Tetrahydrocannabinol Sensing in Complex Biofluid with Portable Raman Spectrometer Using Diatomaceous SERS Substrates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Gold Nanoparticles
2.2. Fabrication of Porous Diatomaceous TLC Plates
2.3. Biofluid Samples Preparation
2.4. TLC-SERS Method
2.5. Portable Raman Spectrometer
2.6. Enhancement Factor Calculation
3. Results and Discussion
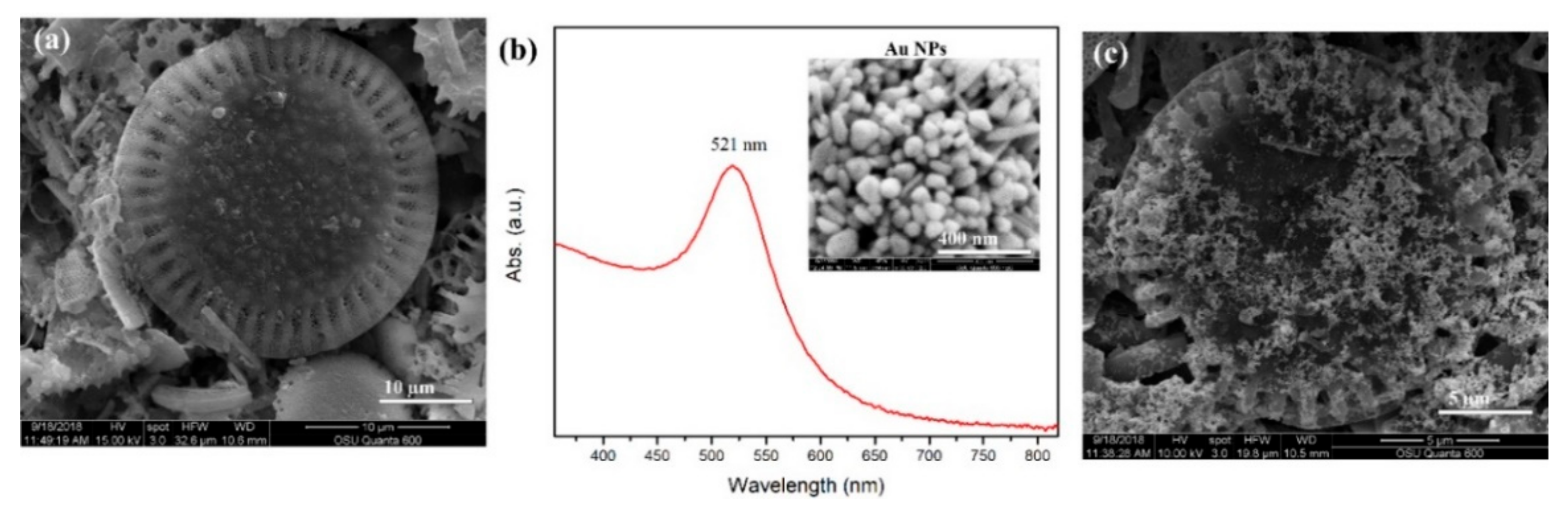
3.1. Characterization of Plasmonic Photonic Materials
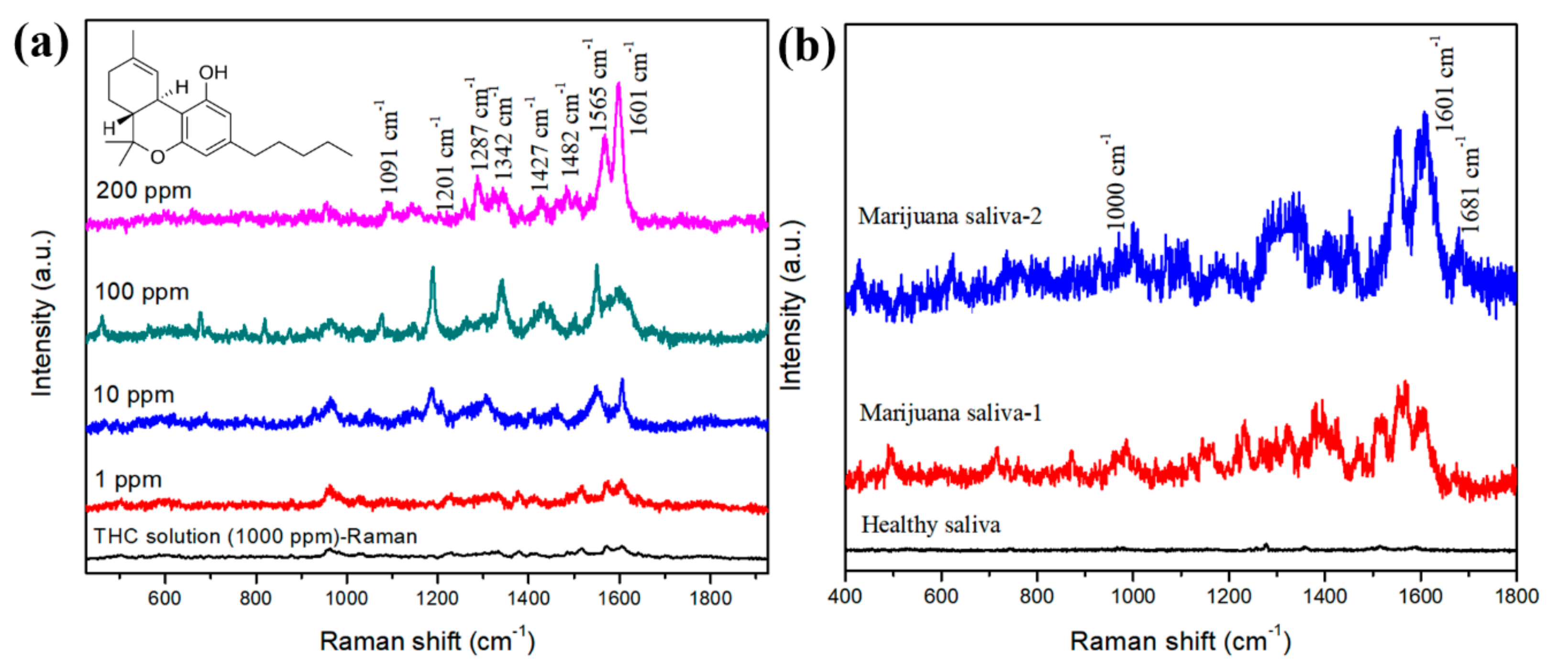
3.2. SERS Analyses of THC in Methanol and Saliva Samples
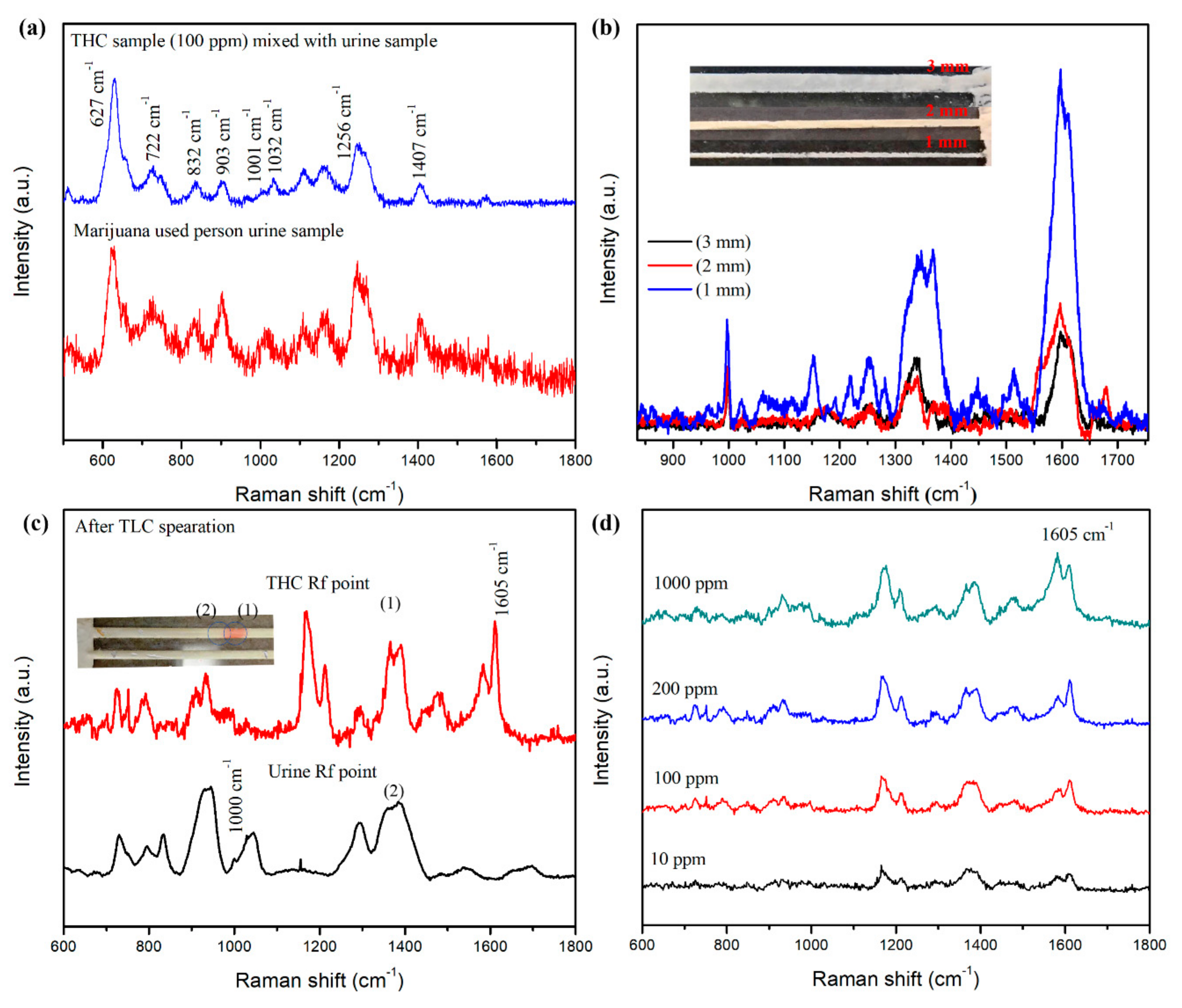
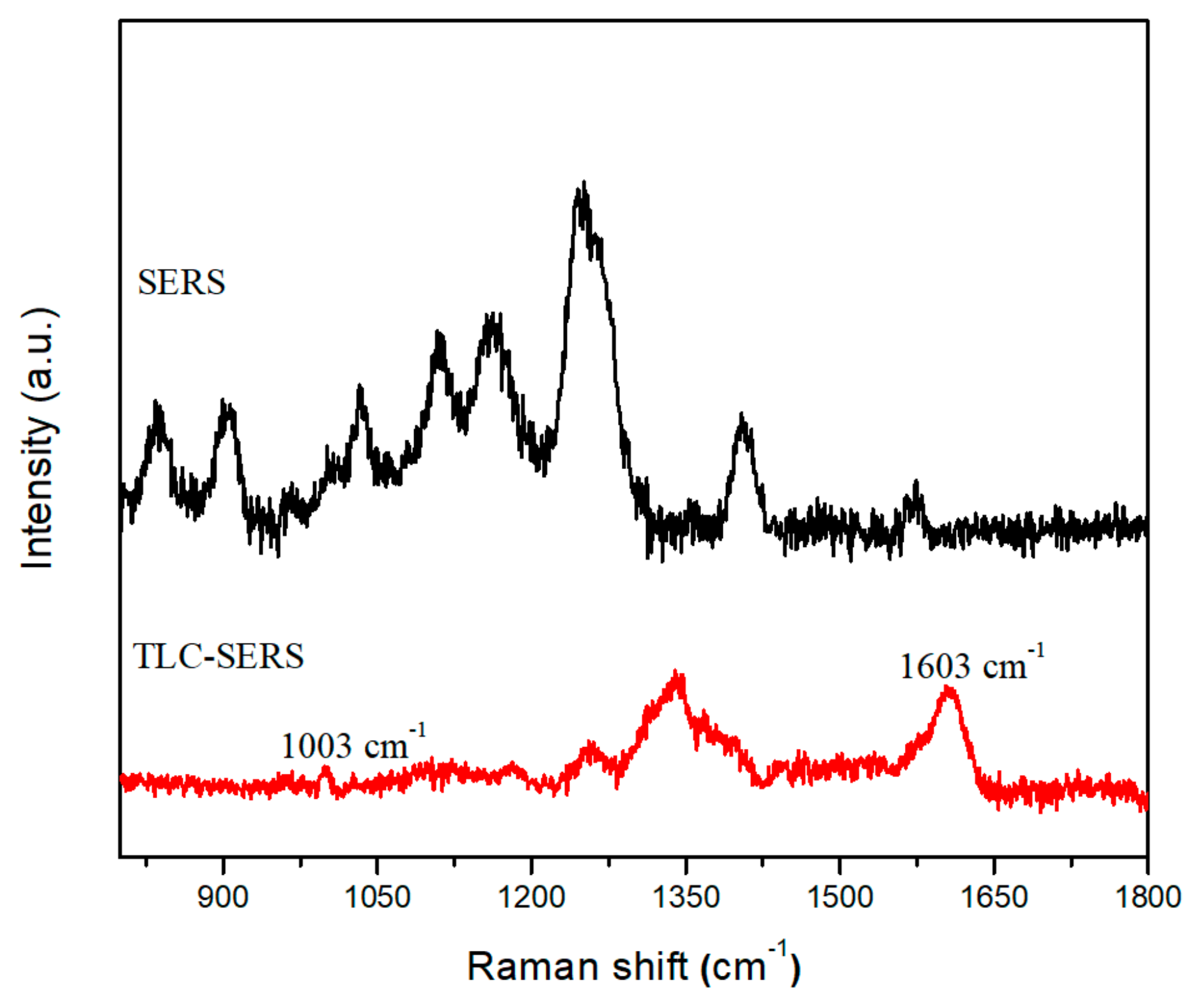
3.3. THC Sensing in Urine Samples Using TLC-SERS
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Murray, R.M.; Morrison, P.D.; Henquet, C.; Forti, M.D. Cannabis, the mind and society: The hash realities. Nat. Rev. Neurosci. 2007, 8, 885. [Google Scholar] [CrossRef] [PubMed]
- Kalant, H. Medicinal Use of Cannabis: History and Current Status. Pain Res. Manag. 2001, 6. [Google Scholar] [CrossRef] [PubMed]
- Grauwiler, S.B.; Scholer, A.; Drewe, J. Development of a LC/MS/MS method for the analysis of cannabinoids in human EDTA-plasma and urine after small doses of Cannabis sativa extracts. J. Chromatogr. B 2007, 850, 515–522. [Google Scholar] [CrossRef]
- Wohlfarth, A.; Mahler, H.; Auwärter, V. Rapid isolation procedure for Δ9-Tetrahydrocannabinolic acid A (THCA) from Cannabis sativa using two flash chromatography systems. J. Chromatogr. B 2011, 879, 3059–3064. [Google Scholar] [CrossRef] [PubMed]
- Sultan, S.; Millar, S.; O’Sullivan, S.; England, T. A Systematic Review and Meta-Analysis of the In Vivo Haemodynamic Effects of Δ9-Tetrahydrocannabinol. Pharmaceuticals 2018, 11, 13. [Google Scholar] [CrossRef] [PubMed]
- Karschner, E.L.; Schwilke, E.W.; Lowe, R.H.; Darwin, W.D.; Herning, R.I.; Cadet, J.L.; Huestis, M.A. Implications of plasma Delta9-tetrahydrocannabinol, 11-hydroxy-THC, and 11-nor-9-carboxy-THC concentrations in chronic cannabis smokers. J. Anal. Toxicol. 2009, 33, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Milman, G.; Schwope, D.M.; Gorelick, D.A.; Huestis, M.A. Cannabinoids and metabolites in expectorated oral fluid following controlled smoked cannabis. Clin. Chim. Acta 2012, 413, 765–770. [Google Scholar] [CrossRef] [Green Version]
- Vinson, J.A.; Patel, D.D.; Patel, A.H. Detection of tetrahydrocannabinol in blood and serum using a fluorescent derivative and thin-layer chromatography. Anal. Chem. 1977, 49, 163–165. [Google Scholar] [CrossRef]
- Scheidweiler, K.B.; Desrosiers, N.A.; Huestis, M.A. Simultaneous quantification of free and glucuronidated cannabinoids in human urine by liquid chromatography tandem mass spectrometry. Clin. Chim. Acta 2012, 413, 1839–1847. [Google Scholar] [CrossRef] [Green Version]
- Lurie, I.S.; Meyers, R.P.; Conver, T.S. Capillary Electrochromatography of Cannabinoids. Anal. Chem. 1998, 70, 3255–3260. [Google Scholar] [CrossRef]
- Abraham, T.T.; Lowe, R.H.; Pirnay, S.O.; Darwin, W.D.; Huestis, M.A. Simultaneous GC-EI-MS determination of Delta9-Tetrahydrocannabinol, 11-hydroxy-Delta9-tetrahydrocannabinol, and 11-nor-9-carboxy-Delta9-tetrahydrocannabinol in human urine following tandem Enzyme-Alkaline hydrolysis. J. Anal. Toxicol. 2007, 31, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.-C.; Sivashanmugan, K.; Liao, J.-D.; Yao, C.-K.; Peng, H.-C. Nanofabricated SERS-active substrates for Single-Molecule to virus detection in vitro: A review. Biosens. Bioelectron. 2014, 61, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Sivashanmugan, K.; Squire, K.; Kraai, J.A.; Tan, A.; Zhao, Y.; Rorrer, G.L.; Wang, A.X. Biological Photonic Crystal-Enhanced Plasmonic Mesocapsules: Approaching Single-Molecule Optofluidic-SERS Sensing. Adv. Opt. Mater. 2019, 7, 1900415. [Google Scholar] [CrossRef]
- Kim, K.; Guo, J.; Liang, Z.; Fan, D. Artificial Micro/Nanomachines for Bioapplications: Biochemical Delivery and Diagnostic Sensing. Adv. Funct. Mater. 2018, 28, 1705867. [Google Scholar] [CrossRef]
- Islam, S.K.; Cheng, Y.P.; Birke, R.L.; Green, O.; Kubic, T.; Lombardi, J.R. Rapid and sensitive detection of synthetic cannabinoids AMB-FUBINACA and α-PVP using surface enhanced Raman scattering (SERS). Chem. Phys. 2018, 506, 31–35. [Google Scholar] [CrossRef]
- Yüksel, S.; Schwenke, A.M.; Soliveri, G.; Ardizzone, S.; Weber, K.; Cialla-May, D.; Hoeppener, S.; Schubert, U.S.; Popp, J. Trace detection of tetrahydrocannabinol (THC) with a SERS-Based capillary platform prepared by the in situ microwave synthesis of AgNPs. Anal. Chim. Acta 2016, 939, 93–100. [Google Scholar] [CrossRef]
- Milliken, S.; Fraser, J.; Poirier, S.; Hulse, J.; Tay, L.-L. Self-Assembled vertically aligned Au nanorod arrays for Surface-Enhanced Raman scattering (SERS) detection of Cannabinol. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 196, 222–228. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, N.; Ji, D.; Song, H.; Liu, Y.; Zhou, L.; Sun, Z.; Jornet, J.M.; Thompson, A.C.; Collins, R.L.; et al. Superabsorbing Metasurfaces with Hybrid Ag–Au Nanostructures for Surface-Enhanced Raman Spectroscopy Sensing of Drugs and Chemicals. Small Methods 2018, 2, 1800045. [Google Scholar] [CrossRef]
- Xu, S.-Q.; Wen, B.-Y.; Zhang, L.-N.; Zhang, H.; Gao, Y.; Nataraju, B.; Xu, L.-P.; Wang, X.; Li, J.-F.; Tian, Z.-Q. Evaluation of cigarette flavoring quality via Surface-Enhanced Raman spectroscopy. Chem. Commun. 2018, 54, 10882–10885. [Google Scholar] [CrossRef]
- Sivashanmugan, K.; Squire, K.; Tan, A.; Zhao, Y.; Kraai, J.A.; Rorrer, G.L.; Wang, A.X. Trace Detection of Tetrahydrocannabinol in Body Fluid via Surface-Enhanced Raman Scattering and Principal Component Analysis. ACS Sens. 2019, 4, 1109–1117. [Google Scholar] [CrossRef]
- Huang, R.; Han, S.; Li, X.S. Detection of Tobacco-Related biomarkers in urine samples by Surface-Enhanced Raman spectroscopy coupled with Thin-Layer chromatography. Anal. Bioanal. Chem. 2013, 405, 6815–6822. [Google Scholar] [CrossRef] [PubMed]
- Sivashanmugan, K.; Huang, W.-L.; Lin, C.-H.; Liao, J.-D.; Lin, C.-C.; Su, W.-C.; Wen, T.-C. Bimetallic nanoplasmonic Gap-Mode SERS substrate for lung normal and Cancer-Derived exosomes detection. J. Taiwan Inst. Chem. Eng. 2017, 80, 149–155. [Google Scholar] [CrossRef]
- Sivashanmugan, K.; Liao, J.-D.; Shao, P.-L.; Haochih Liu, B.; Tseng, T.-Y.; Chang, C.-Y. Intense Raman scattering on hybrid Au/Ag nanoplatforms for the distinction of MMP-9-digested collagen Type-I fiber detection. Biosens. Bioelectron. 2015, 72, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Chong, X.; Squire, K.; Wang, A.X. Microfluidic diatomite analytical devices for illicit drug sensing with ppb-Level sensitivity. Sens. Actuators B Chem. 2018, 259, 587–595. [Google Scholar] [CrossRef]
- Kong, X.; Squire, K.; Chong, X.; Wang, A.X. Ultra-Sensitive lab-on-a-chip detection of Sudan I in food using Plasmonics-Enhanced diatomaceous thin film. Food Control 2017, 79, 258–265. [Google Scholar] [CrossRef]
- Kong, X.; Squire, K.; Li, E.; LeDuff, P.; Rorrer, G.L.; Tang, S.; Chen, B.; McKay, C.P.; Navarro-Gonzalez, R.; Wang, A.X. Chemical and Biological Sensing Using Diatom Photonic Crystal Biosilica with In-Situ Growth Plasmonic Nanoparticles. IEEE Trans. NanoBiosci. 2016, 15, 828–834. [Google Scholar] [CrossRef]
- Tan, A.; Zhao, Y.; Sivashanmugan, K.; Squire, K.; Wang, A.X. Quantitative TLC-SERS detection of histamine in seafood with support vector machine analysis. Food Control 2019, 103, 111–118. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.-M.; Liu, J.-F.; Liu, R.; Sun, J.-F.; Wei, G.-H. Thin Layer Chromatography Coupled with Surface-Enhanced Raman Scattering as a Facile Method for On-Site Quantitative Monitoring of Chemical Reactions. Anal. Chem. 2014, 86, 7286–7292. [Google Scholar] [CrossRef]
- Leonardo, S.; Prieto-Simón, B.; Campàs, M. Past, present and future of diatoms in biosensing. TrAC Trends Anal. Chem. 2016, 79, 276–285. [Google Scholar] [CrossRef]
- Yang, W.; Lopez, P.J.; Rosengarten, G. Diatoms: Self assembled silica nanostructures, and templates for bio/chemical sensors and biomimetic membranes. Analyst 2011, 136, 42–53. [Google Scholar] [CrossRef]
- Dong, R.; Weng, S.; Yang, L.; Liu, J. Detection and direct readout of drugs in human urine using dynamic Surface-Enhanced Raman spectroscopy and support vector machines. Anal. Chem. 2015, 87, 2937–2944. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Huang, M.; Wang, K.; Song, B.; Wang, Y.; Chen, J.; Liu, X.; Li, X.; Lin, L.; Huang, G. Urine Surface-Enhanced Raman spectroscopy for Non-Invasive diabetic detection based on a portable Raman spectrometer. Laser Phys. Lett. 2016, 13. [Google Scholar] [CrossRef]
- Moreira, L.P.; Silveira, L., Jr.; da Silva, A.G.; Fernandes, A.B.; Pacheco, M.T.T.; Rocco, D. Raman spectroscopy applied to identify metabolites in urine of physically active subjects. J. Photochem. Photobiol. B 2017, 176, 92–99. [Google Scholar] [CrossRef] [PubMed]


| Raman Shifts (cm−1) | Raman Bands Assignments | |
|---|---|---|
| Urea | THC | |
| 627,727 | C-H bending vibration | |
| 780 | C-C stretching vibration | |
| 830 | C-C stretching vibration | |
| 903,937 | 932,958 | C-O-H stretching vibration |
| 1000,1038 | N-C-N stretching vibration | |
| 998,1001 | C-C stretching vibration | |
| 1091 | C-C stretching vibration | |
| 1153,1164,1171,1192 | C-C stretching vibration | |
| 1201,1210,1250 | CH deformation | |
| 1245,1256,1293 | H-C-H bending vibration | |
| 1343,1366,1367 | C-H deformation | |
| 1382,1407 | 1384,1392 | H-C-H bending vibration |
| 1448,1475,1512 | -C-H deformation | |
| 1582,1584 | C-C stretching | |
| 1600–1605 | C-C stretching | |
| 1681 | O-C = O stretching | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sivashanmugan, K.; Zhao, Y.; Wang, A.X. Tetrahydrocannabinol Sensing in Complex Biofluid with Portable Raman Spectrometer Using Diatomaceous SERS Substrates. Biosensors 2019, 9, 125. https://doi.org/10.3390/bios9040125
Sivashanmugan K, Zhao Y, Wang AX. Tetrahydrocannabinol Sensing in Complex Biofluid with Portable Raman Spectrometer Using Diatomaceous SERS Substrates. Biosensors. 2019; 9(4):125. https://doi.org/10.3390/bios9040125
Chicago/Turabian StyleSivashanmugan, Kundan, Yong Zhao, and Alan X. Wang. 2019. "Tetrahydrocannabinol Sensing in Complex Biofluid with Portable Raman Spectrometer Using Diatomaceous SERS Substrates" Biosensors 9, no. 4: 125. https://doi.org/10.3390/bios9040125




