Author Contributions
Min Huang conceived and supervised the study; Min Huang and Yukun Fang designed experiments; Yukun Fang, Anhong Ning, Shaozheng Zhou, Lei Liu, Mintao Zhong, and Jilong Jiao performed the experiments; Min Huang, Yukun Fang, Thomson Patric Joseph, Yonghui Shi, and Meishan Zhang analyzed and interpreted the data; Yukun Fang, Li Sha, and Wei Zhang wrote and revised the manuscript; Min Huang and Li Sha reviewed the manuscript.
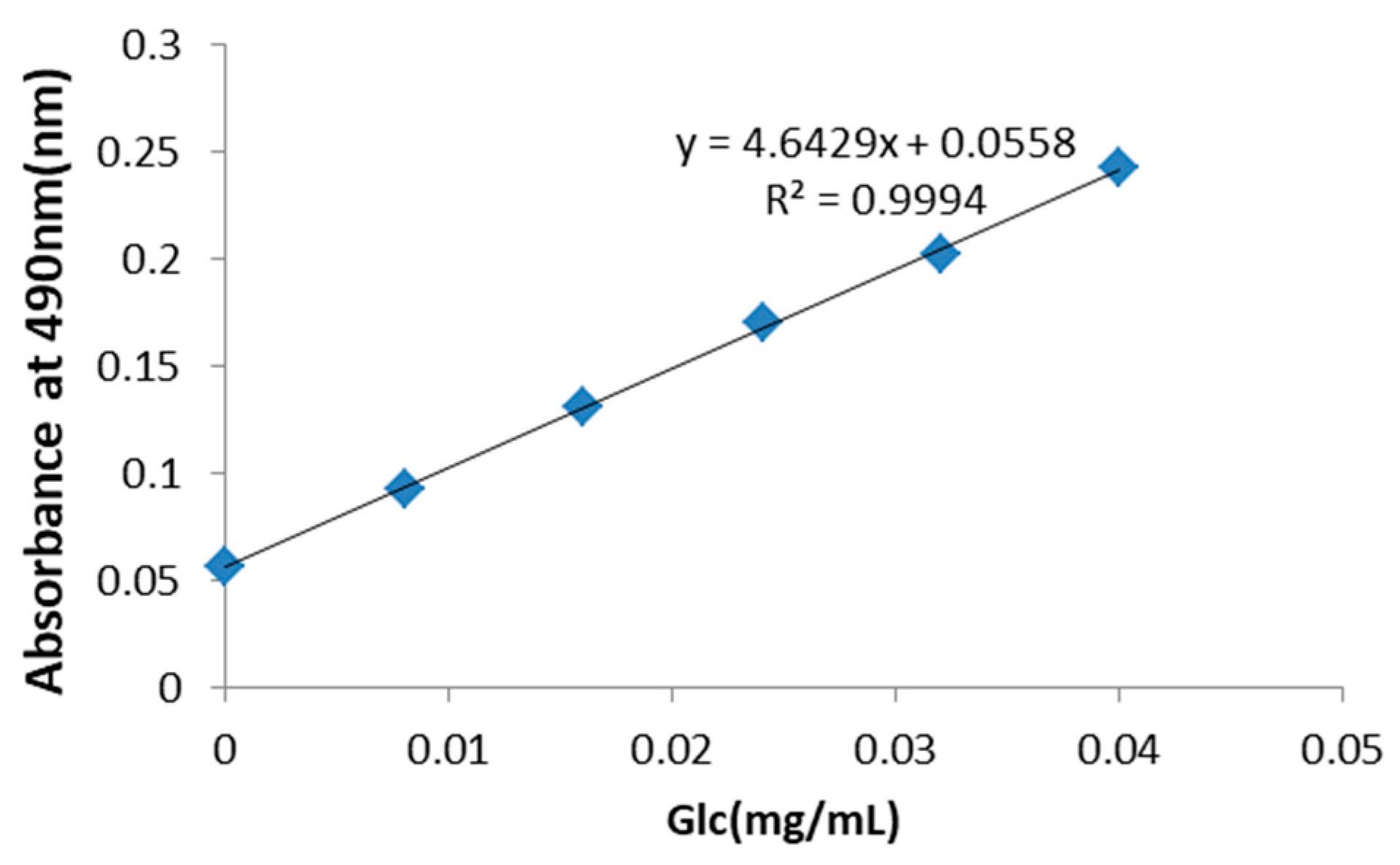
Figure 1.
Standard curve for the determination of the total concentration of polysaccharides in Rhizoma Pleionis (PRP). The total saccharide concentration in PRP was determined to be 30 mg/mL using d-glucose as a standard.
Figure 1.
Standard curve for the determination of the total concentration of polysaccharides in Rhizoma Pleionis (PRP). The total saccharide concentration in PRP was determined to be 30 mg/mL using d-glucose as a standard.
Figure 2.
Effects of PRP treatment on the H22 malignant ascites model (Control group, PRP treatment groups, Cyclophosphamide(CTX) group). (A) H22 malignant ascites mouse model. (B) Abdominal perimeter of mice bearing H22 ascites tumors in five different experimental groups. (C) Mean mouse weight in each group. (D) Mice survival curve in each group. (E) Survival rate of each group by the 50th day. The data are presented as the mean ± SD (n = 8). (* p < 0.05).
Figure 2.
Effects of PRP treatment on the H22 malignant ascites model (Control group, PRP treatment groups, Cyclophosphamide(CTX) group). (A) H22 malignant ascites mouse model. (B) Abdominal perimeter of mice bearing H22 ascites tumors in five different experimental groups. (C) Mean mouse weight in each group. (D) Mice survival curve in each group. (E) Survival rate of each group by the 50th day. The data are presented as the mean ± SD (n = 8). (* p < 0.05).
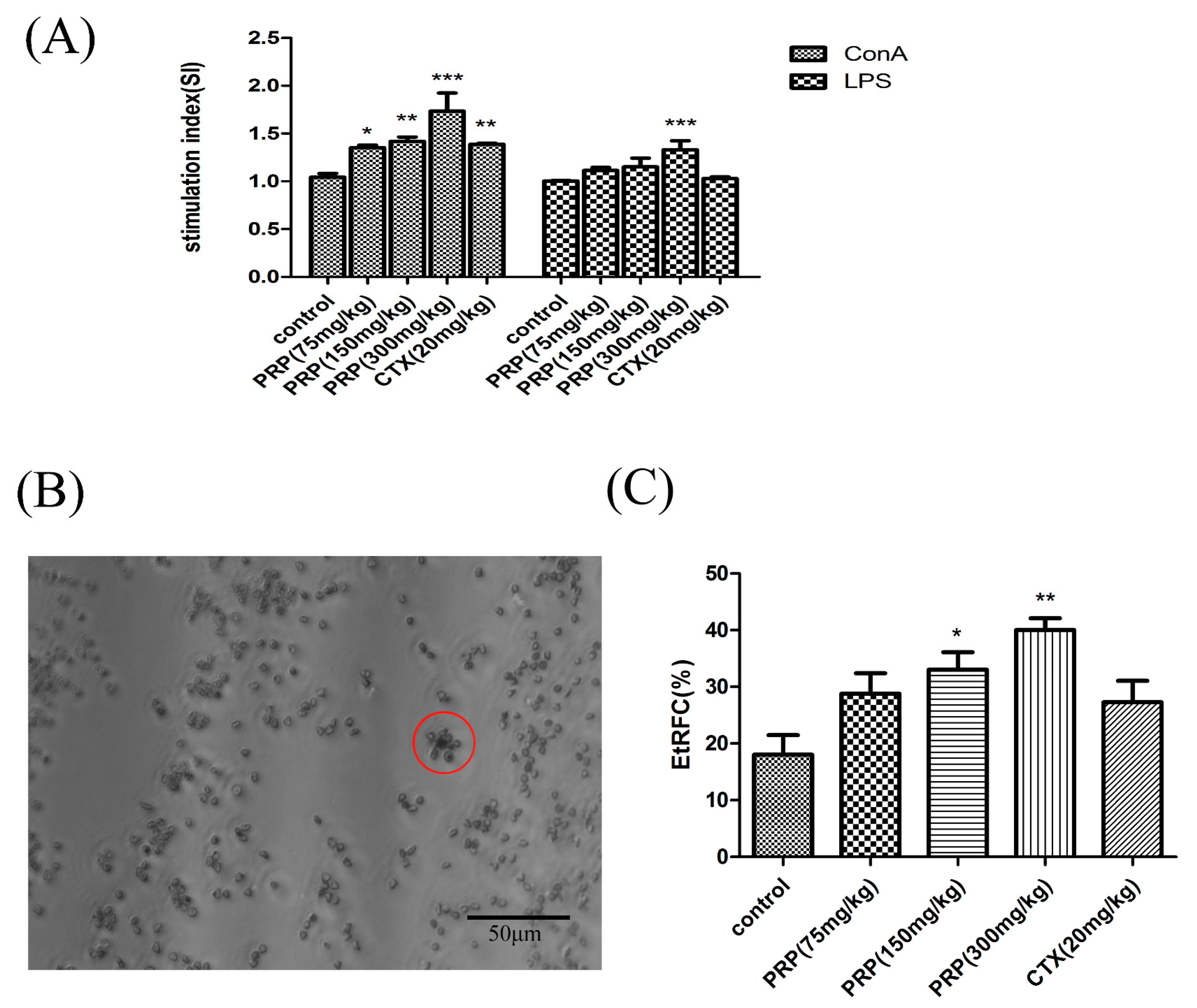
Figure 3.
Effect of PRP treatment on T and B cell activation and proliferation. (A) Effects of PRP on spleen lymphocyte proliferation. (B,C) Garland structure of a sheep red blood cell with several T lymphocytes in red circle and the rate of E-rosette-forming cells (EtRFC) in each experimental group. The ratio of activation and proliferation was significantly higher in the treatment group compared to the control group. The data are shown as mean ± SD (n = 8). (* p < 0.05, ** p < 0.01, *** p < 0.001).
Figure 3.
Effect of PRP treatment on T and B cell activation and proliferation. (A) Effects of PRP on spleen lymphocyte proliferation. (B,C) Garland structure of a sheep red blood cell with several T lymphocytes in red circle and the rate of E-rosette-forming cells (EtRFC) in each experimental group. The ratio of activation and proliferation was significantly higher in the treatment group compared to the control group. The data are shown as mean ± SD (n = 8). (* p < 0.05, ** p < 0.01, *** p < 0.001).
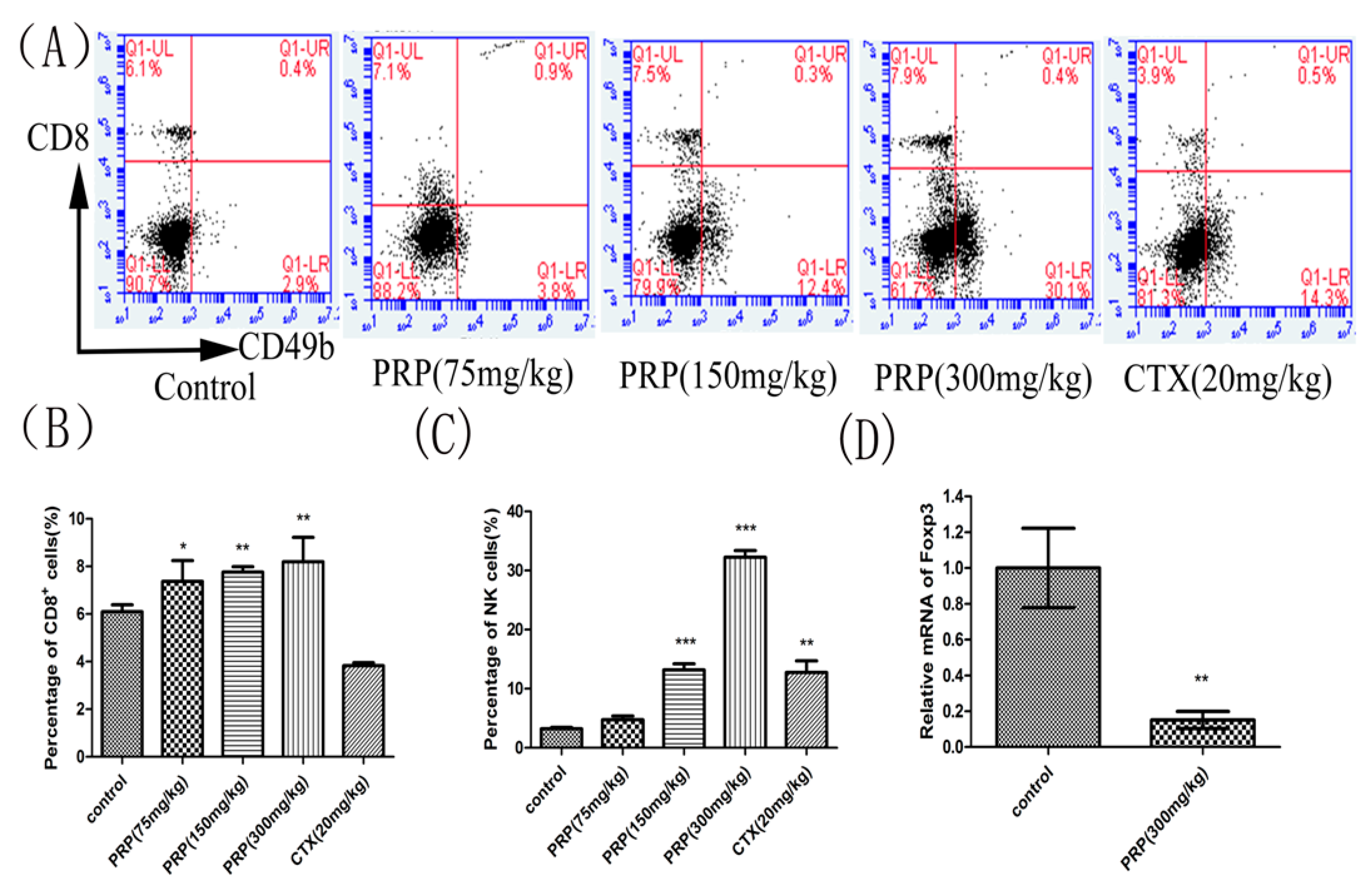
Figure 4.
Effects of PRP treatment on spleen lymphocyte and Foxp3 mRNA expression. (A) Flow cytometry analysis of CD8+ T cells and CD49b+NK cells in different groups. (B,C) Effects of PRP on the percentage of splenic CD8+ T lymphocytes and NK cells. (D) Effects of PRP on spleen lymphocyte relative to Foxp3 mRNA expression. The data are presented as the mean ± SD (n = 8); (* p < 0.05, ** p < 0.01, *** p < 0.001) significantly different compared to the control group.
Figure 4.
Effects of PRP treatment on spleen lymphocyte and Foxp3 mRNA expression. (A) Flow cytometry analysis of CD8+ T cells and CD49b+NK cells in different groups. (B,C) Effects of PRP on the percentage of splenic CD8+ T lymphocytes and NK cells. (D) Effects of PRP on spleen lymphocyte relative to Foxp3 mRNA expression. The data are presented as the mean ± SD (n = 8); (* p < 0.05, ** p < 0.01, *** p < 0.001) significantly different compared to the control group.
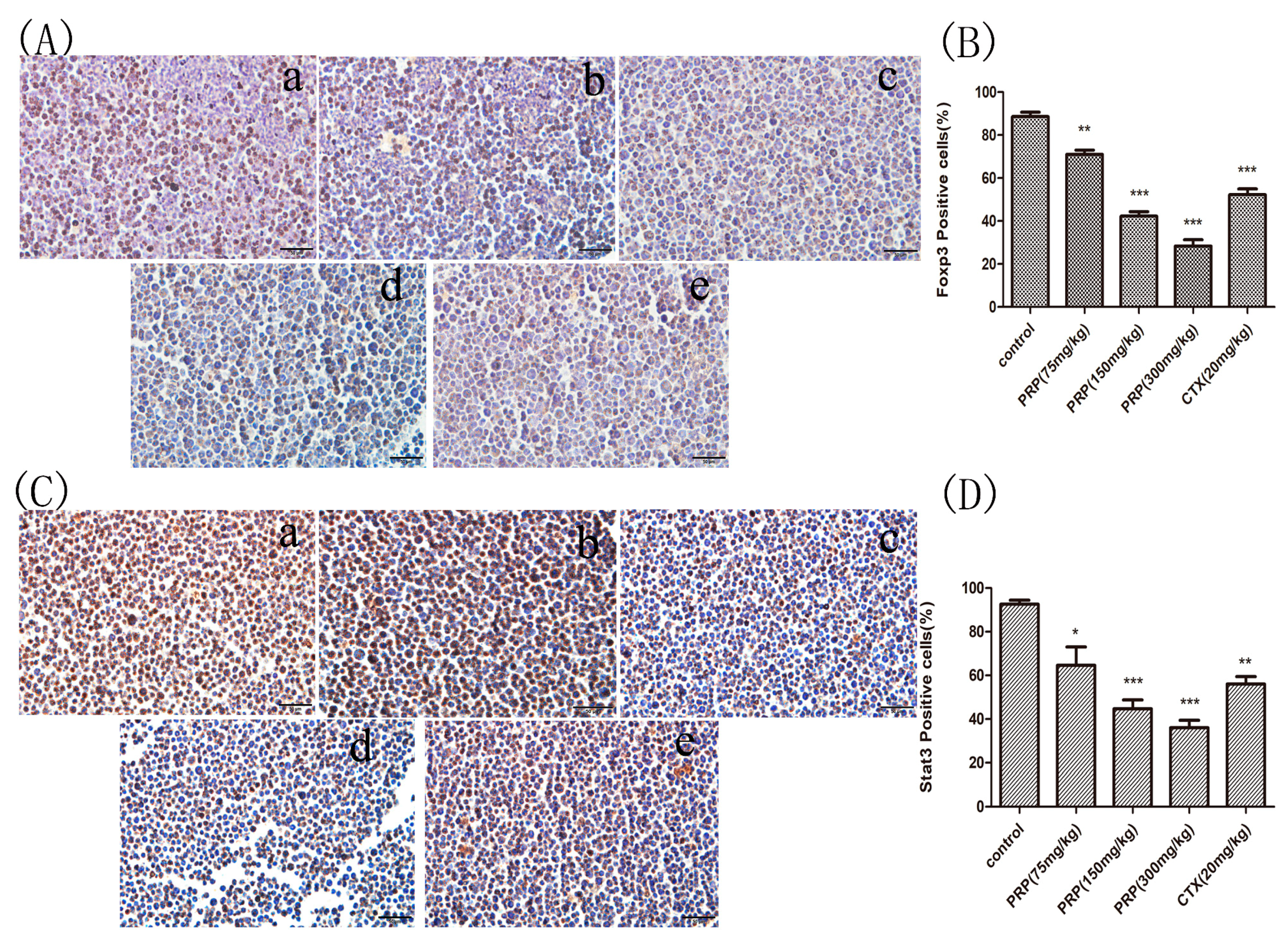
Figure 5.
Foxp3 and Stat3 immunocytochemistry staining in ascites cells from mice. (A,C) Foxp3 and Stat3 immunocytochemistry images of ascites cells from mice in the various groups; (a) Control group; (b) low PRP treatment (75 mg/kg); (c) moderate PRP treatment (150 mg/kg); (d) high PRP treatment (300 mg/kg); (e) CTX (20 mg/kg). (B,D) The number of Foxp3- and Stat3-positive cells (moderate and strong brownish cytoplasmic staining) was calculated in five randomly selected areas in each experimental group as the number of positive cells/total compared to the control group. The data are presented as the mean ± SD (* p < 0.05, ** p < 0.01, *** p < 0.001) Original magnification, ×400.
Figure 5.
Foxp3 and Stat3 immunocytochemistry staining in ascites cells from mice. (A,C) Foxp3 and Stat3 immunocytochemistry images of ascites cells from mice in the various groups; (a) Control group; (b) low PRP treatment (75 mg/kg); (c) moderate PRP treatment (150 mg/kg); (d) high PRP treatment (300 mg/kg); (e) CTX (20 mg/kg). (B,D) The number of Foxp3- and Stat3-positive cells (moderate and strong brownish cytoplasmic staining) was calculated in five randomly selected areas in each experimental group as the number of positive cells/total compared to the control group. The data are presented as the mean ± SD (* p < 0.05, ** p < 0.01, *** p < 0.001) Original magnification, ×400.
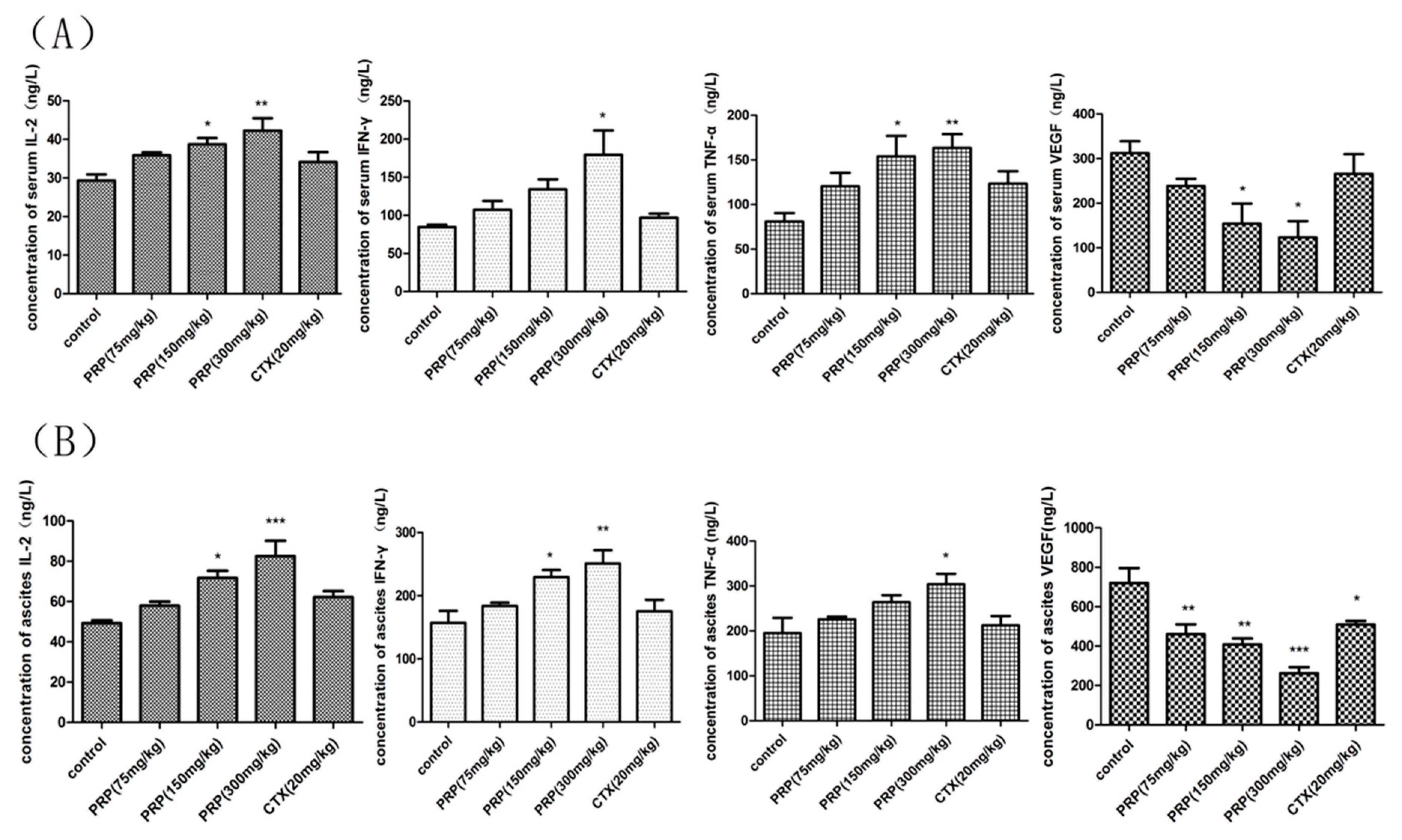
Figure 6.
Effects of PRP on serum and ascites cytokines in different experimental groups. (A) Effects of PRP on serum cytokine levels in mice treated with different PRP concentrations or with CTX. (B) Effects of PRP on ascites cytokine levels in mice treated with different PRP concentrations or with CTX. The data are presented as the mean ± SD (n = 8); (* p < 0.05, ** p < 0.01, *** p < 0.001).
Figure 6.
Effects of PRP on serum and ascites cytokines in different experimental groups. (A) Effects of PRP on serum cytokine levels in mice treated with different PRP concentrations or with CTX. (B) Effects of PRP on ascites cytokine levels in mice treated with different PRP concentrations or with CTX. The data are presented as the mean ± SD (n = 8); (* p < 0.05, ** p < 0.01, *** p < 0.001).
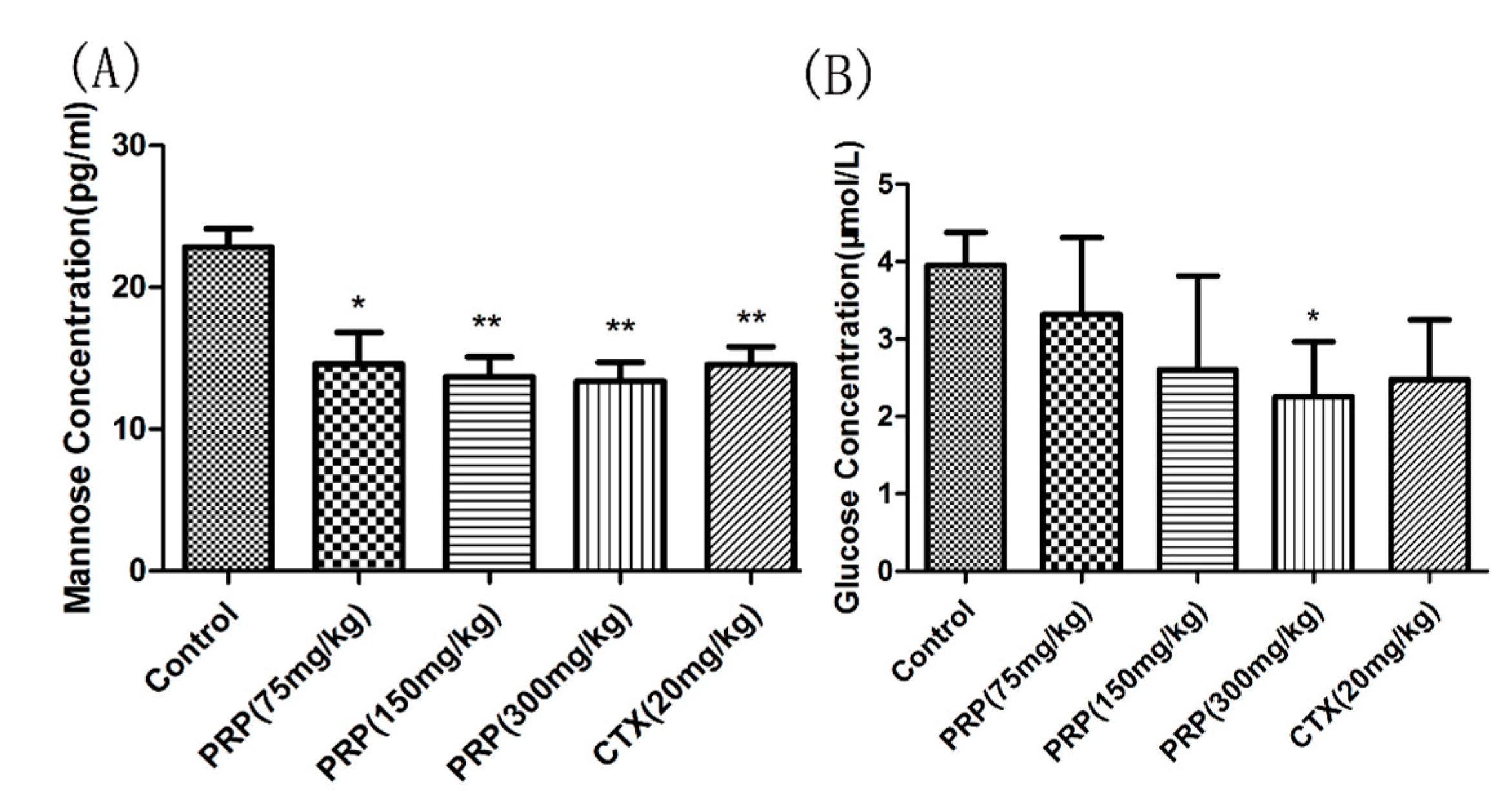
Figure 7.
Mannose (A) and Glucose (B) serum concentrations. The data are means ± SD. (* p < 0.05, ** p < 0.01).
Figure 7.
Mannose (A) and Glucose (B) serum concentrations. The data are means ± SD. (* p < 0.05, ** p < 0.01).
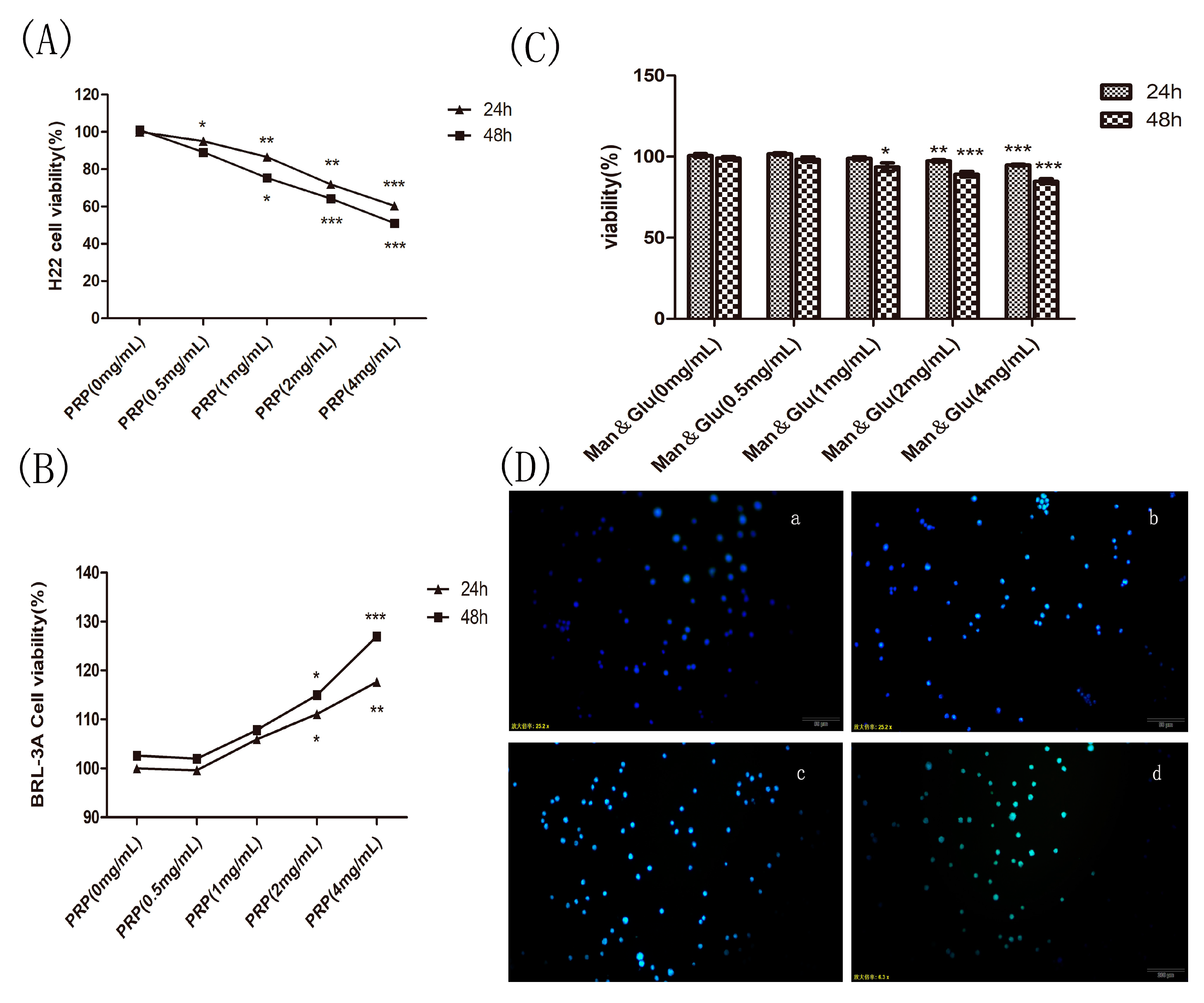
Figure 8.
Effect of PRP and mannose and glucose (Man & Glu) on the proliferation of H22 cells and PRP toxicity test. (A,B) Effects of PRP on H22 cells and BRL-3A normal cell. (C) Effect of Man & Glu on the viability of H22 cells. (D) Fluorescence micrographs of H22 cells stained with Hoechst 33258. The cells were treated with (a) 0 mg/mL, (b) 1 mg/mL, (c) 2 mg/mL, and (d) 4 mg/mL PRP for 24 h (×400 magnification). The data are presented as the mean ± SD (* p < 0.05, ** p < 0.01, *** p < 0.001).
Figure 8.
Effect of PRP and mannose and glucose (Man & Glu) on the proliferation of H22 cells and PRP toxicity test. (A,B) Effects of PRP on H22 cells and BRL-3A normal cell. (C) Effect of Man & Glu on the viability of H22 cells. (D) Fluorescence micrographs of H22 cells stained with Hoechst 33258. The cells were treated with (a) 0 mg/mL, (b) 1 mg/mL, (c) 2 mg/mL, and (d) 4 mg/mL PRP for 24 h (×400 magnification). The data are presented as the mean ± SD (* p < 0.05, ** p < 0.01, *** p < 0.001).
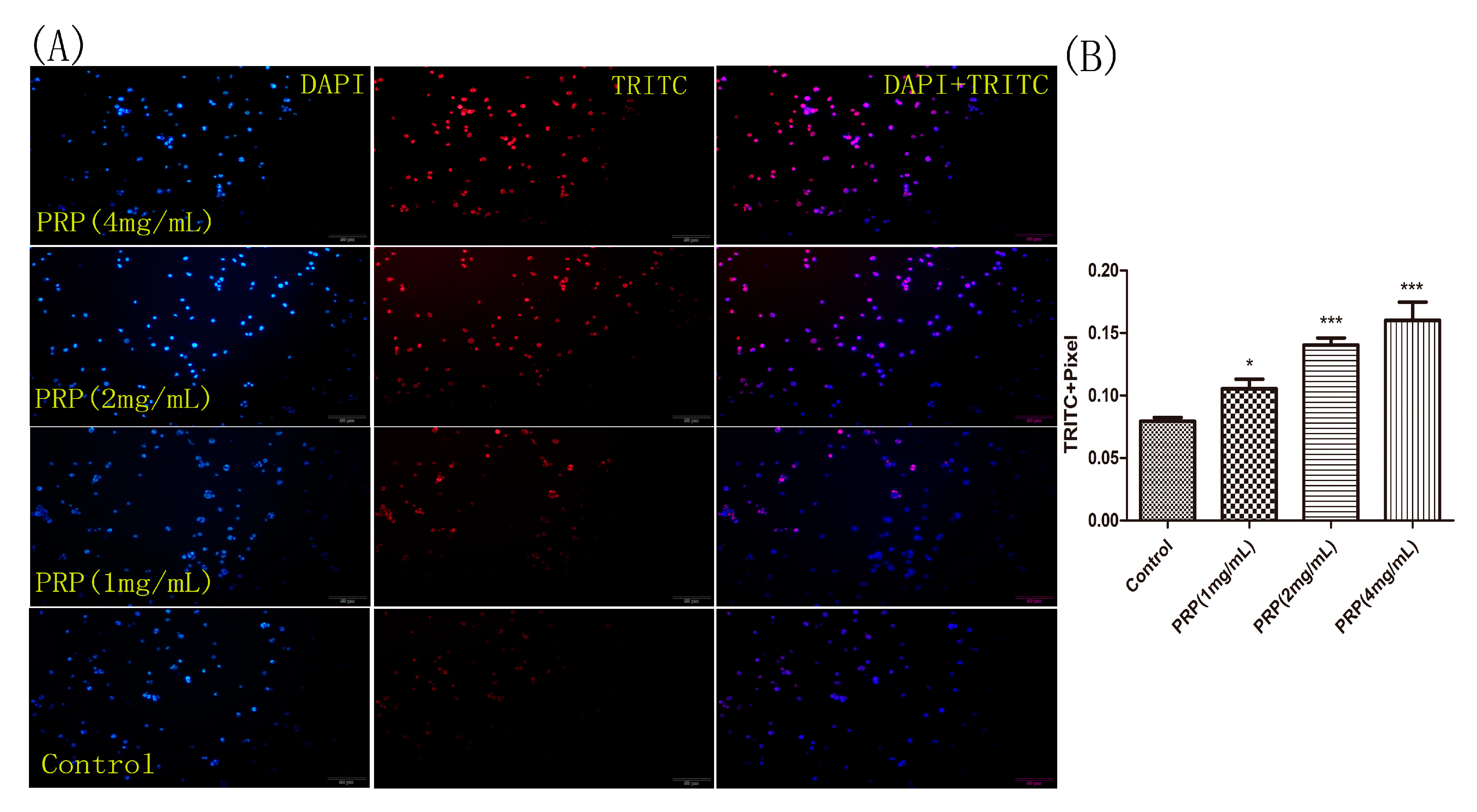
Figure 9.
H22 cell staining for apoptosis using the TUNEL assay. (A) Representative images of cells stained with DAPI (left), TRITC (center), and merged (right). H22 cells were treated with different PRP concentrations for 24 h and then stained using the TUNEL assay. Apoptotic cells were examined by fluorescence microscopy. (×400 magnification) (B) The mean red fluorescence TRITC intensity of TUNEL-positive cells was analyzed by ImageJ software. The data are presented as mean ± SD. (*** p < 0.001, * p < 0.05).
Figure 9.
H22 cell staining for apoptosis using the TUNEL assay. (A) Representative images of cells stained with DAPI (left), TRITC (center), and merged (right). H22 cells were treated with different PRP concentrations for 24 h and then stained using the TUNEL assay. Apoptotic cells were examined by fluorescence microscopy. (×400 magnification) (B) The mean red fluorescence TRITC intensity of TUNEL-positive cells was analyzed by ImageJ software. The data are presented as mean ± SD. (*** p < 0.001, * p < 0.05).
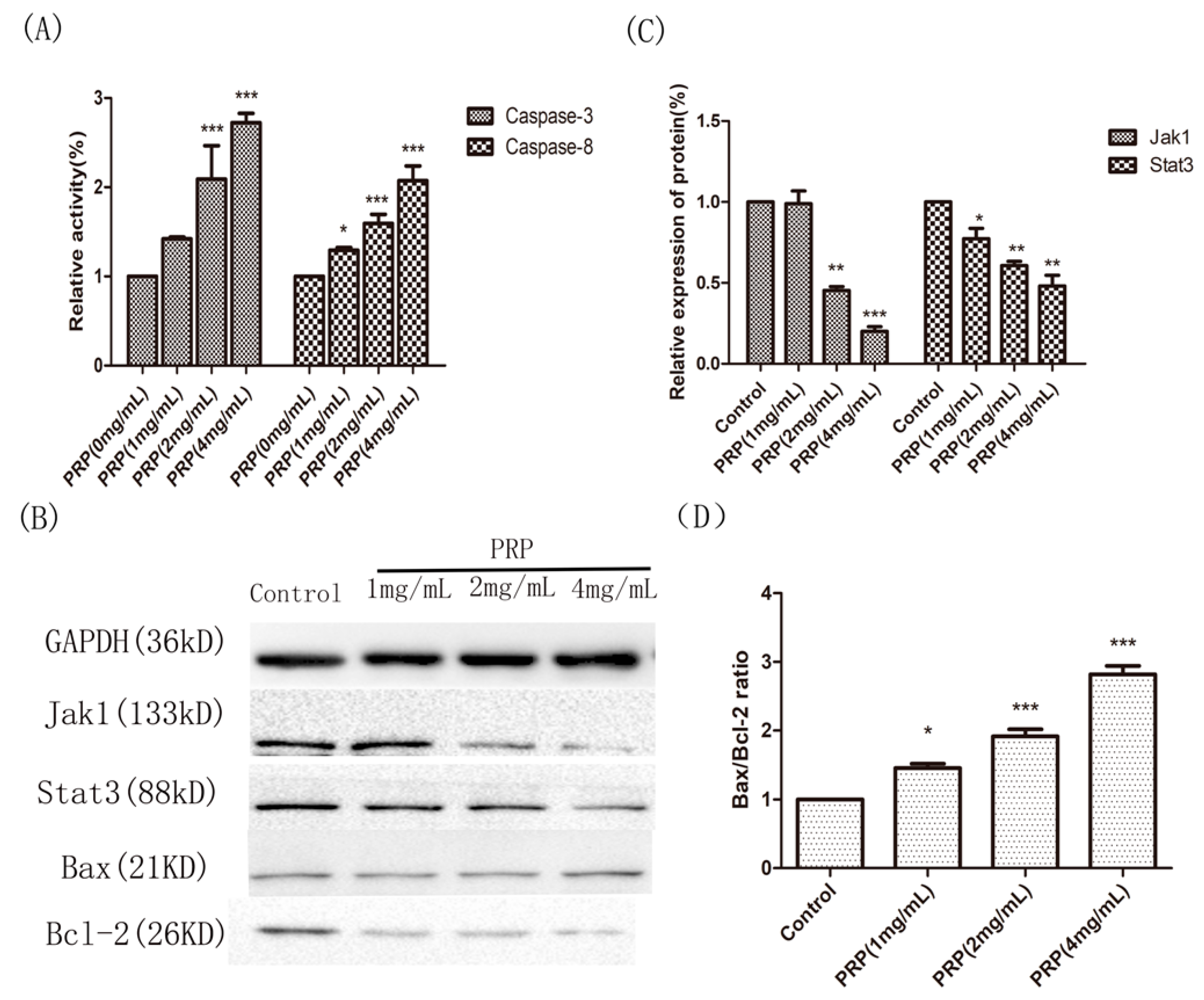
Figure 10.
Effects of PRP treatment on the activation of Caspase-3 and Caspase-8 and on the expression of other related proteins. (A) Relative activities of Caspase-3 and Caspase-8 in H22 cells treated with PRP for 48 h. (B) Western-blot analysis of Jak1, Stat3, Bcl-2, and Bax expression (C,D) Relative quantative analysis ofJak1, Stat3, and the Bax/Bcl-2 ratio in each groups. The data are shown as mean ± SD; (* p < 0.05, ** p < 0.01, *** p < 0.01) significantly different from the control group.
Figure 10.
Effects of PRP treatment on the activation of Caspase-3 and Caspase-8 and on the expression of other related proteins. (A) Relative activities of Caspase-3 and Caspase-8 in H22 cells treated with PRP for 48 h. (B) Western-blot analysis of Jak1, Stat3, Bcl-2, and Bax expression (C,D) Relative quantative analysis ofJak1, Stat3, and the Bax/Bcl-2 ratio in each groups. The data are shown as mean ± SD; (* p < 0.05, ** p < 0.01, *** p < 0.01) significantly different from the control group.
Table 1.
PRP monosaccharide composition.
Table 1.
PRP monosaccharide composition.
| Monosaccharide Composition Analysis | Content (μg/mL) |
|---|
| Mannose | 6067.76 |
| Glucose | 3744.62 |
| Galactose | 60.82 |
| Arabinose | 50.50 |
| Fucose | 21.99 |
| Xylose | 8.00 |
| Rhamnose | 4.34 |
Table 2.
Molecular weight of PRP was determined to be 383.569.
Table 2.
Molecular weight of PRP was determined to be 383.569.
| Molecular | Weight (kDa) |
|---|
| Mn | 39.877 |
| Mw | 383.569 |
| Mz | 2637 |
| Mp | 151.009 |
| Mw/Mn | 9.619 |
Table 3.
Secondary structure of PRP as determined by circular dichroism spectrum.
Table 3.
Secondary structure of PRP as determined by circular dichroism spectrum.
| Structures | 180–260 nm | 185–260 nm | 190–260 nm | 195–260 nm | 200–260 nm | 205–260 nm | 210–260 nm |
|---|
| Helix | 16.60% | 16.30% | 16.90% | 14.70% | 12.40% | 11.60% | 11.10% |
| Antiparallel | 41.30% | 44.30% | 42.50% | 30.00% | 20.70% | 17.40% | 21.00% |
| Parallel | 14.50% | 14.10% | 13.10% | 16.50% | 19.60% | 22.00% | 19.00% |
| Beta-Turn | 21.50% | 21.70% | 20.80% | 21.40% | 22.50% | 22.10% | 23.20% |
| Rndm.Coil | 44.00% | 45.50% | 43.30% | 49.00% | 54.20% | 56.80% | 55.70% |
| Total Sum | 137.90% | 142.00% | 136.50% | 131.60% | 129.40% | 129.90% | 130.10% |
Table 4.
Mouse mean abdominal perimeter in each group.
Table 4.
Mouse mean abdominal perimeter in each group.
| Group (n = 8) | Abdominal (cm) |
|---|
| Day 1 | Day 5 | Day 10 | Day 15 |
|---|
| Control | 5.33 ± 0.61 | 7.06 ± 0.81 | 9.33 ± 0.8 | 12.67 ± 0.98 |
| PRP (75 mg/kg) | 5.42 ± 0.58 | 6.42 ± 0.67 | 8.93 ± 0.79 | 11.17 ± 0.82 |
| PRP (150 mg/kg) | 5.30 ± 0.60 | 6.33 ± 0.52 | 8.13 ± 0.71 | 10.08 * ± 0.83 |
| PRP (300 mg/kg) | 5.41 ± 0.52 | 5.75 ± 0.41 | 7.25 ** ± 0.89 | 8.67 ** ± 1.75 |
| CTX (20 mg/kg) | 5.26 ± 0.61 | 6.41 ± 0.59 | 8.63 ± 0.67 | 10.75 ± 1.40 |
Table 5.
Mouse mean weight in each group.
Table 5.
Mouse mean weight in each group.
| Group (n = 8) | Weight (g) |
|---|
| Day 1 | Day 5 | Day 10 | Day 15 |
|---|
| Control | 20.3 ± 1.03 | 23.7 ± 1.50 | 26.7 ± 1.96 | 30.8 ± 1.94 |
| PRP (75 mg/kg) | 20.5 ± 1.51 | 22.8 ± 1.60 | 25.5 ± 1.64 | 28.8 ± 1.75 |
| PRP (150 mg/kg) | 20.5 ± 1.51 | 22.3 ± 1.21 | 24.2 ± 1.17 | 27.3 * ± 1.97 |
| PRP (300 mg/kg) | 20.3 ± 1.36 | 21.3 ± 1.36 | 22.8 * ± 1.03 | 25.2 ** ± 1.17 |
| CTX (20 mg/kg) | 20.5 ± 1.52 | 22.3 ± 1.75 | 24.7 ± 1.21 | 29.3 ± 1.50 |