Abstract
One of the recent advancements in the field of cardiac electrophysiology is pulsed field ablation (PFA). PFA is a novel energy modality that does not rely on thermal processes to achieve ablation which, in turn, results in limited collateral damage to surrounding structures. In this review, we discuss the mechanisms, safety, efficacy, and clinical applications of PFA for the management of atrial and ventricular arrhythmias. We also summarize the published pre-clinical and clinical studies regarding this new technology.
1. Introduction
Electroporation involves the application of strong pulses of electric fields over a short period of time (micro- or nano-seconds). This results in disruption of cellular homeostasis, increase in cell membrane permeability, apoptosis, and cell death. The earliest clinical applications of electroporation included electrochemotherapy, gene transfer, and the ablation of solid tumors. In recent years, electroporation has earned substantial attention in the field of cardiac electrophysiology and has been shown to be a promising technique for ablation of cardiac arrhythmias. In this review, we discuss the mechanisms of pulsed field ablation (PFA) and review the evidence behind its safety and efficacy for the management of atrial and ventricular arrhythmias.
2. Methods
A systematic search was performed in PubMed regarding cardiac electroporation using the search words ‘electroporation AND atrial OR electroporation AND ventricular OR irreversible electroporation OR IRE OR pulsed field ablation’ for studies published in English.
3. Mechanisms of PFA
Electroporation is achieved by applying high voltages or currents across electrodes. In 1982, Scheinman et al. first described the use of high-energy direct current (DC) shock for ablation of the atrioventricular node in humans []. The main difference between electroporation and procedures in the 1980s is the lower current density at the electrode surface, decreasing the risks of arcing and barotrauma. Electroporation can be reversible or irreversible. The induced electric field results in the accumulation and redistribution of charges across the cell membrane, the formation of pores in the cell membrane, and the loss of cellular homeostasis. If the cell cannot return to its normal function, it then dies via various programmed cell death pathways (Figure 1). The mechanism of pore formation during electroporation was studied using molecular dynamic simulations and explained by local electric field gradients at the water–lipid interface []. These effects result in water defects penetrating the bilayer interior, which further increases the local electric field [] (Figure 2, Video S1). On a cellular level, the exact mechanism of cell death is not fully understood, but it has been attributed to adenosine triphosphate (ATP) depletion, proteolysis, and calcium overload. The apoptotic effect of electroporation depends on several factors, including the energy delivery parameters, the electrode–tissue interface, and the tissue characteristics (Figure 3). It is worth noting that even though cardiac electroporation is often described as a non-thermal ablation modality, there is still some tissue heating with the high-voltage pulses delivered, which is proportional to the product of the local electric field and current density [].
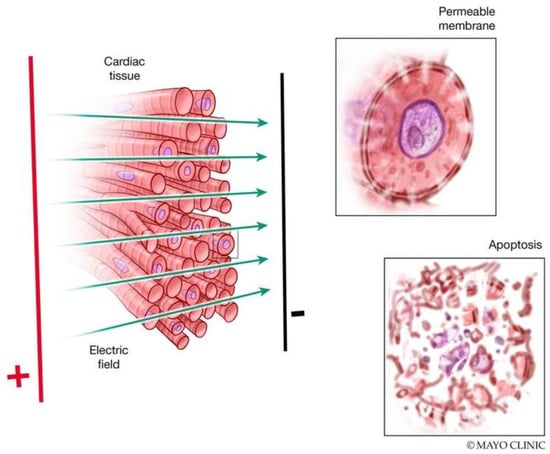
Figure 1.
Mechanisms of electroporation. Delivery of a strong pulsed electric field (PEF) results in pore formation and increased cell membrane permeability. These changes may be reversible with a return to normal cell function or irreversible with progression to cell death.
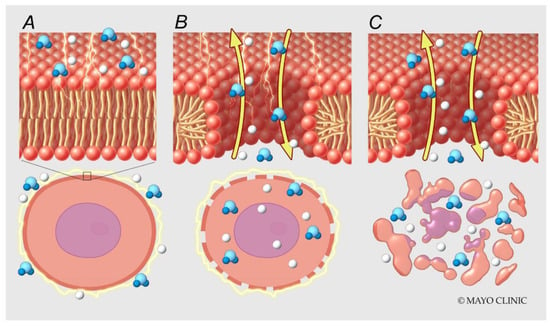
Figure 2.
Molecular mechanisms of electroporation. High voltage is applied across electrodes (A). Water molecules move along local electric field gradients resulting in pore formation (B) and cell death (C).
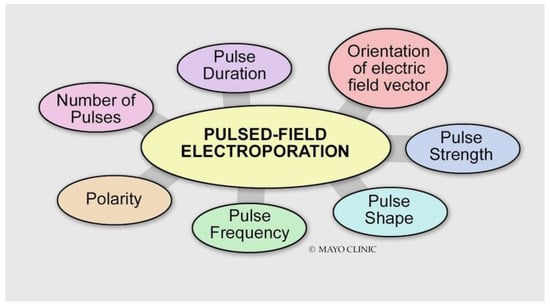
Figure 3.
Various energy delivery parameters can affect the magnitude of the electroporation effect.
Energy Delivery Parameters
- Voltage: Different types of tissue have different electroporation thresholds []. Atrial cardiomyocytes have lower electroporation thresholds than surrounding structures (400 V/cm) []. Increasing the voltage increases the electroporation effect.
- Pulse duration: Increasing the pulse duration increases the magnitude of the electric field delivered, which results in a larger electroporation effect.
- Number of pulses (frequency): The efficacy of biphasic PFA is dependent on the frequency of the waveform. Higher frequencies result in smaller PFA lesions []. This may be explained by the decreased ability of the applied electric field to generate a sufficiently high transmembrane potential for electroporation to occur.
- Biphasic versus monophasic: Monophasic energy delivery results in a larger electroporation effect as compared with biphasic energy delivery at the expense of substantial skeletal muscle, diaphragmatic engagement, and pain [,,].
- Bipolar versus unipolar: A unipolar configuration creates deeper lesions at the expense of significant skeletal muscle contraction and pain.
Other factors that can affect lesion size:
- Contact: Effective PFA is dependent on proximity, but not necessarily greater electrode contact force to the target tissue. Hence, PFA may be more successful at ablating trabeculated regions and sites without optimal contact and stability. Even though PFA is not contact-dependent per se, several studies have shown that at constant pulsed electric field current and pulse duration, lesion depth increased significantly with increasing contact force [,].
- Electrode surface area: The smaller the surface area of the catheter electrode is, the greater the electroporation effect.
- Electrode arrangement: Lesion depth is greater with an ablation hoop than with a single ablation electrode. The large total electrode surface area of a multielectrode hoop catheter decreases the risk of arcing. The current delivered by a multielectrode hoop is predominantly directed outward rather than to the center of the hoop [].
- Cellular orientation in relation to the direction of the electric field: Using nanosecond pulses, there is a greater electroporation effect with a perpendicular orientation than parallel. With millisecond pulses, there is a greater electroporation with a parallel orientation than perpendicular. Using microsecond pulses, cells of both orientations were electroporated to the same extent [].
Although considerable progress has been made with moving PFA from bench to bedside for the management of atrial arrhythmias, there are limited data on PFA for ventricular ablation. Most devices are currently geared towards thin-walled atria, and thicker ventricular tissue may require different PFA configurations. For instance, bipolar ablation in the atria may not be as contact-dependent but ventricular tissue may benefit from unipolar ablation which is more contact-dependent.
4. Efficacy of PFA
4.1. PFA for Atrial Arrhythmias
Table 1 and Table 2 summarize the main findings of pre-clinical and clinical studies assessing the outcomes of cardiac electroporation for atrial ablation and the management of atrial arrhythmias. In 2007, Lavee et al. first described the intentional use of irreversible electroporation (IRE) as an ablation energy source for surgical epicardial atrial ablation []. Since then, several studies using different catheters, pulsed electric field (PEF) generators, and setups have shown the efficacy of IRE in creating transmural atrial lesions while sparing adjacent structures.

Table 1.
Summary of pre-clinical studies on atrial PFA.

Table 2.
Summary of clinical studies on atrial PFA.
In 2018, Reddy et al. reported the first clinical experience with PFA for the management of paroxysmal atrial fibrillation (AF) []. This study showed that PEF-based ablation of the pulmonary veins (PVs) and left atrium (LA) was safe and feasible, whether it was performed endocardially or epicardially. In 2019, the results of the IMPULSE (A Safety and Feasibility Study of the IOWA Approach Endocardial Ablation System to Treat Atrial Fibrillation) and PEFCAT (A Safety and Feasibility Study of the FARAPULSE (Boston Scientific) Endocardial Ablation System to Treat Paroxysmal Atrial Fibrillation) trials were published []. The ablation protocol underwent consecutive modifications: from monophasic to biphasic pulses, followed by optimization of the biphasic waveform morphology and pulse sequence composition with improvement in durable PVI success rates at 3-month follow-up. The 12-month freedom from arrhythmia was 87.4 ± 5.6% []. These findings were in line with the 1-year outcomes of the IMPULSE, PEFCAT, and PEFCAT II (Expanded Safety and Feasibility Study of the FARAPULSE Endocardial Multi Ablation System to Treat Paroxysmal Atrial Fibrillation) studies [].
In 2020, Reddy et al. demonstrated that PFA was safe and feasible for the management of persistent AF with durable lesions on remapping at two and a half-month follow-up []. The PVs and LA posterior wall remained isolated in 96 and 100% of the cases, respectively []. Isolation was defined by entrance block. Similarly, Schiavone et al. showed that LA posterior wall isolation (PWI) using PFA is safe and feasible in patients with persistent AF []. During a median follow-up of 273 days, 41 (16.5%) patients had an arrhythmic recurrence with no differences noted among the ablation strategies (PVI only versus PVI + LAPWI) []. Mitral isthmus ablation has also been reported to be feasible with the FARAPULSE PFA system (Boston Scientific) with a 4.4% risk of coronary artery spasm []. However, this catheter appears to be less suited for ablation of the mitral isthmus and the anterior line as compared with the LA posterior wall and roof [].
In 2022, the PUSLED AF Pilot trial was published showing the safety and feasibility of acute PVI with the PulseSelect PFA system (Medtronic) in patients with paroxysmal as well as persistent AF []. This was followed by the PULSED AF Pivotal trial in 2023, which showed a freedom from arrhythmia recurrence of 66.2% in patients with paroxysmal AF and 55.1% in patients with persistent AF at 1 year []. The rate of primary safety adverse events was low at 0.7% [].
In a recent meta-analysis by Aldaas et al., which included six comparative studies and a total of 1012 patients comparing PFA to other thermal energy sources, PFA was associated with shorter procedural times and longer fluoroscopy times []. There was no difference in periprocedural complications or rates of recurrent AF between the two groups []. This was consistent with the results of the ADVENT trial, which is a randomized controlled trial that compared PFA to conventional thermal ablation []. The trial showed that in patients with paroxysmal AF undergoing catheter ablation, PFA was non-inferior to conventional thermal ablation with respect to freedom from a composite of initial procedural failure, documented atrial tachyarrhythmia after a 3-month blanking period, antiarrhythmic drug use, cardioversion, or repeat ablation and with respect to periprocedural serious adverse events at 1 year []. When compared to cryoballoon PVI in particular, PFA had similar acute and chronic success rates but was associated with a shorter procedure time and no phrenic nerve palsies []. The longer fluoroscopy time with PFA is expected to improve with the integration of PFA systems with three-dimensional mapping systems [].
4.2. PFA for Ventricular Arrhythmias
Table 3 and Table 4 summarize the main findings of pre-clinical and clinical studies assessing the outcomes of cardiac electroporation for ventricular ablation and management of ventricular arrhythmias. In experimental animal studies, epicardial electroporation has been shown to be feasible and effective at creating transmural ventricular lesions with adequate contact, which is not surprising in the absence of a blood pool that causes significant current leakage [,,]. However, it remains unclear whether electroporation can create significant myocardial lesions through thick areas of epicardial fat. Because the mechanism of lesion formation in electroporation is not primarily dependent on thermal injury, there is no sparing of myocardial injury surrounding arteries. The ability of epicardial PFA to produce transmural lesions while preserving the coronary arteries is highly appealing [,,].

Table 3.
Summary of pre-clinical studies on ventricular PFA.

Table 4.
Summary of clinical studies on ventricular PFA.
Regarding endocardial ablation, several animal studies have shown that PFA is effective at creating transmural lesions in healthy ventricular myocardia as well as scarred ventricular myocardia, whether the scar was due to a prior infarction or ablation [,]. In scars, lesion depth with PFA was greater than radiofrequency ablation (RFA) []. PFA of common locations of ventricular arrhythmias including the interventricular septum, papillary muscles, and left ventricular summit via the distal coronary sinus has also been shown to be feasible and effective at creating deep lesions [,,]. In the left ventricular summit and papillary muscles, PFA resulted in larger lesions and fewer steam pops as compared with RFA [].
5. Safety of PFA
Figure 4 summarizes the main energy-specific adverse effects that have been reported with PFA. Based on the MANIFEST-17K study, which included 17,642 patients with AF undergoing PFA, PFA energy-specific adverse events included transient phrenic nerve paresis (0.06%), coronary spasm (0.14%), and hemolysis-related renal failure (0.03%) [].
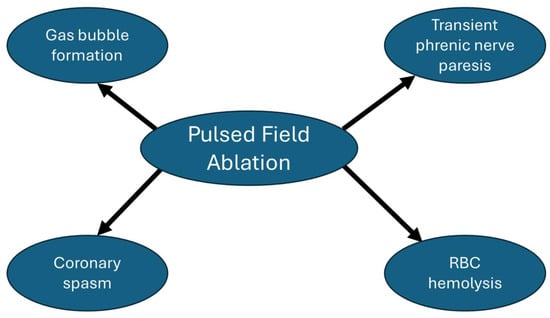
Figure 4.
Pulsed field ablation energy-specific adverse effects.
5.1. Gas Bubble Formation
Gas bubble formation is commonly reported with PFA and seen on intraprocedural intracardiac echocardiography imaging. Potential explanations for gas bubble formation with PFA include heating, electrolysis, and the de-gasification or displacement of gases from the blood. In patients treated with PFA for the management of AF, the incidence of asymptomatic thromboembolic cerebral events or lesions detected on brain imaging ranged between 3 and 9% [,]. The amount of gas bubble formation depends on several parameters of energy delivery. It is directly related to the delivered charge. Anodal IRE applications result in less gas formation than cathodal IRE applications and radiofrequency applications []. Sub-RFA alternating currents (AC) pulses have also been shown to avoid electrolysis-induced gas bubble formation [].
5.2. Phrenic Nerve Injury
Reported findings in the literature regarding the effect of electroporation on nerves are variable with some describing a minimal effect [] and others describing transient damage with recovery at 7 weeks []. The preservation of the endoneurium architecture and proliferation of Schwann cells seen histologically post-ablation reflect the potential for axonal regeneration [,]. Van Driel et al. demonstrated that energy levels that could create myocardial lesions spared the phrenic nerve without histologic or functional evidence of phrenic nerve damage []. The proximity of the catheter to the phrenic nerve and the PFA dose level are predictors of phrenic nerve response to PFA []. Recently, Ollitrault et al. reported transient phrenic nerve stunning without phrenic nerve palsy at the end of the procedure and at hospital discharge in 64% of patients undergoing isolation of the superior vena cava using a pentaspline PFA catheter [].
5.3. Esophageal Injury
Neven et al. demonstrated that the esophageal architecture was unaffected 2 months after delivering IRE directly to the esophageal adventitia []. Similarly, Song et al. showed no histopathologic changes to the esophagus at 4 weeks and 16 weeks after monophasic, bipolar IRE of the esophagus [,]. In an in vivo porcine esophageal injury model, ablation at areas of esophageal contact resulted in no histopathologic esophageal changes with PFA and a spectrum of esophageal lesions with RFA []. In patients with AF undergoing PFA, a dose-dependent rise in esophageal temperature has been reported []. The long-term implications of this finding need further evaluation.
5.4. Coronary Artery Damage
Animal studies showed that epicardial IRE did not result in coronary vessel luminal narrowing at 3 weeks and 3 months post-ablation [,]. Conversely, intracoronary PFA can lead to fixed coronary stenosis []. Furthermore, there have been several reports of coronary artery spasm noted transiently post-PFA [,,]. The exact mechanism of coronary artery spasm post-PFA is not entirely known. Potential explanations include transient activation of vascular smooth muscles by the delivered PEF []. This phenomenon is responsive to nitroglycerin administration either pre- or post-ablation [,].
5.5. Pulmonary Vein Stenosis
In animal studies, multiple circumferential electroporation applications inside the ostia of the pulmonary veins (PVs) did not result in PV narrowing or stenosis at 1-month and 3-month follow-up whereas RFA did [,]. In patients with AF undergoing catheter ablation, Kuroki et al. demonstrated that the incidence and severity of pulmonary vein (PV) narrowing and stenosis were significantly lower with PFA as compared to RFA []. This may be explained by mechanistic differences in the ablation and healing processes between PFA and RFA.
5.6. Hemolysis
Lysis of red blood cells is common with PFA and has been shown to occur in a dose-dependent manner []. In a study comparing the risk of hemolysis during PVI with PFA versus RFA, significant renal injury was uncommon with a number of 70 PFA lesions []. The PFA system that was used in this study was the Farapulse PFA system (Boston Scientific).
6. PFA versus Conventional Ablation Modalities
Table 5 summarizes the technical aspects of the two PFA systems that are currently available on the market in the US. PFA has several advantages and disadvantages when compared to thermal ablation modalities such as RFA and cryoablation. It has been shown to carry a lower risk for collateral damage, reducing complications such as esophageal injury and pulmonary vein stenosis. Furthermore, it can induce immediate cell death, and this rapid response may shorten procedure times compared to RFA and cryoablation, which require time to reach effective thermal thresholds.

Table 5.
Comparison of the pulsed field ablation systems approved for pulmonary vein isolation.
As PFA technology is still evolving, there is less standardization in devices and protocols compared to the well-established RFA and cryoablation systems. Unlike thermal modalities that often allow for real-time temperature and electrogram monitoring, PFA lacks direct indicators for successful ablation during the procedure, making it more difficult to assess the completeness of lesion formation in real-time. Furthermore, while PFA carries a lower risk for collateral damage, there is still a risk of unintended effects, such as coronary spasms and gas bubble formation, which require further research and the refinement of ablation parameters to mitigate. Lastly, the PFA systems available currently use larger sheaths which carry a higher risk for vascular access issues, cardiac perforation, and air emboli.
7. Future Directions
Despite the high level of enthusiasm, there are still many aspects of cardiac electroporation that need to be understood and optimized. The notions of the non-thermal nature and tissue selectivity of cardiac electroporation have been debunked. Future research should aim to enhance our ability to titrate the ablative effects of cardiac electroporation and distinguish between acute outcomes of reversible versus IRE. Additionally, efforts should focus on mitigating potential energy-specific adverse effects, including gas bubble formation and coronary spasm, optimizing PFA configurations for ventricular ablation, and integration of PFA systems with mapping systems.
8. Conclusions
In conclusion, cardiac electroporation represents a promising new modality in the field of cardiac electrophysiology, offering potential safety advantages over traditional ablation techniques. By leveraging the mechanisms of reversible and IRE, this technology provides a novel approach to achieve precise and targeted tissue ablation with minimal collateral damage. Early studies and clinical trials assessing the outcomes of PFA for the management of arrhythmias have demonstrated encouraging results. However, further research is needed to optimize PFA protocols, understand long-term effects, and establish comprehensive guidelines for clinical application. As the field continues to evolve, cardiac electroporation may become a valuable addition to the therapeutic arsenal for treating cardiac arrhythmias.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm13175191/s1, Video S1: Mechanisms of cardiac electroporation.
Author Contributions
Writing—original draft preparation, F.M.E.; writing—review and editing, S.J.A. and D.T.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
All authors have no conflicts of interest to disclose relevant to the content of this manuscript.
References
- Scheinman, M.M.; Morady, F.; Hess, D.S.; Gonzalez, R. Catheter-induced ablation of the atrioventricular junction to control refractory supraventricular arrhythmias. Jama 1982, 248, 851–855. [Google Scholar] [CrossRef]
- Tieleman, D.P. The molecular basis of electroporation. BMC Biochem. 2004, 5, 10. [Google Scholar] [CrossRef]
- Davalos, R.V.; Mir, I.L.; Rubinsky, B. Tissue ablation with irreversible electroporation. Ann. Biomed. Eng. 2005, 33, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Avazzadeh, S.; Dehkordi, M.H.; Owens, P.; Jalali, A.; O’Brien, B.; Coffey, K.; O’Halloran, M.; Fernhead, H.O.; Keane, D.; Quinlan, L.R. Establishing electroporation thresholds for targeted cell specific cardiac ablation in a 2D culture model. J. Cardiovasc. Electrophysiol. 2022, 33, 2050–2061. [Google Scholar] [CrossRef] [PubMed]
- Tabaja, C.; Younis, A.; Hussein, A.A.; Taigen, T.L.; Nakagawa, H.; Saliba, W.I.; Wazni, O.M. Catheter-Based Electroporation: A Novel Technique for Catheter Ablation of Cardiac Arrhythmias. JACC Clin. Electrophysiol. 2023, 9, 2008–2023. [Google Scholar] [CrossRef] [PubMed]
- García-Sánchez, T.; Amorós-Figueras, G.; Jorge, E.; Campos, M.C.; Maor, E.; Guerra, J.M.; Ivorra, A. Parametric Study of Pulsed Field Ablation with Biphasic Waveforms in an In Vivo Heart Model: The Role of Frequency. Circ. Arrhythm. Electrophysiol. 2022, 15, e010992. [Google Scholar] [CrossRef]
- Thomson, K.R.; Cheung, W.; Ellis, S.J.; Federman, D.; Kavnoudias, H.; Loader-Oliver, D.; Roberts, S.; Evans, P.; Ball, C.; Haydon, A. Investigation of the safety of irreversible electroporation in humans. J. Vasc. Interv. Radiol. 2011, 22, 611–621. [Google Scholar] [CrossRef]
- Arena, C.B.; Sano, M.B.; Rossmeisl, J.H., Jr.; Caldwell, J.L.; Garcia, P.A.; Rylander, M.N.; Davalos, R.V. High-frequency irreversible electroporation (H-FIRE) for non-thermal ablation without muscle contraction. Biomed. Eng. Online. 2011, 10, 102. [Google Scholar] [CrossRef]
- Nakagawa, H.; Ikeda, A.; Yokoyama, K.; An, Y.; Hussein, A.A.; Saliba, W.I.; Wazni, O.M.; Castellvi, Q. Improvement in Lesion Formation with Radiofrequency Energy and Utilization of Alternate Energy Sources (Cryoablation and Pulsed Field Ablation) for Ventricular Arrhythmia Ablation. Card. Electrophysiol. Clin. 2022, 14, 757–767. [Google Scholar] [CrossRef]
- Di Biase, L.; Marazzato, J.; Govari, A.; Altman, A.; Beeckler, C.; Keyes, J.; Sharma, T.; Grupposo, V.; Zou, F.; Sugawara, M.; et al. Pulsed Field Ablation Index-Guided Ablation for Lesion Formation: Impact of Contact Force and Number of Applications in the Ventricular Model. Circ. Arrhythm. Electrophysiol. 2024, 17, e012717. [Google Scholar] [CrossRef]
- Wittkampf, F.H.; van Driel, V.J.; van Wessel, H.; Neven, K.G.; Gründeman, P.F.; Vink, A.; Loh, P.; Doevendans, P.A. Myocardial lesion depth with circular electroporation ablation. Circ. Arrhythm. Electrophysiol. 2012, 5, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Dermol-Černe, J.; Batista Napotnik, T.; Reberšek, M.; Miklavčič, D. Short microsecond pulses achieve homogeneous electroporation of elongated biological cells irrespective of their orientation in electric field. Sci. Rep. 2020, 10, 9149. [Google Scholar] [CrossRef] [PubMed]
- Lavee, J.; Onik, G.; Mikus, P.; Rubinsky, B. A novel nonthermal energy source for surgical epicardial atrial ablation: Irreversible electroporation. Heart Surg. Forum. 2007, 10, E162–E167. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.T.; Haines, D.E.; Verma, A.; Kirchhof, N.; Barka, N.; Grassl, E.; Howard, B. Intracardiac pulsed field ablation: Proof of feasibility in a chronic porcine model. Heart Rhythm. 2019, 16, 754–764. [Google Scholar] [CrossRef]
- Ye, X.; Liu, S.; Yin, H.; He, Q.; Xue, Z.; Lu, C.; Su, S. Study on Optimal Parameter and Target for Pulsed-Field Ablation of Atrial Fibrillation. Front. Cardiovasc. Med. 2021, 8, 690092. [Google Scholar] [CrossRef]
- Stewart, M.T.; Haines, D.E.; Miklavčič, D.; Kos, B.; Kirchhof, N.; Barka, N.; Mattison, L.; Martien, M.; Onal, B.; Howard, B.; et al. Safety and chronic lesion characterization of pulsed field ablation in a Porcine model. J. Cardiovasc. Electrophysiol. 2021, 32, 958–969. [Google Scholar] [CrossRef]
- Hsu, J.C.; Gibson, D.; Banker, R.; Doshi, S.K.; Gidney, B.; Gomez, T.; Berman, D.; Datta, K.; Govari, A.; Natale, A. In Vivo porcine characterization of atrial lesion safety and efficacy utilizing a circular pulsed-field ablation catheter including assessment of collateral damage to adjacent tissue in supratherapeutic ablation applications. J. Cardiovasc. Electrophysiol. 2022, 33, 1480–1488. [Google Scholar] [CrossRef]
- Koruth, J.; Verma, A.; Kawamura, I.; Reinders, D.; Andrade, J.G.; Deyell, M.W.; Reddy, V.Y. PV Isolation Using a Spherical Array PFA Catheter: Preclinical Assessment and Comparison to Radiofrequency Ablation. JACC Clin. Electrophysiol. 2023, 9, 652–666. [Google Scholar] [CrossRef]
- Reddy, V.Y.; Koruth, J.; Jais, P.; Petru, J.; Timko, F.; Skalsky, I.; Neuzil, P. Ablation of Atrial Fibrillation with Pulsed Electric Fields: An Ultra-Rapid, Tissue-Selective Modality for Cardiac Ablation. JACC Clin. Electrophysiol. 2018, 4, 987–995. [Google Scholar] [CrossRef]
- Reddy, V.Y.; Neuzil, P.; Koruth, J.S.; Petru, J.; Funosako, M.; Cochet, H.; Jais, P. Pulsed Field Ablation for Pulmonary Vein Isolation in Atrial Fibrillation. J. Am. Coll. Cardiol. 2019, 74, 315–326. [Google Scholar] [CrossRef]
- Reddy, V.Y.; Anic, A.; Koruth, J.; Petru, J.; Funasako, M.; Minami, K.; Neuzil, P. Pulsed Field Ablation in Patients with Persistent Atrial Fibrillation. J. Am. Coll. Cardiol. 2020, 76, 1068–1080. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.Y.; Anter, E.; Rackauskas, G.; Peichl, P.; Koruth, J.S.; Petru, J.; Neuzil, P. Lattice-Tip Focal Ablation Catheter That Toggles between Radiofrequency and Pulsed Field Energy to Treat Atrial Fibrillation: A First-in-Human Trial. Circ. Arrhythm. Electrophysiol. 2020, 13, e008718. [Google Scholar] [CrossRef]
- Loh, P.; van Es, R.; Groen, M.H.A.; Neven, K.; Kassenberg, W.; Wittkampf, F.H.M.; Doevendans, P.A. Pulmonary Vein Isolation with Single Pulse Irreversible Electroporation: A First in Human Study in 10 Patients with Atrial Fibrillation. Circ. Arrhythm. Electrophysiol. 2020, 13, e008192. [Google Scholar] [CrossRef]
- Reddy, V.Y.; Dukkipati, S.R.; Neuzil, P.; Anic, A.; Petru, J.; Funasako, M.; Jais, P. Pulsed Field Ablation of Paroxysmal Atrial Fibrillation: 1-Year Outcomes of IMPULSE, PEFCAT, and PEFCAT II. JACC Clin. Electrophysiol. 2021, 7, 614–627. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, I.; Neuzil, P.; Shivamurthy, P.; Kuroki, K.; Lam, J.; Musikantow, D.; Chu, E.; Turagam, M.K.; Minami, K.; Funasako, M.; et al. How does the level of pulmonary venous isolation compare between pulsed field ablation and thermal energy ablation (radiofrequency, cryo, or laser)? Europace. 2021, 23, 1757–1766. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, I.; Neuzil, P.; Shivamurthy, P.; Petru, J.; Funasako, M.; Minami, K.; Kuroki, K.; Dukkipati, S.R.; Koruth, J.S.; Reddy, V.Y. Does pulsed field ablation regress over time? A quantitative temporal analysis of pulmonary vein isolation. Heart Rhythm. 2021, 18, 878–884. [Google Scholar] [CrossRef]
- Verma, A.; Boersma, L.; Haines, D.E.; Natale, A.; Marchlinski, F.E.; Sanders, P.; Calkins, H.; Packer, D.L.; Hummel, J.; Onal, B.; et al. First-in-Human Experience and Acute Procedural Outcomes Using a Novel Pulsed Field Ablation System: The PULSED AF Pilot Trial. Circ. Arrhythm. Electrophysiol. 2022, 15, e010168. [Google Scholar] [CrossRef]
- Kueffer, T.; Seiler, J.; Madaffari, A.; Mühl, A.; Asatryan, B.; Stettler, R.; Haeberlin, A.; Noti, F.; Servatius, H.; Tanner, H.; et al. Pulsed-field ablation for the treatment of left atrial reentry tachycardia. J. Interv. Card. Electrophysiol. 2023, 66, 1431–1440. [Google Scholar] [CrossRef]
- Verma, A.; Haines, D.E.; Boersma, L.V.; Sood, N.; Natale, A.; Marchlinski, F.E.; Calkins, H.; Sanders, P.; Packer, D.L.; Kuck, K.-H.; et al. Pulsed Field Ablation for the Treatment of Atrial Fibrillation: PULSED AF Pivotal Trial. Circulation 2023, 147, 1422–1432. [Google Scholar] [CrossRef]
- Davong, B.; Adeliño, R.; Delasnerie, H.; Albenque, J.P.; Combes, N.; Cardin, C.; Boveda, S. Pulsed-Field Ablation on Mitral Isthmus in Persistent Atrial Fibrillation: Preliminary Data on Efficacy and Safety. JACC Clin. Electrophysiol. 2023, 9, 1070–1081. [Google Scholar] [CrossRef]
- Urbanek, L.; Bordignon, S.; Schaack, D.; Chen, S.; Tohoku, S.; Efe, T.H.; Ebrahimi, R.; Pansera, F.; Hirokami, J.; Plank, K.; et al. Pulsed Field Versus Cryoballoon Pulmonary Vein Isolation for Atrial Fibrillation: Efficacy, Safety, and Long-Term Follow-Up in a 400-Patient Cohort. Circ. Arrhythm. Electrophysiol. 2023, 16, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.Y.; Gerstenfeld, E.P.; Natale, A.; Whang, W.; Cuoco, F.A.; Patel, C.; Mountantonakis, S.E.; Gibson, D.N.; Harding, J.D.; Ellis, C.R.; et al. Pulsed Field or Conventional Thermal Ablation for Paroxysmal Atrial Fibrillation. N. Engl. J. Med. 2023, 389, 1660–1671. [Google Scholar] [CrossRef] [PubMed]
- Schiavone, M.; Solimene, F.; Moltrasio, M.; Casella, M.; Bianchi, S.; Iacopino, S.; Rossillo, A.; Schillaci, V.; Fassini, G.; Compagnucci, P.; et al. Pulsed field ablation technology for pulmonary vein and left atrial posterior wall isolation in patients with persistent atrial fibrillation. J. Cardiovasc. Electrophysiol. 2024, 35, 1101–1111. [Google Scholar] [CrossRef]
- Duytschaever, M.; De Potter, T.; Grimaldi, M.; Anic, A.; Vijgen, J.; Neuzil, P.; Van Herendael, H.; Verma, A.; Skanes, A.; Scherr, D.; et al. Paroxysmal Atrial Fibrillation Ablation Using a Novel Variable-Loop Biphasic Pulsed Field Ablation Catheter Integrated with a 3-Dimensional Mapping System: 1-Year Outcomes of the Multicenter inspIRE Study. Circ. Arrhythm. Electrophysiol. 2023, 16, e011780. [Google Scholar] [CrossRef] [PubMed]
- Turagam, M.K.; Neuzil, P.; Schmidt, B.; Reichlin, T.; Neven, K.; Metzner, A.; Hansen, J.; Blaauw, Y.; Maury, P.; Arentz, T.; et al. Safety and Effectiveness of Pulsed Field Ablation to Treat Atrial Fibrillation: One-Year Outcomes from the MANIFEST-PF Registry. Circulation 2023, 148, 35–46. [Google Scholar] [CrossRef]
- Aldaas, O.M.; Malladi, C.; Han, F.T.; Hoffmayer, K.S.; Krummen, D.; Ho, G.; Raissi, F.; Birgersdotter-Green, U.; Feld, G.K.; Hsu, J.C. Pulsed field ablation versus thermal energy ablation for atrial fibrillation: A systematic review and meta-analysis of procedural efficiency, safety, and efficacy. J. Interv. Card. Electrophysiol. 2024, 67, 639–648. [Google Scholar] [CrossRef]
- du Pré, B.C.; van Driel, V.J.; van Wessel, H.; Loh, P.; Doevendans, P.A.; Goldschmeding, R.; Wittkampf, F.H.; Vink, A. Minimal coronary artery damage by myocardial electroporation ablation. Europace 2013, 15, 144–149. [Google Scholar] [CrossRef]
- Neven, K.; van Driel, V.; van Wessel, H.; van Es, R.; Doevendans, P.A.; Wittkampf, F. Myocardial lesion size after epicardial electroporation catheter ablation after subxiphoid puncture. Circ. Arrhythm. Electrophysiol. 2014, 7, 728–733. [Google Scholar] [CrossRef]
- Neven, K.; van Driel, V.; van Wessel, H.; van Es, R.; du Pré, B.; Doevendans, P.A.; Wittkampf, F. Safety and feasibility of closed chest epicardial catheter ablation using electroporation. Circ. Arrhythm. Electrophysiol. 2014, 7, 913–919. [Google Scholar] [CrossRef]
- Neven, K.; van Driel, V.; van Wessel, H.; van Es, R.; Doevendans, P.A.; Wittkampf, F. Epicardial linear electroporation ablation and lesion size. Heart Rhythm. 2014, 11, 1465–1470. [Google Scholar] [CrossRef]
- Livia, C.; Sugrue, A.; Witt, T.; Polkinghorne, M.D.; Maor, E.; Kapa, S.; Lehmann, H.I.; DeSimone, C.V.; Behfar, A.; Asirvatham, S.J.; et al. Elimination of Purkinje Fibers by Electroporation Reduces Ventricular Fibrillation Vulnerability. J. Am. Heart Assoc. 2018, 7, e009070. [Google Scholar] [CrossRef] [PubMed]
- Azarov, J.E.; Semenov, I.; Casciola, M.; Pakhomov, A.G. Excitation of murine cardiac myocytes by nanosecond pulsed electric field. J. Cardiovasc. Electrophysiol. 2019, 30, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Koruth, J.S.; Kuroki, K.; Iwasawa, J.; Viswanathan, R.; Brose, R.; Buck, E.D.; Donskoy, E.; Dukkipati, S.R.; Reddy, V.Y. Endocardial ventricular pulsed field ablation: A proof-of-concept preclinical evaluation. Europace 2020, 22, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Neven, K.; van Driel, V.J.H.M.; Vink, A.; du Pré, B.C.; van Wessel, H.; Füting, A.; Doevendans, P.A.; Wittkampf, F.H.M.; van Es, R. Characteristics and time course of acute and chronic myocardial lesion formation after electroporation ablation in the porcine model. J Cardiovasc Electrophysiol. 2022, 33, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, I.; Reddy, V.Y.; Wang, B.J.; Dukkipati, S.R.; Chaudhry, H.W.; Santos-Gallego, C.G.; Koruth, J.S. Pulsed Field Ablation of the Porcine Ventricle Using a Focal Lattice-Tip Catheter. Circ. Arrhythm. Electrophysiol. 2022, 15, e011120. [Google Scholar] [CrossRef]
- Im, S.I.; Higuchi, S.; Lee, A.; Stillson, C.; Buck, E.; Morrow, B.; Schenider, K.; Speltz, M.; Gerstenfeld, E.P. Pulsed Field Ablation of Left Ventricular Myocardium in a Swine Infarct Model. JACC Clin. Electrophysiol. 2022, 8, 722–731. [Google Scholar] [CrossRef]
- van Zyl, M.; Ladas, T.P.; Tri, J.A.; Yasin, O.Z.; Ladejobi, A.O.; Tan, N.Y.; Asirvatham, S.J. Bipolar Electroporation Across the Interventricular Septum: Electrophysiological, Imaging, and Histopathological Characteristics. JACC Clin. Electrophysiol. 2022, 8, 1106–1118. [Google Scholar] [CrossRef]
- Tan, N.Y.; Ladas, T.P.; Christopoulos, G.; Sugrue, A.M.; van Zyl, M.; Ladejobi, A.O.; Lodhi, F.K.; Hu, T.Y.; Ezzeddine, F.M.; Agboola, K.; et al. Ventricular nanosecond pulsed electric field delivery using active fixation leads: A proof-of-concept preclinical study. J. Interv. Card. Electrophysiol. 2022.
- Verma, A.; Neal, R.; Evans, J.; Castellvi, Q.; Vachani, A.; Deneke, T.; Nakagawa, H. Characteristics of pulsed electric field cardiac ablation porcine treatment zones with a focal catheter. J. Cardiovasc. Electrophysiol. 2023, 34, 99–107. [Google Scholar] [CrossRef]
- Chaigne, S.; Sigg, D.C.; Stewart, M.T.; Hocini, M.; Batista Napotnik, T.; Miklavčič, D.; Bernus, O.; Benoist, D. Reversible and Irreversible Effects of Electroporation on Contractility and Calcium Homeostasis in Isolated Cardiac Ventricular Myocytes. Circ. Arrhythm. Electrophysiol. 2022, 15, e011131. [Google Scholar] [CrossRef]
- Kawamura, I.; Reddy, V.Y.; Santos-Gallego, C.G.; Wang, B.J.; Chaudhry, H.W.; Buck, E.D.; Mavroudis, G.; Jerrell, S.; Schneider, C.W.; Speltz, M.; et al. Electrophysiology, Pathology, and Imaging of Pulsed Field Ablation of Scarred and Healthy Ventricles in Swine. Circ. Arrhythm. Electrophysiol. 2023, 16, e011369. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, I.; Wang, B.J.; Nies, M.; Watanabe, K.; Chaudhry, H.W.; Maejima, Y.; Sasano, T.; Gordon, R.; Dukkipati, S.R.; Reddy, V.Y. Ultrastructural insights from myocardial ablation lesions from microsecond pulsed field vs radiofrequency energy. Heart Rhythm. 2024, 21, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Kos, B.; Mattison, L.; Ramirez, D.; Cindrič, H.; Sigg, D.C.; Iaizzo, P.A.; Miklavčič, D. Determination of lethal electric field threshold for pulsed field ablation in ex vivo perfused porcine and human hearts. Front. Cardiovasc. Med. 2023, 10, 1160231. [Google Scholar] [CrossRef] [PubMed]
- Aryana, A.; Hata, C.; de la Rama, A.; Nguyen, K.; Panescu, D. A novel pulsed field ablation system using linear and spiral ablation catheters can create large and durable endocardial and epicardial ventricular lesions In Vivo. J. Interv. Card. Electrophysiol. 2023. [Google Scholar] [CrossRef]
- Younis, A.; Buck, E.; Santangeli, P.; Tabaja, C.; Garrott, K.; Lehn, L.; Wazni, O.M. Efficacy of Pulsed Field vs Radiofrequency for the Reablation of Chronic Radiofrequency Ablation Substrate: Redo Pulsed Field Ablation. JACC Clin. Electrophysiol. 2024, 10, 222–234. [Google Scholar] [CrossRef]
- Nies, M.; Watanabe, K.; Kawamura, I.; Santos-Gallego, C.G.; Reddy, V.Y.; Koruth, J.S. Preclinical Study of Pulsed Field Ablation of Difficult Ventricular Targets: Intracavitary Mobile Structures, Interventricular Septum, and Left Ventricular Free Wall. Circ. Arrhythm. Electrophysiol. 2024, 17, e012734. [Google Scholar] [CrossRef]
- Younis, A.; Tabaja, C.; Kleve, R.; Garrott, K.; Lehn, L.; Buck, E.; Hussein, A.A.; Nakhla, S.; Nakagawa, H.; Krywanczyk, A.; et al. Comparative Efficacy and Safety of Pulsed Field Ablation Versus Radiofrequency Ablation of Idiopathic LV Arrhythmias. JACC Clin. Electrophysiol. 2024. [CrossRef]
- Schmidt, B.; Chen, S.; Tohoku, S.; Bordignon, S.; Bologna, F.; Chun, K.R.J. Single shot electroporation of premature ventricular contractions from the right ventricular outflow tract. Europace 2022, 24, 597. [Google Scholar] [CrossRef]
- Adragão, P.; Matos, D.; Carmo, P.; Costa, F.M.; Ramos, S. Pulsed-field ablation vs radiofrequency ablation for ventricular tachycardia: First in-human case of histologic lesion analysis. Heart Rhythm. 2023, 20, 1395–1398. [Google Scholar] [CrossRef]
- Hansen, J.; Haugdal, M.A.; Johannessen, A.; Hansen, M.L.; Worck, R.; Ruwald, M.H. Focal pulsed field electroporation of left ventricular premature contractions after failed radiofrequency ablation. Hear. Case Rep. 2023, 9, 581–585. [Google Scholar] [CrossRef]
- Worck, R.; Haugdal, M.A.; Johannessen, A.; Hansen, M.L.; Ruwald, M.H.; Hansen, J. A case of safe and durable focal pulsed-field electroporation treatment of outflow tract premature ventricular contractions. Heart Rhythm. O2 2023, 4, 463–465. [Google Scholar] [CrossRef]
- Ekanem, E.; Neuzil, P.; Reichlin, T.; Kautzner, J.; van der Voort, P.; Jais, P.; Chierchia, G.-B.; Bulava, A.; Blaauw, Y.; Skala, T.; et al. Safety of pulsed field ablation in more than 17,000 patients with atrial fibrillation in the MANIFEST-17K study. Nat. Med. 2024, 30, 2020–2029. [Google Scholar] [CrossRef] [PubMed]
- Reinsch, N.; Füting, A.; Höwel, D.; Bell, J.; Lin, Y.; Neven, K. Cerebral safety after pulsed field ablation for paroxysmal atrial fibrillation. Heart Rhythm. 2022, 19, 1813–1818. [Google Scholar] [CrossRef] [PubMed]
- Patel, C.; Gerstenfeld, E.P.; Gupta, S.K.; Winterfield, J.; Woods, C.; Natale, A.; Reddy, V.Y. Comparison of cerebral safety following atrial fibrillation using pulsed field and thermal ablation: Results of the Neurological Assessment Subgroup in the ADVENT Trial. Heart Rhythm. 2024.
- Groen, M.H.A.; van Es, R.; van Klarenbosch, B.R.; Stehouwer, M.; Loh, P.; Doevendans, P.A.; Neven, K. In Vivo analysis of the origin and characteristics of gaseous microemboli during catheter-mediated irreversible electroporation. Europace 2021, 23, 139–146. [Google Scholar] [CrossRef]
- Ziv, R.; Steinhardt, Y.; Pelled, G.; Gazit, D.; Rubinsky, B. Micro-electroporation of mesenchymal stem cells with alternating electrical current pulses. Biomed. Microdevices. 2009, 11, 95–101. [Google Scholar] [CrossRef]
- Onik, G.; Mikus, P.; Rubinsky, B. Irreversible electroporation: Implications for prostate ablation. Technol. Cancer Res. Treat. 2007, 6, 295–300. [Google Scholar] [CrossRef]
- Li, W.; Fan, Q.; Ji, Z.; Qiu, X.; Li, Z. The effects of irreversible electroporation (IRE) on nerves. PLoS ONE. 2011, 6, e18831. [Google Scholar] [CrossRef]
- Schoellnast, H.; Monette, S.; Ezell, P.C.; Deodhar, A.; Maybody, M.; Erinjeri, J.P.; Stubblefield, M.D.; Single, G.W.; Hamilton, W.C.; Solomon, S.B. Acute and subacute effects of irreversible electroporation on nerves: Experimental study in a pig model. Radiology 2011, 260, 421–427. [Google Scholar] [CrossRef]
- Schoellnast, H.; Monette, S.; Ezell, P.C.; Maybody, M.; Erinjeri, J.P.; Stubblefield, M.D.; Single, G.; Solomon, S.B. The delayed effects of irreversible electroporation ablation on nerves. Eur. Radiol. 2013, 23, 375–380. [Google Scholar] [CrossRef]
- van Driel, V.J.; Neven, K.; van Wessel, H.; Vink, A.; Doevendans, P.A.; Wittkampf, F.H. Low vulnerability of the right phrenic nerve to electroporation ablation. Heart Rhythm. 2015, 12, 1838–1844. [Google Scholar] [CrossRef]
- Howard, B.; Haines, D.E.; Verma, A.; Kirchhof, N.; Barka, N.; Onal, B.; Stewart, M.T.; Sigg, D.C. Characterization of Phrenic Nerve Response to Pulsed Field Ablation. Circ. Arrhythm. Electrophysiol. 2022, 15, e010127. [Google Scholar] [CrossRef]
- Ollitrault, P.; Chaumont, C.; Font, J.; Manninger, M.; Conti, S.; Matusik, P.T.; Mulder, B.A.; Ferchaud, V.; Pellissier, A.; Al Khoury, M.; et al. Superior vena cava isolation using a pentaspline pulsed-field ablation catheter: Feasibility and safety in patients undergoing atrial fibrillation catheter ablation. Europace 2024, 26, euae160. [Google Scholar] [CrossRef] [PubMed]
- Neven, K.; van Es, R.; van Driel, V.; van Wessel, H.; Fidder, H.; Vink, A.; Wittkampf, F. Acute and Long-Term Effects of Full-Power Electroporation Ablation Directly on the Porcine Esophagus. Circ. Arrhythm. Electrophysiol. 2017, 10, e004672. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zheng, J.; Fan, L. Nonthermal Irreversible Electroporation to the Esophagus: Evaluation of Acute and Long-Term Pathological Effects in a Rabbit Model. J. Am. Heart Assoc. 2021, 10, e020731. [Google Scholar] [CrossRef]
- Song, Y.; Yang, L.; He, J.; Zhao, X.; Zheng, J.; Fan, L. Ultra-microhistological study of nonthermal irreversible electroporation on the esophagus. Heart Rhythm. 2023, 20, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Koruth, J.S.; Kuroki, K.; Kawamura, I.; Brose, R.; Viswanathan, R.; Buck, E.D.; Reddy, V.Y. Pulsed Field Ablation Versus Radiofrequency Ablation: Esophageal Injury in a Novel Porcine Model. Circ. Arrhythm. Electrophysiol. 2020, 13, e008303. [Google Scholar] [CrossRef]
- Kirstein, B.; Heeger, C.H.; Vogler, J.; Eitel, C.; Feher, M.; Phan, H.L.; Mushfiq, I.; Traub, A.; Hatahet, S.; Samara, O.; et al. Impact of pulsed field ablation on intraluminal esophageal temperature. J. Cardiovasc. Electrophysiol. 2024, 35, 78–85. [Google Scholar] [CrossRef]
- Ladejobi, A.; Christopoulos, G.; Tan, N.; Ladas, T.P.; Tri, J.; van Zyl, M.; Yasin, O.; Sugrue, A.; Khabsa, M.; Uecker, D.R.; et al. Effects of Pulsed Electric Fields on the Coronary Arteries in Swine. Circ. Arrhythm. Electrophysiol. 2022, 15, e010668. [Google Scholar] [CrossRef]
- Koruth, J.S.; Kawamura, I.; Buck, E.; Jerrell, S.; Brose, R.; Reddy, V.Y. Coronary Arterial Spasm and Pulsed Field Ablation: Preclinical Insights. JACC Clin. Electrophysiol. 2022, 8, 1579–1580. [Google Scholar] [CrossRef]
- Reddy, V.Y.; Petru, J.; Funasako, M.; Kopriva, K.; Hala, P.; Chovanec, M.; Neuzil, P. Coronary Arterial Spasm During Pulsed Field Ablation to Treat Atrial Fibrillation. Circulation 2022, 146, 1808–1819. [Google Scholar] [CrossRef] [PubMed]
- Menè, R.; Boveda, S.; Della Rocca, D.G.; Sousonis, V.; Vetta, G.; Zeriouh, S.; Doundoulakis, I.; Betancur, A.; Benadel, M.; Combes, N.; et al. Efficacy of Intravenous Nitrates for the Prevention of Coronary Artery Spasm During Pulsed Field Ablation of the Mitral Isthmus. Circ. Arrhythm. Electrophysiol. 2024, 17, e012426. [Google Scholar] [CrossRef] [PubMed]
- van Driel, V.J.; Neven, K.G.; van Wessel, H.; du Pré, B.C.; Vink, A.; Doevendans, P.A.; Wittkampf, F.H. Pulmonary vein stenosis after catheter ablation: Electroporation versus radiofrequency. Circ. Arrhythm. Electrophysiol. 2014, 7, 734–738. [Google Scholar] [CrossRef] [PubMed]
- Witt, C.M.; Sugrue, A.; Padmanabhan, D.; Vaidya, V.; Gruba, S.; Rohl, J.; DeSimone, C.V.; Killu, A.M.; Naksuk, N.; Pederson, J.; et al. Intrapulmonary Vein Ablation without Stenosis: A Novel Balloon-Based Direct Current Electroporation Approach. J. Am. Heart Assoc. 2018, 7, e009575. [Google Scholar] [CrossRef]
- Kuroki, K.; Whang, W.; Eggert, C.; Lam, J.; Leavitt, J.; Kawamura, I.; Reddy, A.; Morrow, B.; Schneider, C.; Petru, J.; et al. Ostial dimensional changes after pulmonary vein isolation: Pulsed field ablation vs radiofrequency ablation. Heart Rhythm. 2020, 17, 1528–1535. [Google Scholar] [CrossRef]
- Nies, M.; Koruth, J.S.; Mlček, M.; Watanabe, K.; Tibenská, V.C.; Královec, Š.; Tejkl, L.; Neuzil, P.; Reddy, V.Y. Hemolysis After Pulsed Field Ablation: Impact of Lesion Number and Catheter-Tissue Contact. Circ. Arrhythm. Electrophysiol. 2024, 17, e012765. [Google Scholar] [CrossRef]
- Osmancik, P.; Bacova, B.; Herman, D.; Hozman, M.; Fiserova, I.; Hassouna, S.; Reddy, V.Y. Periprocedural Intravascular Hemolysis During Atrial Fibrillation Ablation: A Comparison of Pulsed Field with Radiofrequency Ablation. JACC Clin. Electrophysiol. 2024, 10, 1660–1671. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).