Predictive Modeling for Voxel-Based Quantification of Imaging-Based Subtypes of Pancreatic Ductal Adenocarcinoma (PDAC): A Multi-Institutional Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
2.1. Patient Demographics
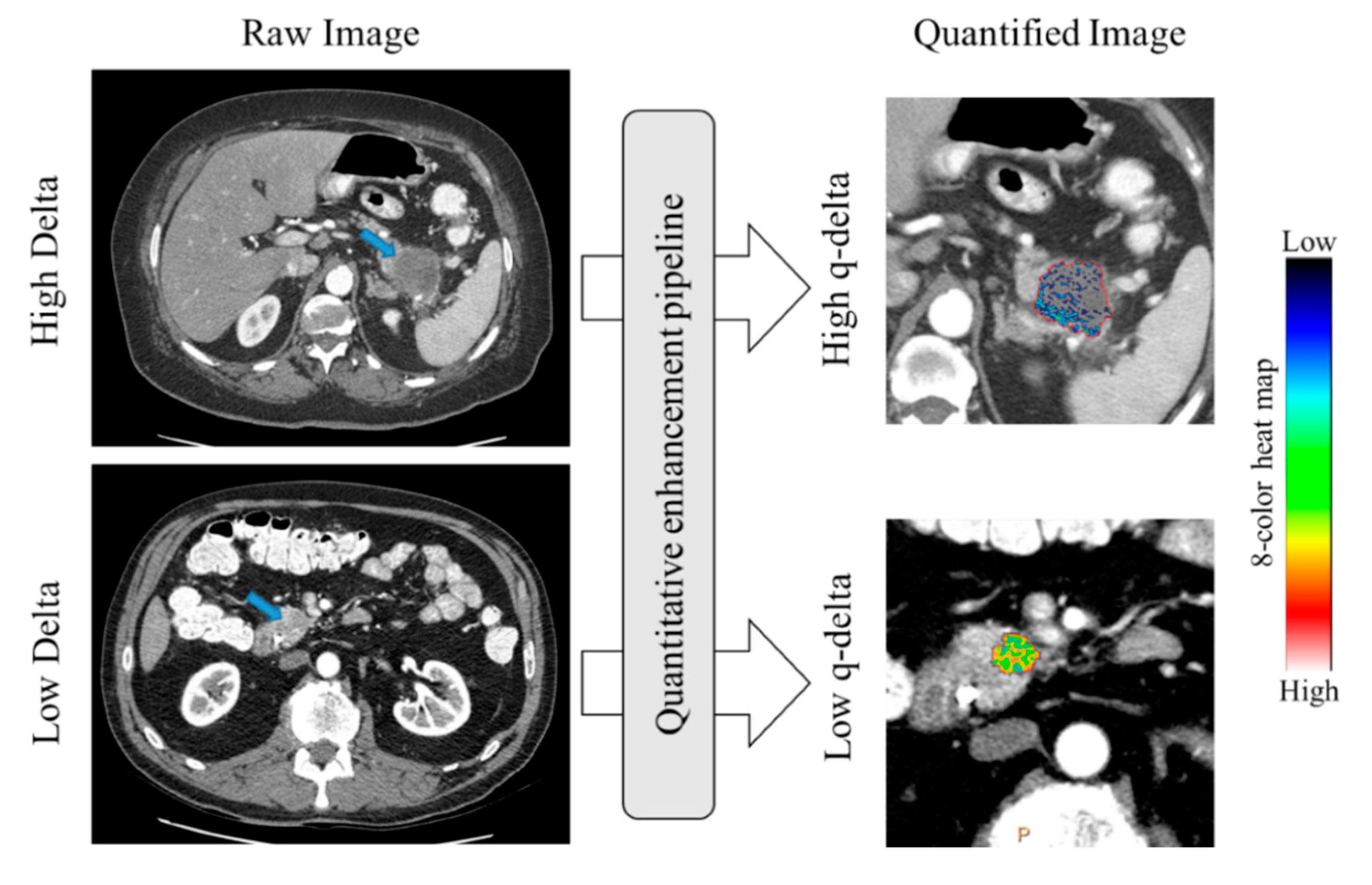
2.2. Association between Qualitative Delta and q-delta
2.3. Association between Quantitative Delta and nAUC
2.4. Inter- and Intrarater Variability Assessment
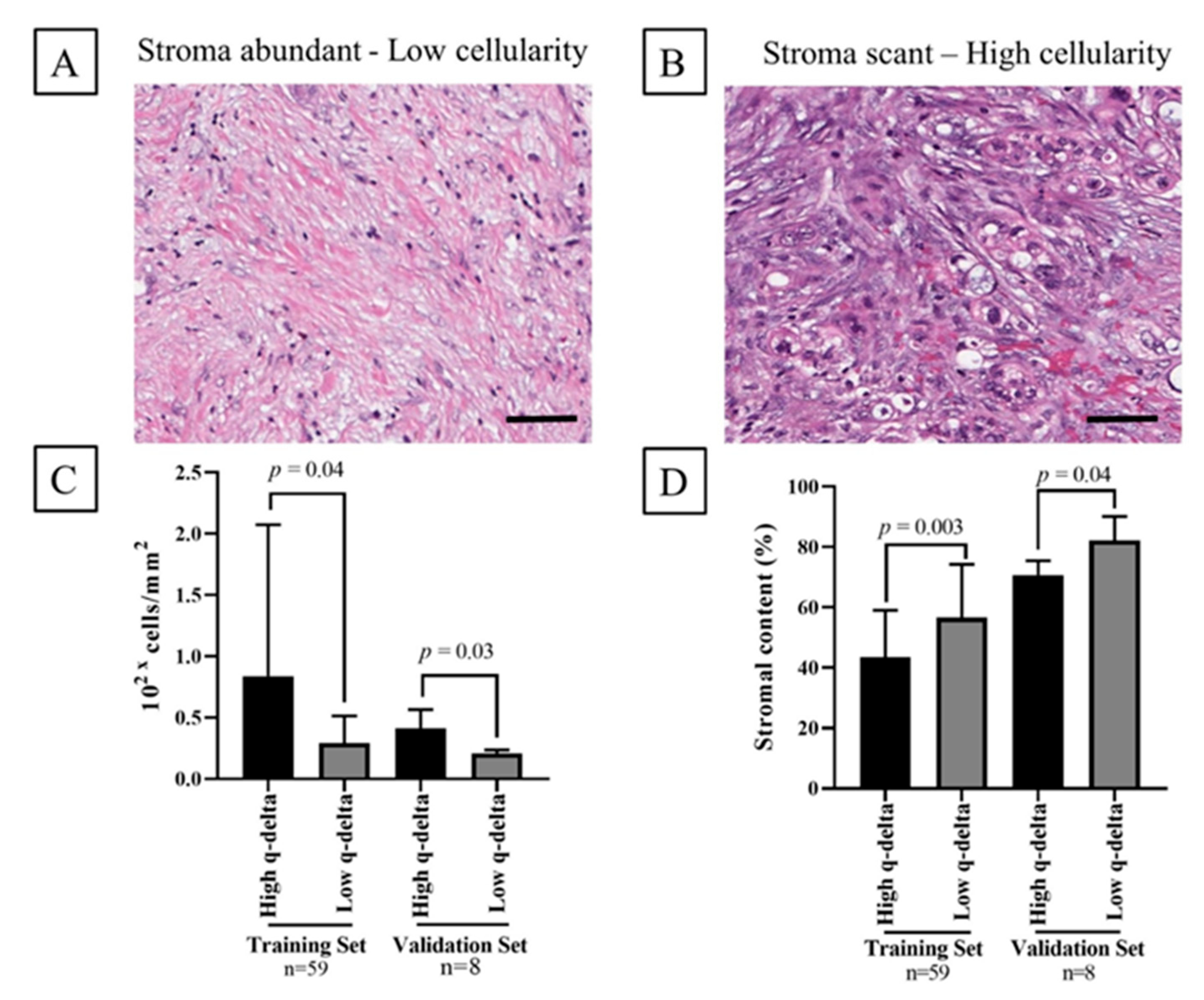
2.5. Association between the q-delta and Tumor Stromal Content
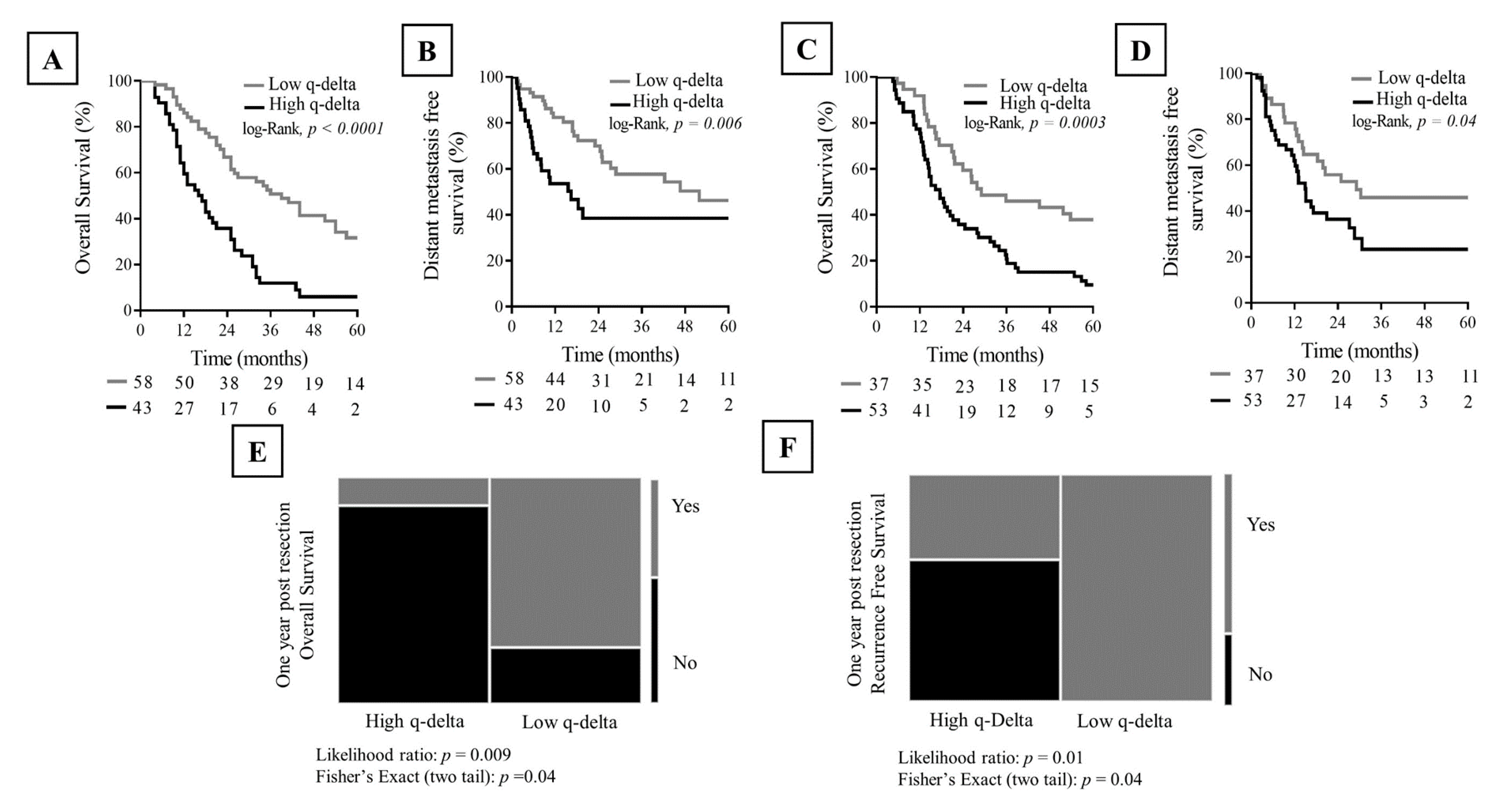
2.6. Association between q-delta and Clinical Outcome
3. Discussion
4. Materials and Methods
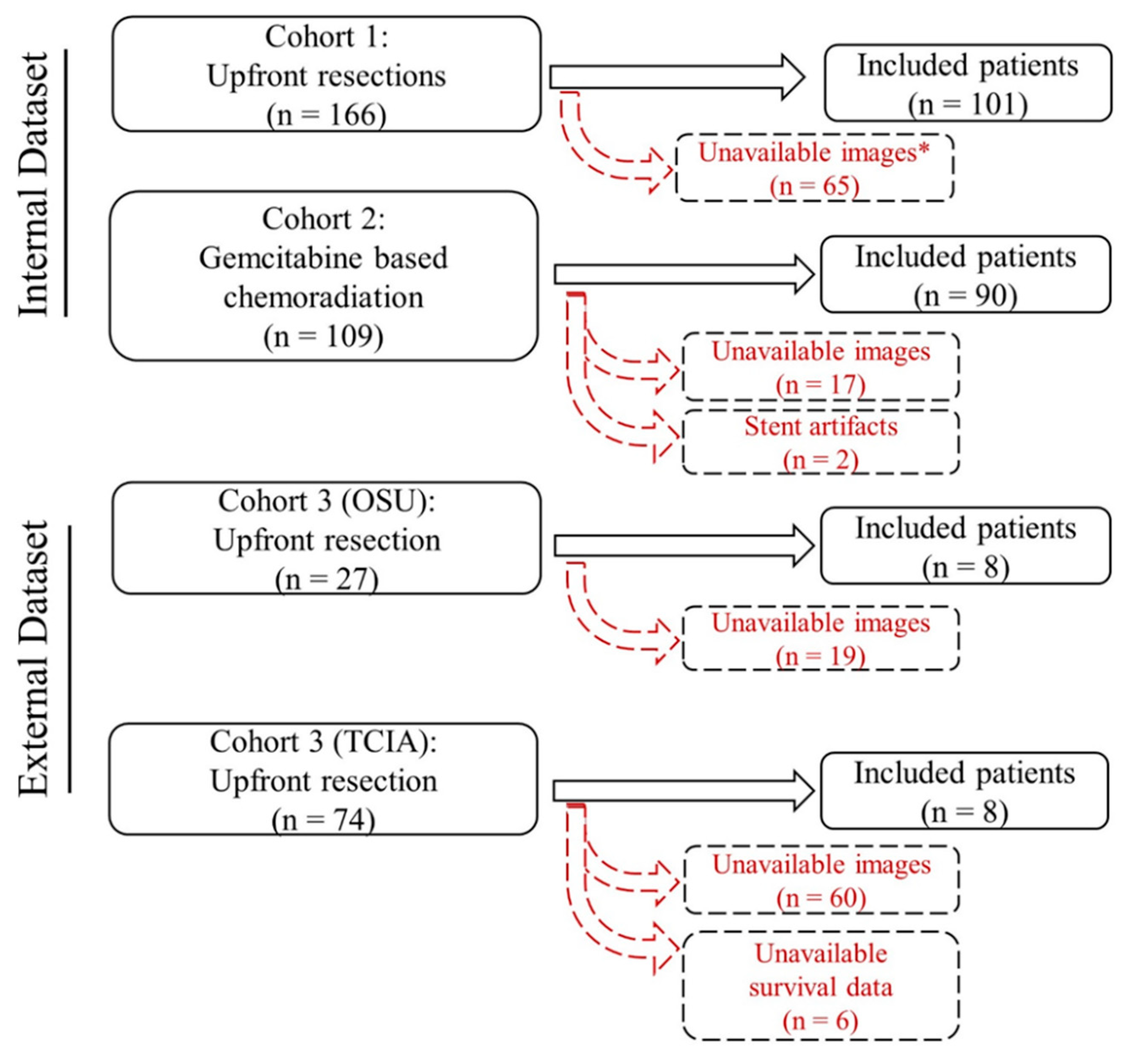
4.1. Patients
4.2. CT Acquisition
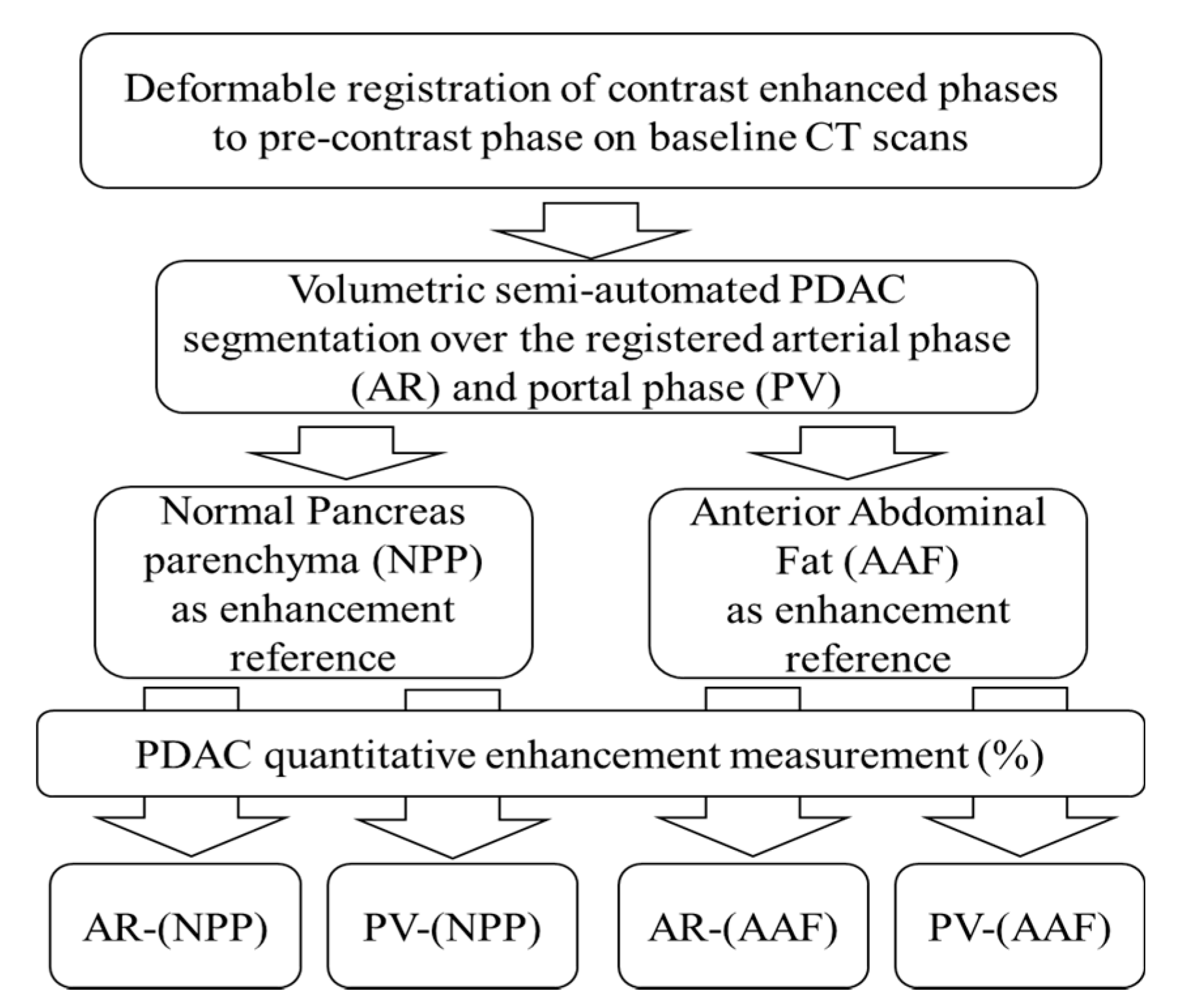
4.3. CT Analysis: Normalized Area under the Curve (nAUC)
4.4. CT Analysis: Qualitative Delta Scoring
4.5. CT Analysis: Quantitative Normalized Enhancement (q-delta)
4.6. Inter- and IntraRater Agreement
4.7. Histopathological Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Hidalgo, M. Pancreatic cancer. N. Engl. J. Med. 2010, 362, 1605–1617. [Google Scholar] [CrossRef] [Green Version]
- Evans, D.B.; Varadhachary, G.R.; Crane, C.H.; Sun, C.C.; Lee, J.E.; Pisters, P.W.T.; Vauthey, J.-N.; Wang, H.; Cleary, K.R.; Staerkel, G.A.; et al. Preoperative Gemcitabine-Based Chemoradiation for Patients with Resectable Adenocarcinoma of the Pancreatic Head. J. Clin. Oncol. 2008, 26, 3496–3502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varadhachary, G.R.; Wolff, R.A.; Crane, C.H.; Sun, C.C.; Lee, J.E.; Pisters, P.W.T.; Vauthey, J.-N.; Abdalla, E.; Wang, H.; Staerkel, G.A.; et al. Preoperative Gemcitabine and Cisplatin Followed by Gemcitabine-Based Chemoradiation for Resectable Adenocarcinoma of the Pancreatic Head. J. Clin. Oncol. 2008, 26, 3487–3495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koay, E.J.; Truty, M.J.; Cristini, V.; Thomas, R.M.; Chen, R.; Chatterjee, D.; Kang, Y.; Bhosale, P.R.; Tamm, E.P.; Crane, C.H.; et al. Transport properties of pancreatic cancer describe gemcitabine delivery and response. J. Clin. Investig. 2014, 124, 1525–1536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katz, M.H.; Varadhachary, G.R.; Fleming, J.B.; Wolff, R.A.; Lee, J.E.; Pisters, P.W.; Vauthey, J.N.; Abdalla, E.K.; Sun, C.C.; Wang, H.; et al. Serum CA 19-9 as a marker of resectability and survival in patients with potentially resectable pancreatic cancer treated with neoadjuvant chemoradiation. Ann. Surg. Oncol. 2010, 17, 1794–1801. [Google Scholar] [CrossRef]
- Wynne, J.F.; Colbert, L.E.; Seldon, C.; Zaid, M.; Yu, D.S.; Landry, J.C.; Koay, E.J. External Validation of an Imaging-Based Biomarker of Pancreatic Ductal Adenocarcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, e79. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Elganainy, D.; Zaid, M.; Butner, J.; Cristini, V.; Koay, E. Mass Transport Model of Radiation Response: Calibration and Application to Chemoradiation for Pancreatic Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, E48–E49. [Google Scholar] [CrossRef]
- Sharib, J.; Zaid, M.; Dai, A.; Widman, L.; Behr, S.; Kirkwood, K.; Koay, E. Spatial Computation of the Immune and Stromal Characteristics of Biophysical Subtypes of Pancreatic Ductal Adenocarcinoma. In Annals of Surgical Oncology; Springer: New York, NY, USA, 2020; pp. S127–S128. [Google Scholar]
- Koay, E.J.; Lee, Y.; Cristini, V.; Lowengrub, J.S.; Kang, Y.; Lucas, F.A.S.; Hobbs, B.P.; Ye, R.; Elganainy, D.; Almahariq, M.; et al. A Visually Apparent and Quantifiable CT Imaging Feature Identifies Biophysical Subtypes of Pancreatic Ductal Adenocarcinoma. Clin. Cancer Res. 2018, 24, 5883–5894. [Google Scholar] [CrossRef] [Green Version]
- Koay, E.J.; Katz, M.H.G.; Wang, H.; Wang, X.; Prakash, L.; Javle, M.; Shroff, R.; Fogelman, D.; Avila, S.; Zaid, M.; et al. Computed Tomography–Based Biomarker Outcomes in a Prospective Trial of Preoperative FOLFIRINOX and Chemoradiation for Borderline Resectable Pancreatic Cancer. JCO Precis. Oncol. 2019, 1–15. [Google Scholar] [CrossRef]
- Tacher, V.; Lin, M.; Duran, R.; Yarmohammadi, H.; Lee, H.; Chapiro, J.; Chao, M.; Wang, Z.; Frangakis, C.; Sohn, J.H.; et al. Comparison of Existing Response Criteria in Patients with Hepatocellular Carcinoma Treated with Transarterial Chemoembolization Using a 3D Quantitative Approach. Radiology 2016, 278, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Pellerin, O.; Bhagat, N.; Rao, P.P.; Loffroy, R.; Ardon, R.; Mory, B.; Reyes, D.K.; Geschwind, J.-F. Quantitative and volumetric European Association for the Study of the Liver and Response Evaluation Criteria in Solid Tumors measurements: Feasibility of a semiautomated software method to assess tumor response after transcatheter arterial chemoembolization. J. Vasc. Interv. Radiol. 2012, 23, 1629–1637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chapiro, J.; Wood, L.D.; Lin, M.; Duran, R.; Cornish, T.; Lesage, D.; Charu, V.; Schernthaner, R.; Wang, Z.; Tacher, V.; et al. Radiologic-pathologic analysis of contrast-enhanced and diffusion-weighted MR imaging in patients with HCC after TACE: Diagnostic accuracy of 3D quantitative image analysis. Radiology 2014, 273, 746–758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chockalingam, A.; Duran, R.; Sohn, J.H.; Schernthaner, R.; Chapiro, J.; Lee, H.; Sahu, S.; Nguyen, S.; Geschwind, J.F.; Lin, M. Radiologic-pathologic analysis of quantitative 3D tumour enhancement on contrast-enhanced MR imaging: A study of ROI placement. Eur. Radiol. 2016, 26, 103–113. [Google Scholar] [CrossRef]
- Zhu, L.; Shi, X.; Xue, H.; Wu, H.; Chen, G.; Sun, H.; He, Y.; Jin, Z.; Liang, Z.; Zhang, Z. CT Imaging Biomarkers Predict Clinical Outcomes after Pancreatic Cancer Surgery. Medicine 2016, 95, e2664. [Google Scholar] [CrossRef]
- Lee, S.; Kim, S.H.; Park, H.K.; Jang, K.T.; Hwang, J.A.; Kim, S. Pancreatic Ductal Adenocarcinoma: Rim Enhancement at MR Imaging Predicts Prognosis after Curative Resection. Radiology 2018, 288, 456–466. [Google Scholar] [CrossRef]
- Hata, H.; Mori, H.; Matsumoto, S.; Yamada, Y.; Kiyosue, H.; Tanoue, S.; Hongo, N.; Kashima, K. Fibrous stroma and vascularity of pancreatic carcinoma: Correlation with enhancement patterns on CT. Abdom. Imaging 2010, 35, 172–180. [Google Scholar] [CrossRef]
- Tamm, E.P.; Balachandran, A.; Bhosale, P.; Szklaruk, J. Update on 3D and multiplanar MDCT in the assessment of biliary and pancreatic pathology. Abdom. Imaging 2009, 34, 64–74. [Google Scholar] [CrossRef]
- Kamaya, A.; Federle, M.P.; Desser, T.S. Imaging Manifestations of Abdominal Fat Necrosis and Its Mimics. RadioGraphics 2011, 31, 2021–2034. [Google Scholar] [CrossRef] [Green Version]
- Broder, J. Chapter 10—Imaging Abdominal and Flank Trauma. In Diagnostic Imaging for the Emergency Physician; Broder, J., Ed.; W.B. Saunders: Saint Louis, MO, USA, 2011; pp. 578–611. [Google Scholar] [CrossRef]
- Rhim, A.D.; Oberstein, P.E.; Thomas, D.H.; Mirek, E.T.; Palermo, C.F.; Sastra, S.A.; Dekleva, E.N.; Saunders, T.; Becerra, C.P.; Tattersall, I.W.; et al. Stromal Elements Act to Restrain, Rather Than Support, Pancreatic Ductal Adenocarcinoma. Cancer Cell 2014, 25, 735–747. [Google Scholar] [CrossRef] [Green Version]
- McNulty, N.J.; Francis, I.R.; Platt, J.F.; Cohan, R.H.; Korobkin, M.; Gebremariam, A. Multi–Detector Row Helical CT of the Pancreas: Effect of Contrast-enhanced Multiphasic Imaging on Enhancement of the Pancreas, Peripancreatic Vasculature, and Pancreatic Adenocarcinoma. Radiology 2001, 220, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.S.; Vedantham, S.; Krasny, R.M.; Kadell, B.; Berger, W.L.; Reber, H.A. Two-phase helical CT for pancreatic tumors: Pancreatic versus hepatic phase enhancement of tumor, pancreas, and vascular structures. Radiology 1996, 199, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Fargnoli, R.; Fusi, I. Computerized tomography of pancreatic tumors. Tumori 1999, 85, S3–S5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boland, G.W.; O’Malley, M.E.; Saez, M.; Fernandez-del-Castillo, C.; Warshaw, A.L.; Mueller, P.R. Pancreatic-phase versus portal vein-phase helical CT of the pancreas: Optimal temporal window for evaluation of pancreatic adenocarcinoma. AJR Am. J. Roentgenol. 1999, 172, 605–608. [Google Scholar] [CrossRef] [Green Version]
- Yoon, S.H.; Lee, J.M.; Cho, J.Y.; Lee, K.B.; Kim, J.E.; Moon, S.K.; Kim, S.J.; Baek, J.H.; Kim, S.H.; Kim, S.H.; et al. Small (</= 20 mm) pancreatic adenocarcinomas: Analysis of enhancement patterns and secondary signs with multiphasic multidetector CT. Radiology 2011, 259, 442–452. [Google Scholar] [CrossRef] [Green Version]
- Park, P.C.; Choi, G.W.; Zaid, M.; Elganainy, D.; Smani, D.A.; Tomich, J.; Samaniego, R.; Ma, J.; Tamm, E.P.; Beddar, S.; et al. Enhancement pattern mapping technique for improving contrast-to-noise ratios and detectability of hepatobiliary tumors on multiphase computed tomography. Med. Phys. 2019. [Google Scholar] [CrossRef]
- Amer, A.M.; Zaid, M.; Chaudhury, B.; Elganainy, D.; Lee, Y.; Wilke, C.T.; Cloyd, J.; Wang, H.; Maitra, A.; Wolff, R.A.; et al. Imaging-based biomarkers: Changes in the tumor interface of pancreatic ductal adenocarcinoma on computed tomography scans indicate response to cytotoxic therapy. Cancer 2018, 124, 1701–1709. [Google Scholar] [CrossRef] [Green Version]
- Zaid, M.; Woermann, S.; Chaudhury, B.; Thunshelle, C.P.; Dai, A.; Agrawal, A.; Widmann, L.; Zhang, J.; Hurd, M.W.; Varadhachary, G.R.; et al. Pancreatic Ductal Adenocarcinoma Immune and Stromal Elements Differ in Spatial Distribution Based on Response to Chemoradiation. Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, e606. [Google Scholar] [CrossRef]
- Thunshelle, C.P.; Zaid, M.; Jaoude, J.A.; Chowdhury, T.; Dai, A.; Kouzy, R.; Mathew, R.; Prakash, L.; Katz, M.H.G.; Varadhachary, G.R.; et al. Neutrophil-Lymphocyte Ratio and Tumor Interface Response as Predictors of Outcomes for Prospective Cohort of Patients with Pancreatic Ductal Adenocarcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, e618. [Google Scholar] [CrossRef]
- Katz, M.W.H.; Pisters, P.W.; Evans, D.B.; Sun, C.C.; Lee, J.E.; Fleming, J.B.; Vauthey, J.N.; Abdalla, E.K.; Crane, C.H.; Wolff, R.A.; et al. Borderline resectable pancreatic cancer: The importance of this emerging stage of disease. J. Am. Coll. Surg. 2008, 206, 833–846. [Google Scholar] [CrossRef] [Green Version]
- Clark, K.; Vendt, B.; Smith, K.; Freymann, J.; Kirby, J.; Koppel, P.; Moore, S.; Phillips, S.; Maffitt, D.; Pringle, M.; et al. The Cancer Imaging Archive (TCIA): Maintaining and Operating a Public Information Repository. J. Digit. Imaging 2013, 26, 1045–1057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- CPTAC. Radiology Data from the Clinical Proteomic Tumor Analysis Consortium Pancreatic Ductal Adenocarcinoma [CPTAC-PDA] Collection [Data set]. Cancer Imaging Arch. 2018. [Google Scholar] [CrossRef]
- Zijdenbos, A.P.; Dawant, B.M.; Margolin, R.A.; Palmer, A.C. Morphometric analysis of white matter lesions in MR images: Method and validation. IEEE Trans. Med. Imaging 1994, 13, 716–724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Characteristic | MDACC Data | External Validation Data | ||
|---|---|---|---|---|
| D1 | D2 | D3 | ||
| Training Set (n = 101) | Validation Set (n = 90) | OSU (n = 8) | TCIA (n = 8) | |
| No. (%) | No. (%) | No. (%) | No. (%) | |
| Age (median, range) | 64 (25–85) | 63 (42–82) | 67.5 (50–88) | 65 (34–80) |
| Sex | ||||
| Women | 46 (47) | 38 (42) | 3 (27) | 3 (37) |
| Men | 55 (53) | 52 (58) | 5 (63) | 5 (63) |
| Pathological T stage | ||||
| T1 | 21 (21) | 24 (37) | 0 (0) | 0 (0) |
| T2 | 67 (66) | 35 (54) | 0 (0) | 0 (0) |
| T3 | 12 (12) | 6 (9) | 8 (100) | 8 (100) |
| T4 | 1 (1) | - | 0 (0) | 0 (0) |
| Pathological N stage | ||||
| Negative (N0) | 19 (19) | 34 (52) | - | 1 (12) |
| Positive (N1) | 30 (30) | 22 (34) | 3 (38) | 7 (88) |
| Positive (N2) | 52 (51) | 9 (14) | 5 (62) | - |
| Overall Stage | ||||
| IA | 8 (8) | 15 (23) | - | - |
| IB | 9 (9) | 16 (25) | - | - |
| IIA | 2 (2) | 3 (5) | - | 1 (12) |
| IIB | 30 (30) | 22 (34) | 3 (38) | 7 (88) |
| III | 52 (51) | 9 (14) | 5 (62) | - |
| IV | - | - | - | - |
| Surgery | ||||
| Yes | 101 (100) | 65 (72) | 8 (100) | 8 (100) |
| No | - | 25 (28) | - | - |
| Surgical margin | ||||
| Negative (R0) | 87 (86) | 62 (95) | 2 (25) | 5 (62) |
| Positive (R1) | 14 (14) | 3 (5) | 6 (75) | 3 (38) |
| Adjuvant chemotherapy | ||||
| Yes | 76 (75) | - | 6 (75) | NA* |
| No | 25 (25) | - | 2 (25) | NA |
| Adjuvant chemoradiation | ||||
| Yes | 33 (33) | - | - | NA |
| No | 68 (67) | - | 8 (100) | NA |
| Variable | p-Value | AUC (95% CI) | Sensitivity (%) | Specificity (%) | Accuracy (%) | Misclassification (%) |
|---|---|---|---|---|---|---|
| Univariate logistic regression | ||||||
| Enhancement AR-(NPP) | <0.0001 | 0.94 (0.90–0.99) | 89 | 85 | 87 | 13 |
| Enhancement PV-(NPP) | <0.0001 | 0.91 (0.84–0.97) | 89 | 81 | 85 | 15 |
| Enhancement AR-(AFF) | <0.0001 | 0.73 (0.63–0.84) | 65 | 74 | 70 | 30 |
| Enhancement PV-(AFF) | 0.0003 | 0.70 (0.60–0.81) | 54 | 78 | 68 | 32 |
| Multivariate logistic regression | ||||||
| Training set (q-delta) | <0.0001 | 0.95 (0.91–0.99) | 91 | 89 | 91 | 9 |
| Internal validation set (q-delta) | <0.0001 | 0.96 (0.92–1) | 100 | 83 | 93 | 7 |
| External validation set (q-delta) | 0.03 | 0.90 (0.69–1) | 85 | 1 | 93 | 7 |
| Characteristic | No. of Patients | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | ||
| q-delta | |||||
| High | 43 | 2.6 (1.6–4.1) | <0.0001 | 2 (1.2–3.4) | 0.004 |
| Low | 58 | - | - | - | - |
| Age | 101 | 1 (0.9–1.03) | 0.3 | - | - |
| Sex | |||||
| Male | 55 | 0.69 (0.4–1.07) | 0.1 | 0.8 (0.5–1.3) | 0.5 |
| Female | 46 | ||||
| Surgical margin | |||||
| Positive (R1) | 14 | 1.6 (0.7–2.8) | 0.18 | 1.3 (0.6–2.5) | 0.3 |
| Negative (R0) | 87 | - | - | - | - |
| Pathologic N Stage | |||||
| N0 vs. N1 | 49 | 0.47 (0.21–0.97) | 0.04 | 0.4 (0.2–1.03) | 0.06 |
| N1 vs. N2 | 82 | 0.6 (0.41–1.1) | 0.1 | 0.7 (0.4–1.1) | 0.2 |
| N0 vs. N2 | 71 | 0.3 (0.1–0.6) | 0.0005 | 0.3 (0.1–0.6) | 0.002 |
| Adjuvant chemotherapy | |||||
| Yes | 76 | 0.5 (0.3–0.9) | 0.02 | 0.5 (0.3–0.9) | 0.02 |
| No | 25 | - | - | - | - |
| Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|
| Characteristic | No. of Patients | HR (95% CI) | p Value | HR (95% CI) | p Value |
| q-delta (†) | |||||
| High | 52 | 2.4 (1.4–3.9) | 0.0003 | 1.9 (1.1–3.2) | 0.01 |
| Low | 37 | - | - | - | - |
| Surgery | |||||
| Yes | 65 | 0.13 (0.07–0.2) | <0.0001 | 0.1 (0.06–0.2) | <0.0001 |
| No | 25 | - | - | - | - |
| q-delta (††) | |||||
| High | 35 | 2.5 (1.4–4.6) | 0.0003 | 2.9 (1.6–5.5) | 0.001 |
| Low | 30 | - | - | - | - |
| Age | 65 | 1.02 (0.9–1.06) | 0.16 | 1 (0.4–7) | 0.3 |
| Sex | |||||
| Male | 36 | 1 (0.62–1.8) | 0.7 | - | - |
| Female | 29 | ||||
| Surgical margin | |||||
| Positive (R1) | 62 | 1.3 (0.4–4.2) | 0.6 | - | - |
| Negative (R0) | 3 | - | - | - | - |
| N Stage | |||||
| N0 vs. N1 | 56 | 1 (0.5–1.9) | 0.8 | - | - |
| N1 vs. N2 | 31 | 0.6 (0.3–1.5) | 0.3 | - | - |
| N0 vs. N2 | 41 | 0.7 (0.3–1.5) | 0.3 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaid, M.; Widmann, L.; Dai, A.; Sun, K.; Zhang, J.; Zhao, J.; Hurd, M.W.; Varadhachary, G.R.; Wolff, R.A.; Maitra, A.; et al. Predictive Modeling for Voxel-Based Quantification of Imaging-Based Subtypes of Pancreatic Ductal Adenocarcinoma (PDAC): A Multi-Institutional Study. Cancers 2020, 12, 3656. https://doi.org/10.3390/cancers12123656
Zaid M, Widmann L, Dai A, Sun K, Zhang J, Zhao J, Hurd MW, Varadhachary GR, Wolff RA, Maitra A, et al. Predictive Modeling for Voxel-Based Quantification of Imaging-Based Subtypes of Pancreatic Ductal Adenocarcinoma (PDAC): A Multi-Institutional Study. Cancers. 2020; 12(12):3656. https://doi.org/10.3390/cancers12123656
Chicago/Turabian StyleZaid, Mohamed, Lauren Widmann, Annie Dai, Kevin Sun, Jie Zhang, Jun Zhao, Mark W. Hurd, Gauri R. Varadhachary, Robert A. Wolff, Anirban Maitra, and et al. 2020. "Predictive Modeling for Voxel-Based Quantification of Imaging-Based Subtypes of Pancreatic Ductal Adenocarcinoma (PDAC): A Multi-Institutional Study" Cancers 12, no. 12: 3656. https://doi.org/10.3390/cancers12123656





