphate in the treatment of iron deficiency anaemia in pregnancy. Semin. Hematol. 2018, 55, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Munoz, M.; Acheson, A.G.; Bisbe, E.; Butcher, A.; Gomez-Ramirez, S.; Khalafallah, A.A.; Kehlet, H.; Kietaibl, S.; Liumbruno, G.M.; Meybohm, P.; et al. An international consensus statement on the management of postoperative anaemia after major surgical procedures. Anaesthesia 2018, 73, 1418–1431. [Google Scholar] [CrossRef] [Green Version]
- van Iperen, C.E.; Kraaijenhagen, R.J.; Biesma, D.H.; Beguin, Y.; Marx, J.J.; van de Wiel, A. Iron metabolism and erythropoiesis after surgery. Br. J. Surg. 1998, 85, 41–45. [Google Scholar] [CrossRef] [Green Version]
- Munoz, M.; Breymann, C.; Garcia-Erce, J.A.; Gomez-Ramirez, S.; Comin, J.; Bisbe, E. Efficacy and safety of intravenous iron therapy as an alternative/adjunct to allogeneic blood transfusion. Vox Sang. 2008, 94, 172–183. [Google Scholar] [CrossRef]
- Parker, M.J. Iron supplementation for anemia after hip fracture surgery: A randomized trial of 300 patients. J. Bone Jt. Surg. Am. 2010, 92, 265–269. [Google Scholar] [CrossRef]
- Kulnigg, S.; Stoinov, S.; Simanenkov, V.; Dudar, L.V.; Karnafel, W.; Garcia, L.C.; Sambuelli, A.M.; D’Haens, G.; Gasche, C. A novel intravenous iron formulation for treatment of anemia in inflammatory bowel disease: The ferric carboxymaltose (FERINJECT) randomized controlled trial. Am. J. Gastroenterol. 2008, 103, 1182–1192. [Google Scholar] [CrossRef]
- Munoz, M.; Garcia-Erce, J.A.; Remacha, A.F. Disorders of iron metabolism. Part 1: Molecular basis of iron homoeostasis. J. Clin. Pathol. 2011, 64, 281–286. [Google Scholar] [CrossRef]
- Ganzoni, A.M. Intravenous iron-dextran: Therapeutic and experimental possibilities. Schweiz. Med. Wochenschr. 1970, 100, 301–303. [Google Scholar]
| Characteristic | Group F (n = 29) | Group C (n = 29) |
|---|---|---|
| Age (yr) | 65.1 (±10.2) | 62.0 (±9.5) |
| Female | 19 (65.5%) | 21 (72.4%) |
| Height (cm) | 156.9 (±7.1) | 158.2 (±6.4) |
| Weight (kg) | 64.2 (±9.8) | 67.1 (±11.8) |
| BMI (kg/m2) | 26.2 (±4.0) | 26.9 (±4.8) |
| Diabetes mellitus | 4 (13.8%) | 5 (17.2%) |
| Hypertension | 13 (44.8%) | 14 (48.3%) |
| Total hip arthroplasty | 19 (65.5%) | 20 (69.0%) |
| Total knee arthroplasty | 10 (34.5%) | 9 (31.0%) |
| Regional anesthesia | 21 (72.4%) | 17 (58.6%) |
| Anesthesia time (min) | 164.6 (±23.7) | 160.3 (±24.8) |
| Operation time (min) | 136.1 (±21.9) | 129.4 (±21.2) |
| Crystalloid (ml) | 950.0 [650.0] | 750.0 [600.0] |
| Tranexamic acid | 19 (65.5%) | 20 (69.0%) |
| Group F (n = 29) | Group C (n = 29) | p-Value | |
|---|---|---|---|
| Patients transfused | 2 (6.9%) | 4 (12.8%) | 0.670 |
| Postoperative anemia | |||
| POD1 | 25 (86.2%) | 24 (82.7%) | 0.717 |
| POD5 | 28 (96.6%) | 26 (89.7%) | 0.300 |
| POD30 | 9 (31.0%) | 9 (31.0%) | 1.000 |
| Hb (g/dl) | |||
| Baseline | 12.5 (±1.3) | 13.4 (±1.1) | 0.007 |
| POD1 | 10.3 (±1.4) | 10.7 (±1.3) | 0.195 |
| POD5 | 9.5 (±1.2) | 9.9 (±1.6) | 0.318 |
| POD30 | 12.8 (±1.3) | 12.6 (±1.1) | 0.433 |
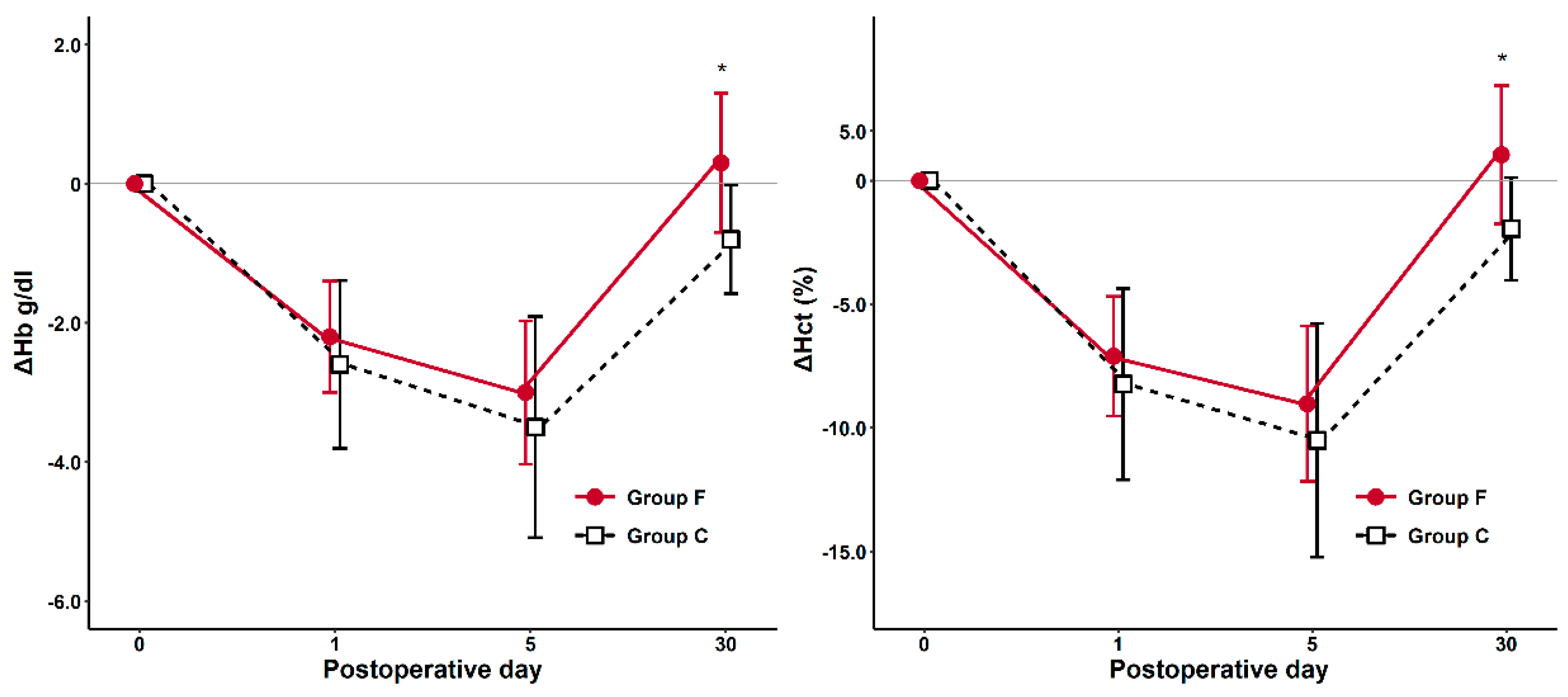
| ΔHb1 | −2.2 (±0.80) | −2.6 (±1.21) | 0.133 |
| ΔHb5 | −3.0 (±1.03) | −3.5 (±1.59) | 0.156 |
| ΔHb30 | 0.3 (±1.00) | −0.8 (±0.78) | <0.001 |
| Hct (%) | |||
| Baseline | 38.1 (±4.0) | 40.5 (±3.0) | 0.014 |
| POD1 | 31.0 (±4.2) | 32.2 (±4.1) | 0.272 |
| POD5 | 29.1 (±3.8) | 29.9 (±4.8) | 0.484 |
| POD30 | 39.2 (±3.9) | 38.5 (±3.1) | 0.470 |
| ΔHct1 | −7.1 (±2.42) | −8.23 (±3.87) | 0.190 |
| ΔHct5 | −9.02 (±3.14) | −10.5 (±4.73) | 0.153 |
| ΔHct30 | 1.04 (±2.80) | −2.0 (±2.10) | <0.001 |
| Preoperative | Postoperative Day5 | |||||
|---|---|---|---|---|---|---|
| Group F | Group C | p-Value | Group F | Group C | p-Value | |
| Ferritin (ng/mL) | 90.6 [82.5] | 87.0 [105.2] | 0.846 | 1248.1 [494.8] | 170.8 [157.2] | < 0.001 |
| TIBC (ug/dL) | 287.0 [55.0] | 288.0 [49.0] | 0.803 | 220.0 [51.0] | 236.0 [58.5] | 0.103 |
| Iron (ug/dL) | 92.4 [37.0] | 101.0 [46.5] | 0.146 | 76.0 [33.0] | 30.0 [30.5] | < 0.001 |
| TSAT (%) | 29.4 [16.3] | 33.2 [17.2] | 0.118 | 38.1 [26.7] | 13.7 [12.0] | < 0.001 |
| Absolute iron deficiency | 2 (6.9%) | 2 (6.9%) | 1 | 0 | 3 (10.3%) | 0.237 |
| Functional iron deficiency | 0 | 0 | 1 | 0 | 14 (48.3%) | < 0.001 |
| CRP | 0.10 [0.16] | 0.11 [0.15] | 0.560 | 7.49 [2.92] | 7.95 [4.63] | 0.936 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.-S.; Kim, T.-Y.; Kim, H.-J.; Ro, Y.-J.; Jang, H.-Y.; Koh, W.U. The Effect of Intraoperative Ferric Carboxymaltose in Joint Arthroplasty Patients: A Randomized Trial. J. Clin. Med. 2019, 8, 1674. https://doi.org/10.3390/jcm8101674
Park H-S, Kim T-Y, Kim H-J, Ro Y-J, Jang H-Y, Koh WU. The Effect of Intraoperative Ferric Carboxymaltose in Joint Arthroplasty Patients: A Randomized Trial. Journal of Clinical Medicine. 2019; 8(10):1674. https://doi.org/10.3390/jcm8101674
Chicago/Turabian StylePark, Hee-Sun, Tae-Yop Kim, Ha-Jung Kim, Young-Jin Ro, Hwa-Young Jang, and Won Uk Koh. 2019. "The Effect of Intraoperative Ferric Carboxymaltose in Joint Arthroplasty Patients: A Randomized Trial" Journal of Clinical Medicine 8, no. 10: 1674. https://doi.org/10.3390/jcm8101674





