-
 Developing a 3D Model Culture of an EBV+/CD30+ B-Anaplastic Large Cell Lymphoma Cell Line to Assay Brentuximab Vedotin Treatment
Developing a 3D Model Culture of an EBV+/CD30+ B-Anaplastic Large Cell Lymphoma Cell Line to Assay Brentuximab Vedotin Treatment -
 Species-Dependent Structural Variations in Single-Domain Antibodies
Species-Dependent Structural Variations in Single-Domain Antibodies -
 A Novel FLI1 Monoclonal Antibody Which Recognizes EWS::FLI1 with High Affinity Is Useful for Detecting Ewing Sarcoma
A Novel FLI1 Monoclonal Antibody Which Recognizes EWS::FLI1 with High Affinity Is Useful for Detecting Ewing Sarcoma -
 Monoclonal Antibodies and Small-Molecule Therapies for Lichen Planus: Targeted Immunomodulation and Emerging Evidence
Monoclonal Antibodies and Small-Molecule Therapies for Lichen Planus: Targeted Immunomodulation and Emerging Evidence -
 Structure-Guided Stapling of Dimeric Conformations and Linker Engineering Enhance Thermostability and Fine-Tune Activity of Bispecific VHH Cytokine Agonists
Structure-Guided Stapling of Dimeric Conformations and Linker Engineering Enhance Thermostability and Fine-Tune Activity of Bispecific VHH Cytokine Agonists
Journal Description
Antibodies
Antibodies
is an international, peer-reviewed, open access journal on immunoglobulins, published bimonthly online by MDPI.
- Open Access— free for readers, with article processing charges (APC) paid by authors or their institutions.
- High Visibility: indexed within Scopus, ESCI (Web of Science), PubMed, PMC, Embase, CAPlus / SciFinder, and other databases.
- Journal Rank: CiteScore - Q2 (Drug Discovery)
- Rapid Publication: manuscripts are peer-reviewed and a first decision is provided to authors approximately 19.5 days after submission; acceptance to publication is undertaken in 5.7 days (median values for papers published in this journal in the second half of 2025).
- Recognition of Reviewers: reviewers who provide timely, thorough peer-review reports receive vouchers entitling them to a discount on the APC of their next publication in any MDPI journal, in appreciation of the work done.
Impact Factor:
2.7 (2024);
5-Year Impact Factor:
4.7 (2024)
Latest Articles
Transplacental Antibody Transfer: Mechanisms, Pregnancy-Related Disruptions, and Emerging Experimental Models
Antibodies 2026, 15(1), 14; https://doi.org/10.3390/antib15010014 - 6 Feb 2026
Abstract
The transplacental transfer of maternal immunoglobulin G from the mother to the foetus is central for providing immunity in early life, resulting in full-term newborns having IgG repertoires and levels similar to those of their mothers. The neonatal Fc receptor is recognised as
[...] Read more.
The transplacental transfer of maternal immunoglobulin G from the mother to the foetus is central for providing immunity in early life, resulting in full-term newborns having IgG repertoires and levels similar to those of their mothers. The neonatal Fc receptor is recognised as the primary transporter of IgGs across the placental epithelium. Understanding the mechanisms of transplacental antibody transfer and factors that affect them is essential in optimising maternal vaccination strategies, ultimately protecting infants from various environmental pathogens. This review first outlines the biological mechanisms governing transplacental IgG transfer, followed by a discussion of how this process may be disrupted by physiological and pathological conditions during pregnancy, including preterm birth, hypergammaglobulinemia, maternal pathogenic IgG, maternal infections, hyperglycaemia, and exposure to biological therapies. We also summarise currently available models used to study transplacental IgG transfer, highlighting existing knowledge gaps and future directions for research in this field.
Full article
(This article belongs to the Section Humoral Immunity)
►
Show Figures
Open AccessArticle
Antibody Avidity Profiles as Diagnostic Biomarkers in Differentiating Acute and Chronic Anisakis simplex—Related Allergic Diseases
by
Juan González-Fernández, Laura Ullate, Marta Rodero, Alvaro Daschner and Carmen Cuéllar
Antibodies 2026, 15(1), 13; https://doi.org/10.3390/antib15010013 - 6 Feb 2026
Abstract
Background/Objectives: Allergic features of anisakiasis, caused by ingestion of third-stage larvae of Anisakis simplex via raw or undercooked fish, manifest clinically as acute gastroallergic anisakiasis (GAA) or chronic urticaria with Anisakis sensitization (CU+). Differentiating these clinical phenotypes remains challenging. This study aimed to
[...] Read more.
Background/Objectives: Allergic features of anisakiasis, caused by ingestion of third-stage larvae of Anisakis simplex via raw or undercooked fish, manifest clinically as acute gastroallergic anisakiasis (GAA) or chronic urticaria with Anisakis sensitization (CU+). Differentiating these clinical phenotypes remains challenging. This study aimed to evaluate the maturation and avidity of specific antibodies (IgE, IgG4, IgG, and IgA) as biomarkers for discriminating between acute and chronic forms of anisakiasis. Methods: A prospective cohort of 65 patients from Madrid, Spain, was classified into three groups: GAA (n = 22), CU+ (n = 22), and chronic urticaria without sensitization (CU−, n = 21). Serum samples were analyzed for antigen-specific immunoglobulins using ELISA and Western blot. Avidity indices (AIs) were quantified through urea dissociation assays. Statistical comparisons and correlation analyses were performed to associate antibody avidity with clinical phenotype and demographic variables. Results: GAA patients exhibited significantly lower IgE avidity indices compared to CU+ individuals (mean AI: 79.9% vs. 88.5%), indicating a less mature IgE response during acute infection. Conversely, IgG4 and IgG avidity were elevated in GAA relative to CU+, reflecting an active but transient immune response. IgA antibodies were detected in both groups, although avidity differences lacked discriminatory capacity. No sex- or age-related differences in antibody avidity were observed. Longitudinal follow-up of GAA patients demonstrated an increase in IgE avidity over time. Conclusions: Quantitative assessment of antibody avidity, particularly for IgE and IgG4, enhances understanding of A. simplex immunopathogenesis and serves as a valuable biomarker for distinguishing acute from chronic clinical presentations. These findings support the use of avidity indices in the diagnosis, staging, and clinical management of anisakiasis.
Full article
(This article belongs to the Section Antibody-Based Diagnostics)
►▼
Show Figures

Graphical abstract
Open AccessArticle
Comparative In Vitro Evaluation of Anti-HIV Immunotoxin, Antibody–Drug Conjugate, and Radioimmunoconjugate Targeted by the Same Antibody
by
Anne-Sophie Kuhlmann, Tami Peters, Donald K. Hamlin, Yawen Li, Xinyi Wang, Megan Stackhouse, Frances M. Cole, Jasmin Martinez-Reyes, Brenda M. Sandmaier, Hans-Peter Kiem, D. Scott Wilbur, Robert D. Harrington and Seth H. Pincus
Antibodies 2026, 15(1), 12; https://doi.org/10.3390/antib15010012 - 28 Jan 2026
Abstract
Background: We are developing cytotoxic immunoconjugates (CICs) to eliminate HIV-infected cells. We investigated the efficacy and kinetics of killing by different forms of CICs targeted by the same monoclonal antibody (mAb), an immunotoxin (IT), antibody-drug conjugate (ADC), and radioimmunoconjugate (RIC). Methods: We compared
[...] Read more.
Background: We are developing cytotoxic immunoconjugates (CICs) to eliminate HIV-infected cells. We investigated the efficacy and kinetics of killing by different forms of CICs targeted by the same monoclonal antibody (mAb), an immunotoxin (IT), antibody-drug conjugate (ADC), and radioimmunoconjugate (RIC). Methods: We compared in vitro effects of CICs made by conjugating anti-gp41 mAb 7B2 to deglycosylated ricin A chain (7B2-dgA), the anthracycline derivative PNU-159682 (7B2-PNU), or the α-emitting isotope actinium-225 (7B2-225Ac). Kinetic analyses of cell growth were performed measuring electrical impedance every 15 min over a 7-day period using cells stably expressing the HIV envelope and Env-negative parent cells. Results: 7B2-dgA and 7B2-225Ac were more potent and acted more rapidly to kill cells than 7B2-PNU. Both the 7B2-PNU and 7B2-225Ac induced bystander-cell killing, whereas the IT did not and consequently allowed the outgrowth of Env-negative cells. Low dose or brief exposure to 7B2-PNU resulted in an increased rate of cell growth. Conclusions: An IT, ADC, and RIC showed substantial differences in the degree of specific toxicity, kinetics, and mechanisms of killing. The results of this side-by-side comparison have implications for the development of CICs to treat HIV, as well as other conditions.
Full article
(This article belongs to the Special Issue A Festschrift Celebrating Dr. Dimiter Stanchev Dimitrov: Antibodies, Innovation, and Impact on Infectious Disease and Cancer Research)
►▼
Show Figures

Figure 1
Open AccessArticle
Antibody Screening and Binding Prediction Analysis Targeting Stx2
by
Jilei Wu, Chenghua Liu, Fenghao Peng, Zeyuan Yu, Chunxia Qiao, Guang Yang, Heng Luo, Keyi Sun, Ziyao Ning, Jing Wang, Yan Wen and Jijun Yu
Antibodies 2026, 15(1), 11; https://doi.org/10.3390/antib15010011 - 27 Jan 2026
Abstract
Background: Shiga toxin (Stx), produced by enterohemorrhagic Escherichia coli (EHEC), is a highly potent exotoxin responsible for severe complications such as hemolytic uremic syndrome (HUS). Among its isoforms, Stx2 exhibits stronger cytotoxicity and poses greater clinical risk, yet no effective therapy currently
[...] Read more.
Background: Shiga toxin (Stx), produced by enterohemorrhagic Escherichia coli (EHEC), is a highly potent exotoxin responsible for severe complications such as hemolytic uremic syndrome (HUS). Among its isoforms, Stx2 exhibits stronger cytotoxicity and poses greater clinical risk, yet no effective therapy currently exists. Methods: In this study, two human monoclonal antibodies, YG12-1 and YG12-2, were identified from a phage display library and systematically characterized using an integrated modeling–validation workflow. Results: Structural modeling with ImmuneBuilder and Rosetta revealed that YG12-2 possessed a longer CDRH3 topology, more short-range hydrogen bonds, and stronger electrostatic complementarity, corresponding to lower binding energy and higher apparent affinity in ELISA and SPR. Although YG12-2 had a better affinity, YG12-1 shows better protective activity in a murine model of acute peritoneal infection. This paradox highlights a non-linear relationship between structural affinity and biological efficacy, emphasizing the importance of functional epitope accessibility and pharmacokinetic behavior in determining neutralization outcomes. Conlusions: Overall, these results indicated that targeting Stx2 with YG12-1 and YG12-2 could serve as a promising protective strategy against E. coli O157:H7 infection.
Full article
(This article belongs to the Section Antibody-Based Therapeutics)
►▼
Show Figures

Figure 1
Open AccessReview
Antibodies as Tools for Characterization, Isolation and Production Enhancement of Anti-Cancer Drugs and Steroidal Hormones from Ginsenoside and Solasodine Glycoside: A Review
by
Yukihiro Shoyama
Antibodies 2026, 15(1), 10; https://doi.org/10.3390/antib15010010 - 19 Jan 2026
Abstract
There are a vast number of monoclonal antibodies (MAbs) against biological components; however, the number for natural products is less than 50. MAbs against ginsenosides, i.e., dammarane triterpene glycosides contained in ginseng, were prepared to develop an Eastern blotting method that can estimate
[...] Read more.
There are a vast number of monoclonal antibodies (MAbs) against biological components; however, the number for natural products is less than 50. MAbs against ginsenosides, i.e., dammarane triterpene glycosides contained in ginseng, were prepared to develop an Eastern blotting method that can estimate the number of bound sugars and pharmacological activity. Meanwhile, as a method for producing ginsenoside Rg3, which is used as an anti-cancer drug, an affinity column for ginsenoside Rb1 was prepared to isolate the raw material ginsenoside Rb1 in a single step, and a method for obtaining ginsenoside Rg3 through fermentation was proposed. A unique MAb capable of detecting all solasodine glycosides contained in Solanum plants was created to prepare an affinity column capable of isolating solasodine glycosides from S. khasianum fruit in a single step. The single-chain variable fragment gene was induced from the MAb against solasodine glycoside and introduced into the hairy root system of S. khasianum, thereby increasing the solasodine glycoside content more than twofold. As a result, we recognized that this method can be used to breed plants with higher concentrations of plant secondary metabolites like solasodine glycosides. The above results collectively demonstrate that solasodine glycoside can be isolated from S. khasianum in high yields and that this compound enables the production of steroids in high yields through a one-step chemical reaction.
Full article
(This article belongs to the Section Antibody Discovery and Engineering)
►▼
Show Figures

Figure 1
Open AccessArticle
Robustness Evaluation of a Legacy N-Glycan Profiling Method for a Therapeutic Antibody Under ICH Q14 Lifecycle Principles
by
Ming-Ching Hsieh, Chao Richard Li, Margaret A. Velardo, Jingming Zhang and Babita S. Parekh
Antibodies 2026, 15(1), 9; https://doi.org/10.3390/antib15010009 - 15 Jan 2026
Abstract
Background: This study assesses the robustness of a legacy N-glycan profiling method for the therapeutic antibody MAB1 with different Peptide-N-glycosidase F (PNGase F) enzyme sources, solid phase extraction (SPE) cartridges, and reagent stability, aligning with ICH Q14 lifecycle management principles. Glycosylation profiling is
[...] Read more.
Background: This study assesses the robustness of a legacy N-glycan profiling method for the therapeutic antibody MAB1 with different Peptide-N-glycosidase F (PNGase F) enzyme sources, solid phase extraction (SPE) cartridges, and reagent stability, aligning with ICH Q14 lifecycle management principles. Glycosylation profiling is critical for therapeutic antibodies as it influences both function and pharmacokinetics. Method: The legacy N-glycan profiling method, 2-aminobenzoic acid (2-AA) labeling combined with normal-phase HPLC, was re-evaluated to confirm consistent analytical performance in the context of evolving regulatory expectations. The evaluation focused on three key factors: PNGase F enzyme sources, solid-phase extraction (SPE) cartridges, and reagent stability. Results: Commercial PNGase F enzymes showed various performances, with some sources yielding significant differences. Several SPE cartridges were also tested, with certain formats displaying poor recovery and high variability, particularly for sialylated glycans. In addition, reagent stability studies revealed rapid degradation of the labeling reagent within a few days. Conclusions: These results underscore the importance of risk control, continual improvement, and lifecycle management to ensure reliable glycosylation analysis and the sustained robustness of legacy methods.
Full article
(This article belongs to the Special Issue Therapeutic Antibodies: New Trends in Discovery, Developability and Characterization)
►▼
Show Figures

Graphical abstract
Open AccessReview
Paraneoplastic Neurological Syndromes: Advances and Future Perspectives in Immunopathogenesis and Management
by
Stoimen Dimitrov, Mihael Tsalta-Mladenov, Plamena Kabakchieva, Tsvetoslav Georgiev and Silva Andonova
Antibodies 2026, 15(1), 8; https://doi.org/10.3390/antib15010008 - 14 Jan 2026
Abstract
Paraneoplastic neurological syndromes (PNSs) are immune-mediated disorders caused by an antitumor response that cross-reacts with the nervous system, leading to severe and often irreversible neurological disability. Once considered exceedingly rare, PNSs are now increasingly recognized owing to the identification of novel neural autoantibodies,
[...] Read more.
Paraneoplastic neurological syndromes (PNSs) are immune-mediated disorders caused by an antitumor response that cross-reacts with the nervous system, leading to severe and often irreversible neurological disability. Once considered exceedingly rare, PNSs are now increasingly recognized owing to the identification of novel neural autoantibodies, wider use of commercial testing, and the emergence of immune checkpoint inhibitor (ICI)-related neurotoxicity that phenotypically overlaps with classic PNS. In this narrative review, we performed a structured search of PubMed/MEDLINE, Scopus, Web of Science, and Google Scholar, without date restrictions, to summarize contemporary advances in the epidemiology, pathogenesis, diagnosis, and management of PNS. Population-based data show rising incidence, largely reflecting improved ascertainment and expanding indications for ICIs. Pathogenetically, we distinguish T-cell-mediated syndromes associated with intracellular antigens from antibody-mediated disorders targeting neuronal surface proteins, integrating emerging concepts of molecular mimicry, tumor genetics, and HLA-linked susceptibility. The 2021 PNS-Care criteria are also reviewed, which replace earlier “classical/non-classical” definitions with risk-stratified phenotypes and antibodies, and demonstrate superior diagnostic performance while underscoring that “probable” and “definite” PNS should be managed with equal urgency. Newly described antibodies and methodological innovations such as PhIP-Seq, neurofilament light chain, and liquid biopsy are highlighted, which refine tumor search strategies and longitudinal monitoring. Management principles emphasize early tumor control, prompt immunotherapy, and a growing repertoire of targeted agents, alongside specific considerations for ICI-associated neurological syndromes. Remaining challenges include diagnostic delays, limited high-level evidence, and the paucity of validated biomarkers of disease activity. Future work should prioritize prospective, biomarker-driven trials and multidisciplinary pathways to shorten time to diagnosis and improve long-term outcomes in patients with PNS.
Full article
(This article belongs to the Section Humoral Immunity)
►▼
Show Figures

Graphical abstract
Open AccessReview
Autoantibodies as Precision Tools in Connective Tissue Diseases: From Epiphenomenon to Endophenotype
by
Muhammad Soyfoo and Julie Sarrand
Antibodies 2026, 15(1), 7; https://doi.org/10.3390/antib15010007 - 13 Jan 2026
Abstract
Autoantibodies have long been regarded as passive reflections of immune dysregulation in connective tissue diseases (CTDs). Recent advances in systems immunology and molecular pathology have fundamentally redefined them as active molecular fingerprints that delineate distinct disease endophenotypes with predictive power for clinical trajectories
[...] Read more.
Autoantibodies have long been regarded as passive reflections of immune dysregulation in connective tissue diseases (CTDs). Recent advances in systems immunology and molecular pathology have fundamentally redefined them as active molecular fingerprints that delineate distinct disease endophenotypes with predictive power for clinical trajectories and therapeutic responses. Rather than mere epiphenomena, autoantibodies encode precise information about dominant immune pathways, organ tropism, and pathogenic mechanisms. This review synthesizes emerging evidence that autoantibody repertoires—defined by specificity, structural properties, and functional characteristics—stratify patients beyond traditional clinical taxonomy into discrete pathobiological subsets. Specific signatures such as anti-MDA5 in rapidly progressive interstitial lung disease, anti-RNA polymerase III in scleroderma renal crisis, and anti-Ro52/TRIM21 in systemic overlap syndromes illustrate how serological profiles predict outcomes with remarkable precision. Mechanistically, autoantibody pathogenicity is modulated by immunoglobulin isotype distribution, Fc glycosylation patterns, and tissue-specific receptor expression—variables that determine whether an antibody functions as a biomarker or pathogenic effector. The structural heterogeneity of autoantibodies, shaped by cytokine microenvironments and B-cell subset imprinting, creates a dynamic continuum between pro-inflammatory and regulatory states. The integration of serological, transcriptomic, and imaging data establishes a precision medicine framework: autoantibodies function simultaneously as disease classifiers and therapeutic guides. This endophenotype-driven approach is already influencing trial design and patient stratification in systemic lupus erythematosus, systemic sclerosis, and inflammatory myopathies, and is reshaping both clinical practice and scientific taxonomy in CTDs. Recognizing autoantibodies as endophenotypic determinants aligns disease classification with pathogenic mechanism and supports the transition towards immunologically informed therapeutic strategies.
Full article
(This article belongs to the Special Issue Antibody and Autoantibody Specificities in Autoimmunity)
►▼
Show Figures

Graphical abstract
Open AccessReview
Oral Immunotherapy-Induced Changes in IgE, IgG, and IgA: A Review of Antibody Isotype Shifts and Their Clinical Relevance in Food Allergy
by
Giovanni Lasagni, Laura Vetrugno, Chiara Maria Maggiore, Chiara Galassetti, Giulia Di Colo, Francesco Pavan, Andrea Costantino and Lorenzo Dagna
Antibodies 2026, 15(1), 6; https://doi.org/10.3390/antib15010006 - 7 Jan 2026
Abstract
Background: Food allergy is a growing public health concern, and oral immunotherapy (OIT) has emerged as a promising approach to induce desensitization and potentially sustained unresponsiveness to allergenic foods. Changes in humoral immunity, particularly in allergen-specific immunoglobulin levels, play a central role in
[...] Read more.
Background: Food allergy is a growing public health concern, and oral immunotherapy (OIT) has emerged as a promising approach to induce desensitization and potentially sustained unresponsiveness to allergenic foods. Changes in humoral immunity, particularly in allergen-specific immunoglobulin levels, play a central role in the immunological mechanisms underlying OIT. This review aims to summarize the current evidence on how OIT modulates allergen-specific immunoglobulin E (IgE), G (IgG) and A (IgA) responses in individuals with food allergy. Methods: We conducted a review of original research articles reporting longitudinal data on allergen-specific IgE, IgG, and/or IgA in patients undergoing OIT for common food allergens. Results: OIT was consistently associated with a transient increase in allergen-specific IgE levels during early phases, followed by a gradual decline. In contrast, Allergen-specific IgG4 levels showed a robust and sustained increase, correlating with desensitization and proposed to function as blocking antibodies. Several studies also reported an increase in allergen-specific IgA, particularly secretory IgA at mucosal sites, suggesting a potential role in enhancing mucosal tolerance and immune exclusion of allergens. Conclusions: Humoral immune responses during OIT are characterized by distinct and dynamic changes in immunoglobulin patterns. In particular, the rise in IgG4 and, in some cases, IgA suggests a role in promoting tolerance. Monitoring these biomarkers may offer insights into treatment efficacy and support individualized approaches to OIT.
Full article
(This article belongs to the Section Humoral Immunity)
►▼
Show Figures

Figure 1
Open AccessReview
Bispecific Antibodies: Strategies Available to Optimize Their Safe Delivery in Patients with Multiple Myeloma
by
Hannah Victoria Giles and Bhuvan Kishore
Antibodies 2026, 15(1), 5; https://doi.org/10.3390/antib15010005 - 5 Jan 2026
Abstract
►▼
Show Figures
Bispecific antibodies (BsAbs) have emerged as an important new class drugs for the treatment of multiple myeloma (MM) over the last few years. Currently, BsAbs are only licensed for use as monotherapy in patients with relapsed/refractory MM who have had at least three
[...] Read more.
Bispecific antibodies (BsAbs) have emerged as an important new class drugs for the treatment of multiple myeloma (MM) over the last few years. Currently, BsAbs are only licensed for use as monotherapy in patients with relapsed/refractory MM who have had at least three prior lines of treatment and are triple class-exposed (patients who have received an anti-CD38 monoclonal antibody, an immunodulatory drug, and a proteasome inhibitor). However, their use in earlier lines, including in the upfront setting, is being explored in multiple ongoing clinical trials with promising early results. The BsAbs have specific toxicities, including a high rate of low-grade cytokine release syndrome and, less commonly, immune effector cell-associated neurotoxicity syndrome. These immune-related toxicities occur almost exclusively during the initiation phase of the BsAbs. This has led to frequent hospitalization of patients for the duration of the initial step-up dosing phase. Strategies that could facilitate outpatient step-up dosing, such as tocilizumab prophylaxis, will become even more critical if BsAbs move into earlier lines of treatment and are used in larger numbers of patients. Optimizing infection prophylaxis is critical for ensuring the safe delivery of BsAbs as infection is the leading cause of non-relapse mortality in patients being treated with BsAbs. Multiple strategies to minimize the infection risk, including antimicrobial prophylaxis, immunoglobulin replacement, vaccination and reduced dosing frequency, have been evaluated. The clinical data on the efficacy of these supportive measures are described in this review article alongside the available strategies for mitigating and managing CRS and ICANS.
Full article

Figure 1
Open AccessReview
Updates on Antibody Drug Conjugates and Bispecific T-Cell Engagers in SCLC
by
Kinsley Wang, Kyle Taing and Robert Hsu
Antibodies 2026, 15(1), 4; https://doi.org/10.3390/antib15010004 - 4 Jan 2026
Abstract
Background/Objectives: Small-cell lung cancer (SCLC) is an aggressive neuroendocrine malignancy characterized by rapid proliferation, early metastasis, and near-universal relapse after initial therapy. While chemo-immunotherapy modestly improves first-line outcomes, survival after progression remains poor and highlights the urgent need for biomarker-directed strategies. Methods
[...] Read more.
Background/Objectives: Small-cell lung cancer (SCLC) is an aggressive neuroendocrine malignancy characterized by rapid proliferation, early metastasis, and near-universal relapse after initial therapy. While chemo-immunotherapy modestly improves first-line outcomes, survival after progression remains poor and highlights the urgent need for biomarker-directed strategies. Methods: A comprehensive literature search was conducted using major medical databases looking at key relevant studies on SCLC antibody studies. All authors reviewed the literature, assessed study quality, and interpreted the results from each study. Results: Recent advances in antibody–drug conjugates (ADCs) and T-cell engagers (TCEs) have transformed therapeutic development by targeting antigens selectively expressed on SCLC cells, enabling more precise and potentially durable tumor control. DLL3 has emerged as the most clinically relevant target to date, with the bispecific TCE tarlatamab demonstrating meaningful and durable response, manageable cytokine-release toxicity, and ultimately achieving accelerated FDA approval for previously treated extensive-stage SCLC. Concurrently, DLL3-directed ADCs have shown variable efficacy, underscoring the importance of payload selection, linker chemistry, and antigen density. Beyond DLL3, next-generation ADCs targeting TROP2, B7-H3, and SEZ6 have reported encouraging early-phase activity, including response rates exceeding those of existing second-line cytotoxic options, though myelosuppression, interstitial lung disease, and hepatic toxicity remain key considerations. Conclusions: Collectively, these emerging immunotherapies illustrate a shift toward antigen-specific targeting in a disease historically defined by limited therapeutic innovation. Continued optimization of antigen selection, payload and linker engineering, and biomarker-driven trial design will be critical for translating early promise into durable clinical benefit and reshaping the treatment landscape for SCLC.
Full article
(This article belongs to the Section Antibody-Based Therapeutics)
►▼
Show Figures

Figure 1
Open AccessReview
Antibody-Based Therapeutics in Breast Cancer: Clinical and Translational Perspectives
by
Anna Balata and Katarzyna Pogoda
Antibodies 2026, 15(1), 3; https://doi.org/10.3390/antib15010003 - 25 Dec 2025
Abstract
Breast cancer remains the most common malignancy and one of the leading causes of cancer-related death among women worldwide. Advances in antibody-based therapies have improved outcomes across all biological subtypes: HER2-positive, triple-negative, and luminal breast cancer. Monoclonal antibodies such as trastuzumab and pertuzumab
[...] Read more.
Breast cancer remains the most common malignancy and one of the leading causes of cancer-related death among women worldwide. Advances in antibody-based therapies have improved outcomes across all biological subtypes: HER2-positive, triple-negative, and luminal breast cancer. Monoclonal antibodies such as trastuzumab and pertuzumab have established HER2-targeted therapy as a standard of care, while immune checkpoint inhibitors have introduced immunotherapy into the treatment of triple-negative breast cancer. The emergence of antibody–drug conjugates (ADCs), including trastuzumab deruxtecan, sacituzumab govitecan, and datopotamab deruxtecan, has further expanded the available therapeutic options. Bispecific antibodies represent a new generation of agents with the potential to overcome resistance and enhance immune activation. Despite impressive progress, important challenges remain, including resistance mechanisms and the management of treatment-related toxicities. This review summarizes the biological rationale, clinical evidence, resistance mechanisms, and safety profiles of therapies based on monoclonal antibodies, bispecific antibodies, and antibody–drug conjugates in breast cancer. The development of these treatment modalities fosters the implementation of personalized, immunologically informed treatment strategies that are redefining precision oncology in breast cancer.
Full article
(This article belongs to the Section Antibody-Based Therapeutics)
Open AccessArticle
Antibodies Against SARS-CoV-2 Nucleocapsid Protein Possess Autoimmune Properties
by
Alexandra Rak, Yana Zabrodskaya, Pei-Fong Wong and Irina Isakova-Sivak
Antibodies 2026, 15(1), 2; https://doi.org/10.3390/antib15010002 - 22 Dec 2025
Abstract
Background/Objectives: Notwithstanding the declaration by the World Health Organization in May 2023 regarding the conclusion of the COVID-19 pandemic, new cases of this potentially lethal infection continue to be documented globally, exerting a sustained influence on the worldwide economy and social structures. Contemporary
[...] Read more.
Background/Objectives: Notwithstanding the declaration by the World Health Organization in May 2023 regarding the conclusion of the COVID-19 pandemic, new cases of this potentially lethal infection continue to be documented globally, exerting a sustained influence on the worldwide economy and social structures. Contemporary SARS-CoV-2 variants, while associated with a reduced propensity for severe acute pathology, retain the capacity to induce long-term post-COVID syndrome, including in ambulatory patient populations. This clinical phenomenon may be attributable to potential autoimmune reactions hypothetically triggered by antiviral antibodies, thereby underscoring the need for developing novel, universal vaccines against COVID-19. The nucleocapsid protein (N), being one of its most conserved and highly immunogenic components of SARS-CoV-2, presents a promising target for such investigative efforts. However, the protective role of anti-N antibodies, generated during natural infection or through immunization with N-based vaccines, alongside the potential adverse effects associated with their production, remains to be fully elucidated. In the present study, we aim to identify potential sites of homology in structures or sequences between the SARS-CoV-2 N protein and human antigens detected using hyperimmune sera against N protein obtained from mice, rabbits, and hamsters. Methods: We employed Western blot analysis of lysates from human cell lines (MCF7, HEK293T, THP-1, CaCo2, Hep2, T98G, A549) coupled with mass spectrometric identification to assess the cross-reactivity of polyclonal and monoclonal antibodies generated against recombinant SARS-CoV-2 N protein with human self-antigens. Results: We showed that anti-N antibodies developed in mice and rabbits exhibit pronounced immunoreactivity towards specific components of the human proteome. In contrast, anti-N immunoglobulins from hamsters showed no non-specific cross-reactivity with either hamster or human proteomic extracts because of the lack of autoreactivity or immunogenicity differences. Subsequent mass spectrometric analysis of the immunoreactive bands identified principal autoantigenic targets, which were predominantly heat shock proteins (including HSP90-beta, HSP70, mitochondrial HSP60, and HSPA8), histones (H2B, H3.1–3), and key metabolic enzymes (G6PD, GP3, PKM, members of the 1st family of aldo-keto reductases). Conclusions: The results obtained herein highlight the differences in the development of anti-N humoral responses in humans and in the Syrian hamster model. These data provide a foundational basis for formulating clinical recommendations to predict possible autoimmune consequences in COVID-19 convalescents and are of critical importance for the rational design of future N protein-based, cross-protective vaccine candidates against novel coronavirus infections.
Full article
(This article belongs to the Section Humoral Immunity)
►▼
Show Figures

Figure 1
Open AccessReview
Nanobody Therapeutics in Alzheimer’s Disease: From Molecular Mechanisms to Translational Approaches
by
Deepika Godugu, Kranthi Gattu, Parul Suri, Abel B. Daartey, Krishna Jadhav and Satish Rojekar
Antibodies 2026, 15(1), 1; https://doi.org/10.3390/antib15010001 - 19 Dec 2025
Abstract
Nanobodies (single-domain antibodies, VHHs) have emerged as versatile tools for evaluating and treating Alzheimer’s disease (AD). They offer distinct engineering benefits compared with traditional antibodies and small molecules, including small size, stability, and specificity. In AD, nanobodies have been shown in preclinical models
[...] Read more.
Nanobodies (single-domain antibodies, VHHs) have emerged as versatile tools for evaluating and treating Alzheimer’s disease (AD). They offer distinct engineering benefits compared with traditional antibodies and small molecules, including small size, stability, and specificity. In AD, nanobodies have been shown in preclinical models to neutralize toxic amyloid-β oligomers, inhibit tau generation and aggregation, and modulate neuroinflammation, thereby demonstrating significant therapeutic potential. However, all nanobody applications in AD are discussed strictly as preclinical therapeutic potential rather than established clinical therapies, and direct clinical evidence in patients with AD is still lacking. Advanced engineering strategies, including intranasal and intrathecal routes, receptor-mediated transport, plasma protein binding with albumin, and focused ultrasound to facilitate brain penetration. Additionally, to improve nanobody delivery precision, half-life, and efficacy, strategies such as integrating nanobodies with nanoparticles, dendrimers, liposomes, and viral vectors are being employed. In fact, nanobodies are applied beyond monotherapy across multiple technological platforms to optimize brain delivery and target multiple targets. Nanobodies have been used on bispecific and trispecific antibody platforms, as well as in CRISPR/Cas9 editing and AI-driven technologies, to expand their applications. Recently, preclinical evidence has been mounting on the efficacy of nanobodies in clearing Aβ and tau, preserving synapses, and normalizing biomarkers. Comparison with FDA-approved anti-Aβ monoclonal antibodies (aducanumab, lecanemab, and donanemab) highlights opportunities and current translational gaps, including safety testing, half-life extension, and delivery optimization. This review critically delineates the current molecular mechanisms, emerging strategies, and delivery platforms, and emphasizes the potential of nanobodies as promising therapeutic and diagnostic molecules in AD therapeutics.
Full article
(This article belongs to the Section Antibody-Based Therapeutics)
►▼
Show Figures

Graphical abstract
Open AccessArticle
Enhancement of Structural Stability and IgG Affinity of a Z34C-Derived α-Helical Peptide via Lactam Stapling
by
Jung Gu Lee, Inseo Lee, Joo-young Kim, Suin Kim, Woo-jin Jeong and Ji-eun Kim
Antibodies 2025, 14(4), 108; https://doi.org/10.3390/antib14040108 - 16 Dec 2025
Abstract
Background: The Fc region of immunoglobulin G (IgG) is a key target in therapeutic and analytical applications, such as antibody purification and site-specific bioconjugation. Although Protein A exhibits strong Fc-binding affinity, its large molecular weight and limited chemical flexibility pose challenges for use
[...] Read more.
Background: The Fc region of immunoglobulin G (IgG) is a key target in therapeutic and analytical applications, such as antibody purification and site-specific bioconjugation. Although Protein A exhibits strong Fc-binding affinity, its large molecular weight and limited chemical flexibility pose challenges for use in compact or chemically defined systems. To address these limitations, we designed two α-helical peptides, SpA h1 and SpA h2, based on the Fc-binding helices of the Z34C domain from Staphylococcus aureus Protein A. Method: To enhance the structural stability and Fc-binding capability of these peptides, a lactam-based stapling strategy was employed by introducing lysine and glutamic acid residues at positions i and i + 4. Result: The resulting stapled peptides, (s)SpA h1 and (s)SpA h2, exhibited significantly improved α-helical content and IgG-binding performance, as demonstrated by circular dichroism (CD) spectroscopy and fluorescence-based IgG capture assays. Surface plasmon resonance (SPR) analysis confirmed specific, concentration-dependent interactions with the Fc region of human IgG, with (s)SpA h1 consistently showing the binding affinity and stability. Proteolytic resistance assays using α-chymotrypsin revealed that (s)SpA h1 maintained its structural integrity over time, exhibiting markedly enhanced resistance to enzymatic degradation compared to its linear counterpart. Furthermore, (s)SpA h1 exhibited strong Fc selectivity with minimal Fab affinity, confirming its suitability as a compact and Fc-specific binding ligand. Conclusions: These results confirm the successful design and development of structurally reinforced Fc-binding peptides that overcome the inherent limitations of short linear sequences through both high-affinity sequence optimization and lactam-based stapling. Among them, (s)SpA h1 demonstrates the most promising characteristics as a compact yet stable Fc-binding ligand, suitable for applications such as antibody purification and site-specific bioconjugation.
Full article
(This article belongs to the Section Antibody Discovery and Engineering)
►▼
Show Figures

Figure 1
Open AccessArticle
Evaluation of Three Recombinant Antigens for the Detection of Anti-Coxiella Antibodies in Cattle
by
Barbara Colitti, Consiglia Longobardi, Gabriela Flores-Ramirez, Chiara Nogarol, Ludovit Skultety and Gianmarco Ferrara
Antibodies 2025, 14(4), 107; https://doi.org/10.3390/antib14040107 - 12 Dec 2025
Abstract
Background/Objectives: The detection of anti-Coxiella antibodies using serological methods is essential for identifying exposed ruminants and preventing this important zoonotic disease in livestock. In recent years, numerous attempts have been made to increase diagnostic performance as well as simplify the production of serological
[...] Read more.
Background/Objectives: The detection of anti-Coxiella antibodies using serological methods is essential for identifying exposed ruminants and preventing this important zoonotic disease in livestock. In recent years, numerous attempts have been made to increase diagnostic performance as well as simplify the production of serological assays. Commercially available tests often use whole-cell antigens, which can decrease specificity and require high-level biosafety facilities for manufacturing. The aim of this work was to produce three Coxiella burnetii (C. burnetii) antigens in recombinant form and assess them for the detection of anti-Coxiella antibodies in ruminants. Methods: Three recombinant C. burnetii antigens (Com-1, MceB, AdaA) were selected among immunodominant antigens and produced in a heterologous system (Escherichia coli). Following purification, the proteins were utilized to coat ELISA plates and evaluated for seroreactivity against sera from both negative and positive cattle. Results: Com-1 demonstrated the greatest agreement with the commercial test, albeit moderate. MceB exhibited nonspecific reactivity against a large number of sera, while the AdaA showed reactivity against only a few positive sera. Conclusions: Our findings are consistent with previous research, indicating that utilizing a single antigen to identify exposed animals is unfeasible with current knowledge, most likely due to the complex immunological response following C. burnetii infection in cattle. Consequently, it is critical to continue testing and identifying immunoreactive antigens in order to further investigate them and, potentially, select the most appropriate.
Full article
(This article belongs to the Section Antibody-Based Diagnostics)
►▼
Show Figures

Figure 1
Open AccessArticle
A Reproducible Sequence-Level Strategy to Enhance Peptide Immunogenicity While Preserving Wild-Type Epitope Recognition
by
Chia-Hung Chen, Yu-Chi Chiu, Kai-Yao Huang, Hsiao-Hsuan Huang, Ta-Wei Kuo, Yu-Chi Liu, Hui-Ju Kao, Chen-Lin Yu, Shun-Long Weng and Kuang-Wen Liao
Antibodies 2025, 14(4), 106; https://doi.org/10.3390/antib14040106 - 12 Dec 2025
Abstract
Background: Short peptide epitopes are valuable for mechanistic studies, yet their intrinsic low immunogenicity and lack of commercial antibodies hinder rapid antibody generation. Methods: We developed a reproducible, sequence-level workflow combining cross-species/structural triage, independent MHC-I/II prioritization, and conservative heteroclitic-style substitutions to enhance predicted
[...] Read more.
Background: Short peptide epitopes are valuable for mechanistic studies, yet their intrinsic low immunogenicity and lack of commercial antibodies hinder rapid antibody generation. Methods: We developed a reproducible, sequence-level workflow combining cross-species/structural triage, independent MHC-I/II prioritization, and conservative heteroclitic-style substitutions to enhance predicted MHC affinity while preserving native epitope features. Using visfatin as a model, two optimized fragments were conjugated to KLH and tested in mice for antibody titers, isotype profiles, and binding kinetics. Results: Mutant peptides improved MHC-binding prediction, elicited stronger antibody titers, and promoted isotype maturation (increased IgG1). Importantly, antibodies maintained measurable binding to wild-type sequences, indicating preserved cross-recognition. Similar effects were reproduced with additional antigens. Conclusions: This proof-of-concept study, based on small exploratory mouse cohorts (n = 3 per group), demonstrates that strategic, minimal sequence edits can significantly enhance peptide immunogenicity while preserving native epitope recognition. This streamlined workflow provides a low-barrier route to generate epitope-directed antibodies when commercial reagents are unavailable.
Full article
(This article belongs to the Section Antibody Discovery and Engineering)
►▼
Show Figures
Graphical abstract
Open AccessEditor’s ChoiceReview
Breakthrough for Anticancer Immunotherapy: Current Advances in Manufacturing Protocols of Chimeric Antigen Receptor-Based Therapies
by
Yuxin Qian, Weiwei Ma and Xiao-Ning Xu
Antibodies 2025, 14(4), 105; https://doi.org/10.3390/antib14040105 - 8 Dec 2025
Abstract
Chimeric antigen receptor (CAR)-based immunotherapy has emerged as a transformative strategy in anticancer treatment, driven by advances in CAR construct design, manufacturing platforms, and expansion to diverse immune cell types. The landmark success of CD19-targeted CAR-T cell therapy in B cell malignancies has
[...] Read more.
Chimeric antigen receptor (CAR)-based immunotherapy has emerged as a transformative strategy in anticancer treatment, driven by advances in CAR construct design, manufacturing platforms, and expansion to diverse immune cell types. The landmark success of CD19-targeted CAR-T cell therapy in B cell malignancies has paved the way for broader clinical applications. As of 2025, the U.S. FDA has approved multiple autologous CAR-T products, underscoring their therapeutic promise. However, challenges persist, including cytokine release syndrome (CRS), neurotoxicity, product inconsistency, and the high cost and complexity of cell manufacturing. Variations in cell source, gene delivery methods, expansion protocols, and CAR design significantly influence the safety, efficacy, and scalability of these therapies. In this review, we comprehensively examine the current advances in manufacturing protocols for CAR-modified T cells, natural killer (NK) cells, and unconventional T cell subsets, including γδ T, invariant natural killer T (iNKT), and mucosal-associated invariant T (MAIT) cells. We also highlight emerging innovations such as in vivo CAR-T generation and off-the-shelf allogeneic approaches. By integrating updated strategies with a critical evaluation of current limitations, this review aims to support the development of standardized, robust, and accessible CAR-based immunotherapies.
Full article
(This article belongs to the Special Issue Emerging Antibody Engineering Strategies and Applications for Immunotherapy of Cancer)
►▼
Show Figures
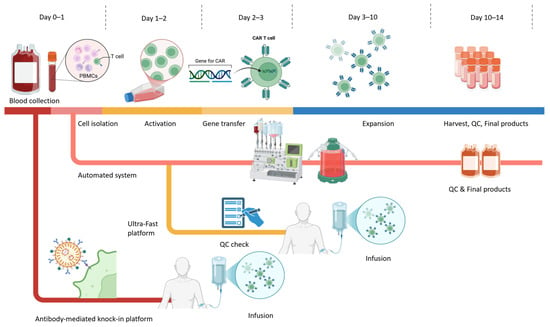
Figure 1
Open AccessReview
Head and Neck Dermatitis in Atopic Dermatitis: A Narrative Review of Pathogenesis, Clinical Challenges, and Therapeutic Strategies
by
Giuseppe Lauletta, Cataldo Patruno, Claudio Brescia, Andrea Cosenza, Carolina D’Elia, Valentina Ventura, Emanuela Martina and Maddalena Napolitano
Antibodies 2025, 14(4), 104; https://doi.org/10.3390/antib14040104 - 5 Dec 2025
Abstract
Background: Head and neck dermatitis (HND) represents a challenging phenotype of atopic dermatitis (AD), often showing suboptimal response or paradoxical worsening during biologic therapy. Objective: To review the efficacy and safety of current systemic treatments for HND, with a focus on
[...] Read more.
Background: Head and neck dermatitis (HND) represents a challenging phenotype of atopic dermatitis (AD), often showing suboptimal response or paradoxical worsening during biologic therapy. Objective: To review the efficacy and safety of current systemic treatments for HND, with a focus on dupilumab, tralokinumab, lebrikizumab, and janus kinase (JAK) inhibitors. Methods: We conducted a narrative review of randomized controlled trials, post hoc analyses, and real-world studies assessing clinical outcomes in patients with moderate-to-severe AD involving the head and neck. Outcomes included Eczema Area and Severity Index (EASI) H&N subscore, erythema grade, patient-reported measures, and adverse events. Results: Dupilumab shows substantial efficacy for HND in both clinical trials and real-life studies; however, responses are often less pronounced than in other anatomical regions, and facial redness (FR) has emerged as a notable adverse event in up to 9% of patients. Tralokinumab and lebrikizumab demonstrate significant improvements in HND involvement, with low incidence of paradoxical reactions. JAK inhibitors, particularly upadacitinib, provide rapid and marked improvement in refractory cases and in patients developing FR during biologic therapy. Conclusions: Systemic therapy for HND should be individualized, balancing efficacy and tolerability. JAK inhibitors represent a valuable alternative in biologic-refractory phenotypes or in patients experiencing dupilumab-associated FR.
Full article
(This article belongs to the Section Antibody-Based Therapeutics)
Open AccessArticle
Comparison of Antigen Conjugation to a Peptidic Carrier or to Bovine Serum Albumin in the Serodiagnosis of Canine Visceral Leishmaniasis via Suspension Array Technology
by
Thais Stelzer Toledo, Pauline Martins Cunha, Josué da Costa Lima-Junior, Monique Paiva De Campos, Alinne R. S. Renzetti, Fabiano Borges Figueiredo, Fernanda Nazaré Morgado, Renato Porrozzi, Fatima da Conceição-Silva, Marta de Almeida Santiago and Paula Mello De Luca
Antibodies 2025, 14(4), 103; https://doi.org/10.3390/antib14040103 - 4 Dec 2025
Abstract
Backgroud/Objectives: Canine Visceral Leishmaniasis (CVL), caused by Leishmania infantum, is a significant public health concern due to dogs serving as reservoirs for human infection. An accurate and rapid diagnostic method to distinguish symptomatic and asymptomatic CVL from healthy and vaccinated animals
[...] Read more.
Backgroud/Objectives: Canine Visceral Leishmaniasis (CVL), caused by Leishmania infantum, is a significant public health concern due to dogs serving as reservoirs for human infection. An accurate and rapid diagnostic method to distinguish symptomatic and asymptomatic CVL from healthy and vaccinated animals is essential for controlling canine and human disease. Developing innovative antibody detection techniques and exploring new antigens are essential for enhancing CVL testing efficiency. Our study focuses on a multiplex flow cytometry technique to detect Leishmania-specific antibodies in canine serum. This involved conjugating small peptides with carrier proteins or peptide tags, sequences designed to facilitate bead coupling. Methods: A peptide from the L. infantum A2 protein was coupled to beads in three forms: unconjugated, conjugated with BSA, and conjugated with a C-terminal β-alanine–lysine (x4)–cysteine TAG. This TAG was previously designed to enhance peptide solubility, improve binding efficiency, and provide functional groups for covalent attachment to the beads, ensuring stable immobilization in the multiplex assay. Results: Our results suggest that the multiplex approach shows promise as a rapid serological test for CVL, particularly with TAG-conjugated peptides, which optimize bead coupling. However, peptide/BSA conjugation revealed anti-BSA antibodies in samples from healthy and CVL dogs. Conclusions: In conclusion, our findings highlight the potential of multiplex methodologies to enhance CVL diagnostics and caution against using BSA as a bead coupling agent in serological tests for canine samples due to its impact on test specificity and sensitivity.
Full article
(This article belongs to the Special Issue Antibodies in Laboratory Diagnostic Techniques)
►▼
Show Figures
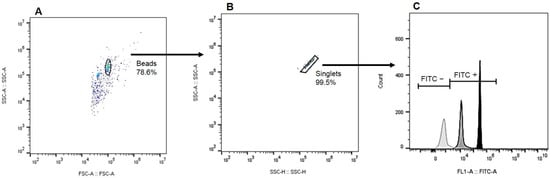
Figure 1

Journal Menu
► ▼ Journal Menu-
- Antibodies Home
- Aims & Scope
- Editorial Board
- Topical Advisory Panel
- Instructions for Authors
- Special Issues
- Topics
- Sections & Collections
- Article Processing Charge
- Indexing & Archiving
- Editor’s Choice Articles
- Most Cited & Viewed
- Journal Statistics
- Journal History
- Journal Awards
- Conferences
- Editorial Office
Journal Browser
► ▼ Journal BrowserHighly Accessed Articles
Latest Books
E-Mail Alert
News
Topics
Topic in
Cancers, CIMB, Current Oncology, Sci. Pharm., Antibodies, IJMS, IJTM
Antibody-Mediated Therapy and Other Emerging Therapies in Cancer Treatment
Topic Editors: Won Sup Lee, Yaewon Yang, Seil GoDeadline: 31 July 2026

Conferences
Special Issues
Special Issue in
Antibodies
Recombinant Binding Proteins and Genetically Engineered T-cells Targeting Intracellular Neoantigens
Guest Editors: Thomas Böldicke, Ana Maria Waaga-GasserDeadline: 25 February 2026
Special Issue in
Antibodies
Selected Papers from The 1st International Online Conference by Antibodies 2025
Guest Editor: Arne SkerraDeadline: 28 February 2026
Special Issue in
Antibodies
Antiphospholipid Antibodies: Beyond Biomarkers
Guest Editors: Md Asiful Islam, Przemysław KotylaDeadline: 25 May 2026
Special Issue in
Antibodies
Antibody-Mediated Rejection in Kidney Transplantation
Guest Editors: Kazuhiro Iwadoh, Hiroto EgawaDeadline: 25 June 2026
Topical Collections
Topical Collection in
Antibodies
Computational Antibody and Antigen Design
Collection Editor: Buyong Ma









