CD4+ T Cells Induced by Tuberculosis Subunit Vaccine H1 Can Improve the HIV-1 Env Humoral Response by Intrastructural Help
Abstract
:1. Introduction
2. Materials and Methods
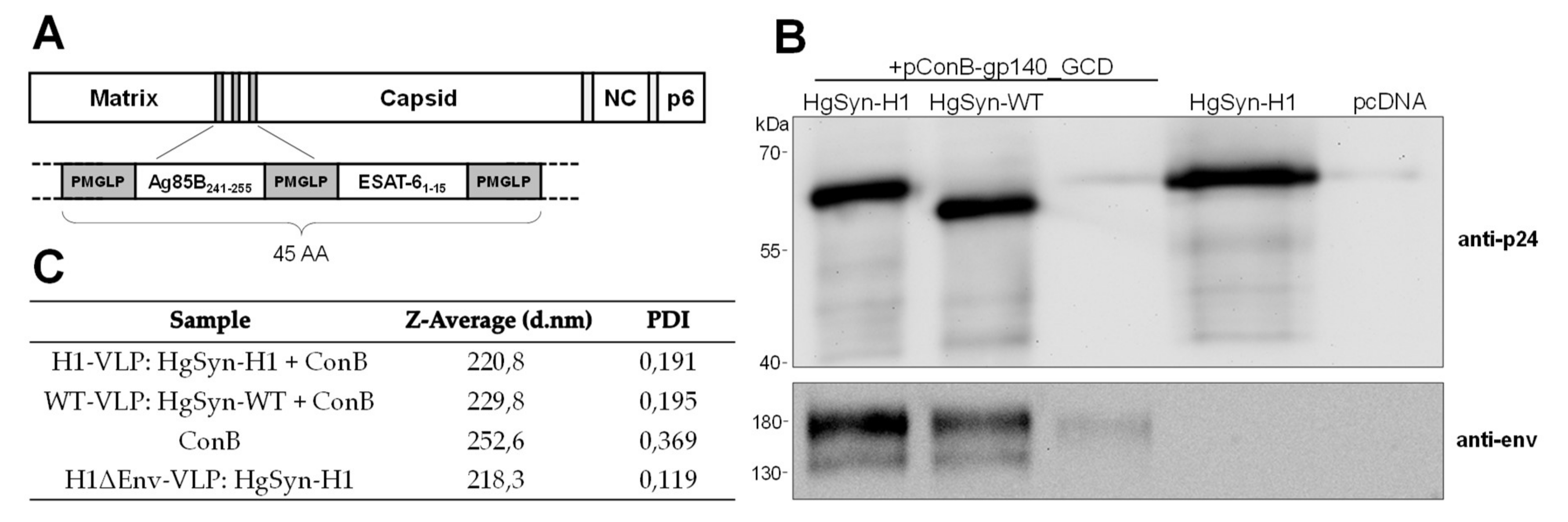
2.1. DNA Plasmids
2.2. Cell Culture
2.3. VLP Purification
2.4. VLP Quantification and Analysis
2.5. Mouse Housing and Ethic Statements
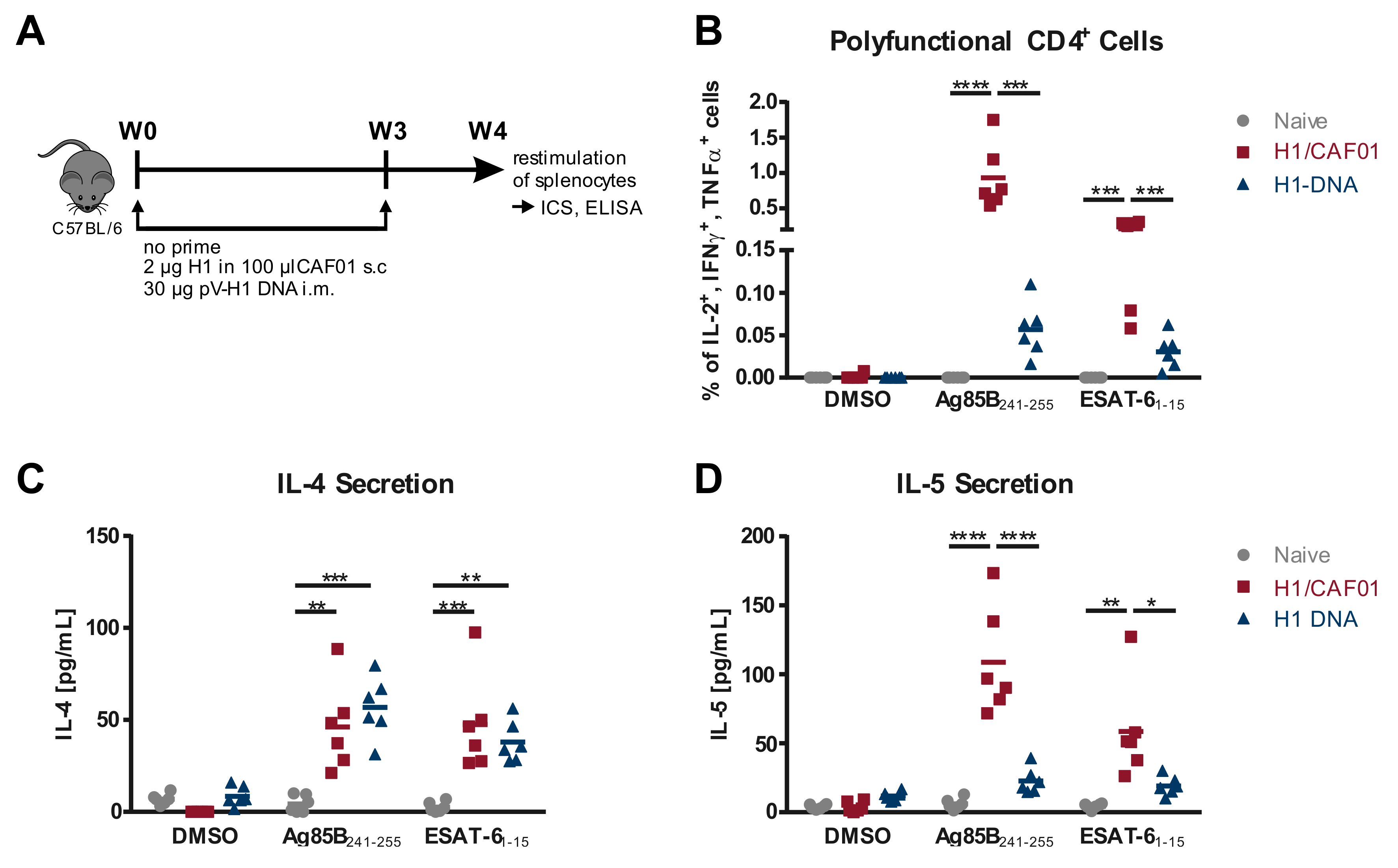
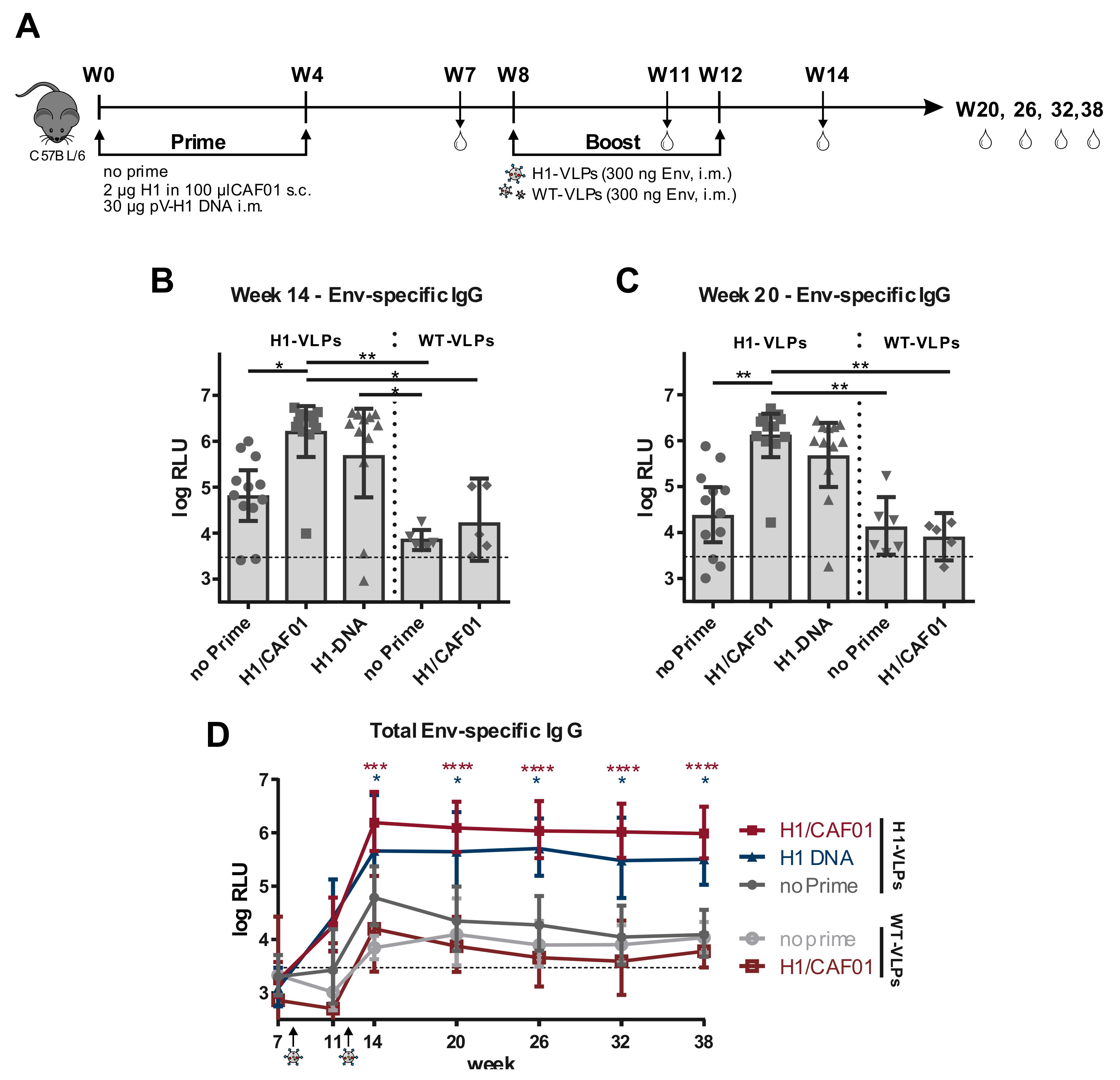
2.6. Immunization
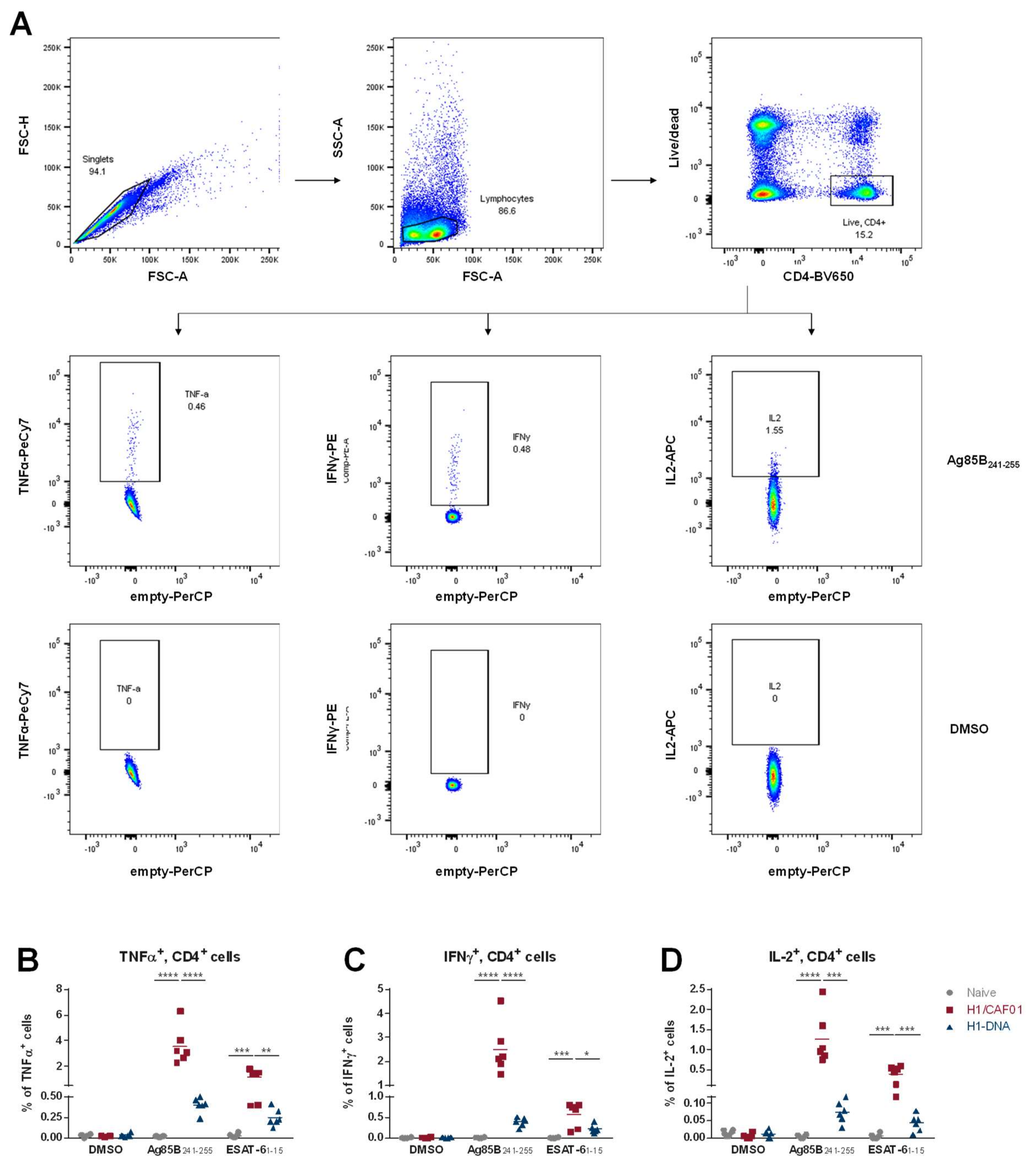
2.7. Analysis of Cellular Immune Response
2.8. Analysis of the Humoral Immune Response
2.9. In Vitro T Cell Activation
2.10. Statistical Analysis
3. Results
3.1. Comparison of T Cell Responses Induced by H1/CAF01 or H1-DNA Immunization
3.2. Generation of Peptide-Bearing VLPs
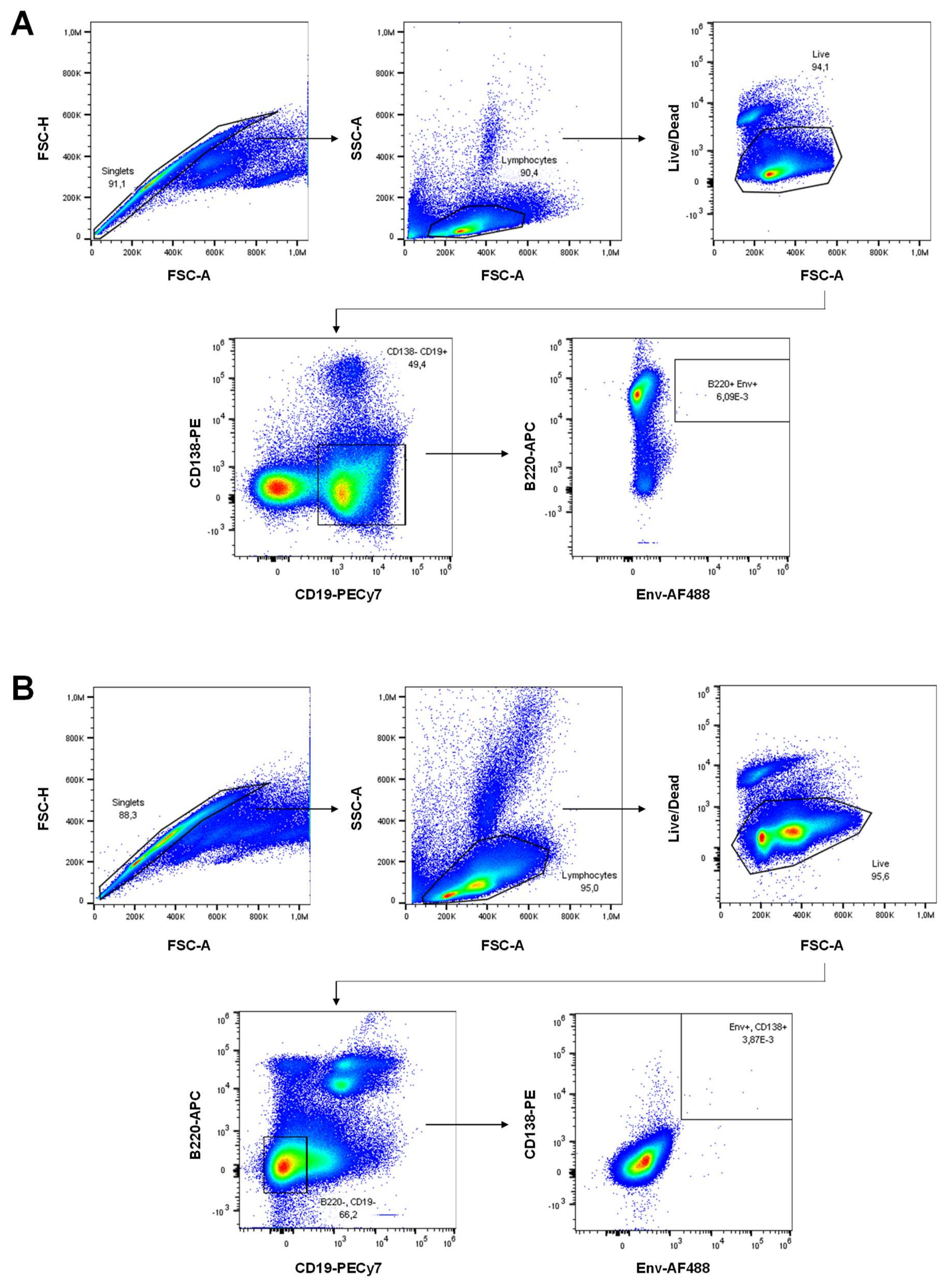
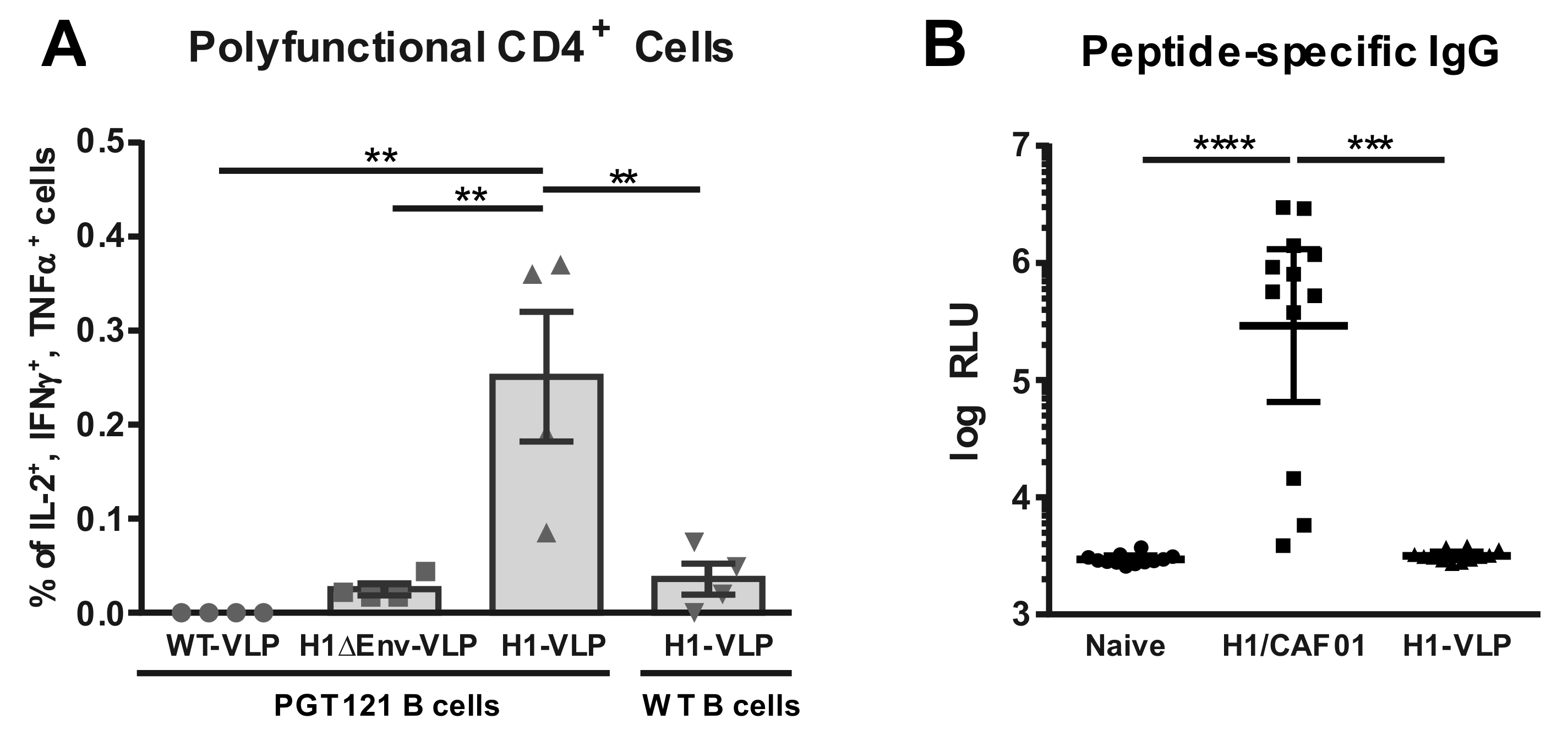
3.3. Analysis of VLP Uptake, Processing, and Peptide Presentation
3.4. H1 Priming Immunization Enhances Env IgG Response by Intrastructural Help
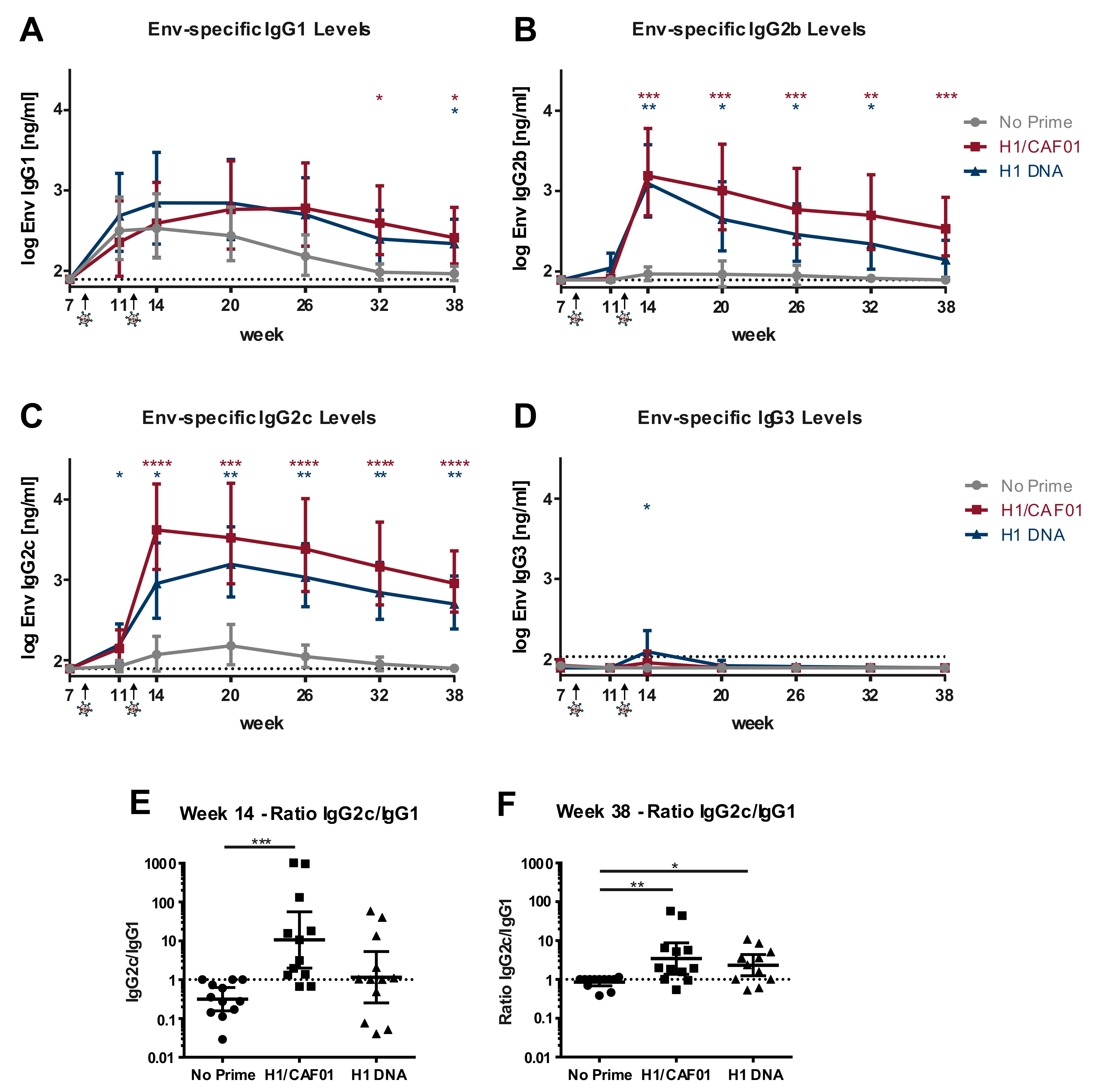
3.5. H1/CAF01 Immunization Can Modulate Env-Specific IgG Isotypes
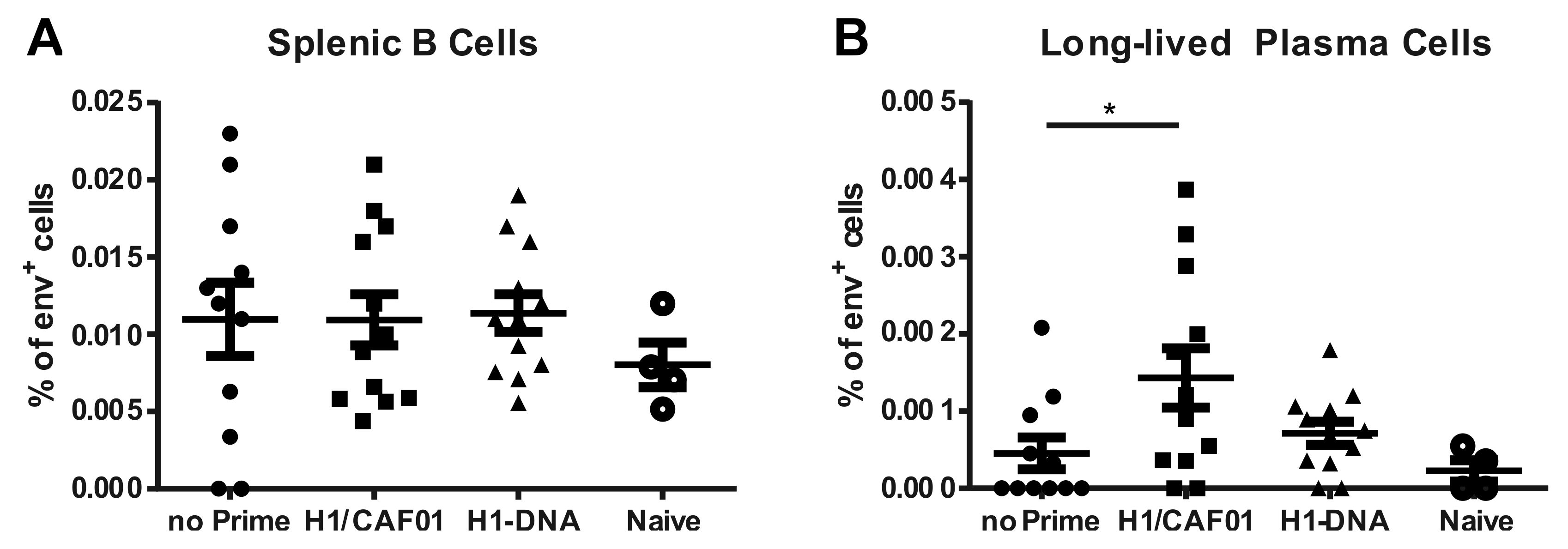
3.6. H1/CAF01 Priming Enhances Frequency of Long-Lived Plasma Cells
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Gao, Y.; McKay, P.F.; Mann, J.F.S. Advances in HIV-1 Vaccine Development. Viruses 2018, 10, 167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rerks-Ngarm, S.; Pitisuttithum, P.; Nitayaphan, S.; Kaewkungwal, J.; Chiu, J.; Paris, R.; Premsri, N.; Namwat, C.; De Souza, M.; Adams, E.; et al. Vaccination with ALVAC and AIDSVAX to Prevent HIV-1 Infection in Thailand. N. Engl. J. Med. 2009, 361, 2209–2220. [Google Scholar] [CrossRef] [PubMed]
- Robb, M.L.; Rerks-Ngarm, S.; Nitayaphan, S.; Pitisuttithum, P.; Kaewkungwal, J.; Kunasol, P.; Khamboonruang, C.; Thongcharoen, P.; Morgan, P.; Benenson, M.; et al. Risk behaviour and time as covariates for efficacy of the HIV vaccine regimen ALVAC-HIV (vCP1521) and AIDSVAX B/E: A post-hoc analysis of the Thai phase 3 efficacy trial RV 144. Lancet Infect. Dis. 2012, 12, 531–537. [Google Scholar] [CrossRef] [Green Version]
- Hessell, A.J.; Hangartner, L.; Hunter, M.; Havenith, C.E.G.; Beurskens, F.J.; Bakker, J.M.; Lanigan, C.M.S.; Landucci, G.; Forthal, D.N.; Parren, P.W.; et al. Fc receptor but not complement binding is important in antibody protection against HIV. Nat. Cell Biol. 2007, 449, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Bournazos, S.; Klein, F.; Pietzsch, J.; Seaman, M.S.; Nussenzweig, M.C.; Ravetch, J.V. Broadly neutralizing anti-HIV-1 antibodies require Fc effector functions for in vivo activity. Cell 2014, 158, 1243–1253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banerjee, K.; Klasse, P.; Sanders, R.W.; Pereyra, F.; Michael, E.; Lu, M.; Walker, B.D.; Moore, J.P. IgG Subclass Profiles in Infected HIV Type 1 Controllers and Chronic Progressors and in Uninfected Recipients of Env Vaccines. AIDS Res. Hum. Retrovir. 2010, 26, 445–458. [Google Scholar] [CrossRef] [Green Version]
- Broliden, A.P.; Morfeldt-Månsson, L.; Rosen, J.; Jondal, M.; Wahren, B. Fine specificity of IgG subclass response to group antigens in HIV-1-infected patients. Clin. Exp. Immunol. 1989, 76, 216–221. [Google Scholar]
- Daly, L.M.; Johnson, P.A.; Donnelly, G.; Nicolson, C.; Robertson, J.; Mills, K.H. Innate IL-10 promotes the induction of Th2 responses with plasmid DNA expressing HIV gp120. Vaccine 2005, 23, 963–974. [Google Scholar] [CrossRef]
- Lu, L.L.; Suscovich, T.J.; Fortune, S.M.; Alter, G. Beyond binding: Antibody effector functions in infectious diseases. Nat. Rev. Immunol. 2017, 18, 46–61. [Google Scholar] [CrossRef]
- Nimmerjahn, F.; Lux, A.; Albert, H.; Woigk, M.; Lehmann, C.; Dudziak, D.; Smith, P.; Ravetch, J.V. FcgammaRIV deletion reveals its central role for IgG2a and IgG2b activity in vivo. Proc. Natl. Acad. Sci. USA 2010, 107, 19396–19401. [Google Scholar] [CrossRef] [Green Version]
- Apostólico, J.D.S.; Boscardin, S.B.; Yamamoto, M.M.; De Oliveira-Filho, J.N.; Kalil, J.; Cunha-Neto, E.; Rosa, D.S. HIV Envelope Trimer Specific Immune Response Is Influenced by Different Adjuvant Formulations and Heterologous Prime-Boost. PLoS ONE 2016, 11, e0145637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banerjee, K.; Andjelic, S.; Klasse, P.J.; Kang, Y.; Sanders, R.W.; Michael, E.; Durso, R.J.; Ketas, T.J.; Olson, W.C.; Moore, J.P. Enzymatic removal of mannose moieties can increase the immune response to HIV-1 gp120 in vivo. Virology 2009, 389, 108–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verma, A.; Schmidt, B.A.; Elizaldi, S.R.; Nguyen, N.K.; Walter, K.A.; Beck, Z.; Trinh, H.V.; Dinasarapu, A.R.; Lakshmanappa, Y.S.; Rane, N.N.; et al. Impact of Th1 CD4 Follicular Helper T Cell Skewing on Antibody Responses to an HIV-1 Vaccine in Rhesus Macaques. J. Virol. 2019, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Visciano, M.L.; Tuen, M.; Gorny, M.K.; Hioe, C.E. In vivo alteration of humoral responses to HIV-1 envelope glycoprotein gp120 by antibodies to the CD4-binding site of gp120. Virology 2008, 372, 409–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nabi, G.; Storcksdieck genannt Bonsmann, M.; Tenbusch, M.; Gardt, O.; Barouch, D.H.; Temchura, V.; Überla, K. GagPol-specific CD4(+) T-cells increase the antibody response to Env by intrastructural help. Retrovirology 2013, 10, 117. [Google Scholar] [CrossRef] [Green Version]
- Storcksdieck genannt Bonsmann, M.; Niezold, T.; Temchura, V.; Pissani, F.; Ehrhardt, K.; Brown, E.P.; Osei-Owusu, N.Y.; Hannaman, E.; Hengel, H.; Ackerman, M.E.; et al. Enhancing the Quality of Antibodies to HIV-1 Envelope by GagPol-Specific Th Cells. J. Immunol. 2015, 195, 4861–4872. [Google Scholar] [CrossRef]
- Temchura, V.; Uberla, K. Intrastructural help: Improving the HIV-1 envelope antibody response induced by virus-like particle vaccines. Curr. Opin. HIV AIDS 2017, 12, 272–277. [Google Scholar] [CrossRef]
- Chamcha, V.; Reddy, P.B.J.; Kannanganat, S.; Wilkins, C.; Gangadhara, S.; Velu, V.; Green, R.; Law, L.; Chang, J.; Bowen, J.R.; et al. Strong TH1-biased CD4 T cell responses are associated with diminished SIV vaccine efficacy. Sci. Transl. Med. 2019, 11, eaav1800. [Google Scholar] [CrossRef]
- Fouts, T.; Bagley, K.; Prado, I.J.; Bobb, K.L.; Schwartz, J.; Xu, R.; Zagursky, R.J.; Egan, M.A.; Eldridge, J.H.; Labranche, C.C.; et al. Balance of cellular and humoral immunity determines the level of protection by HIV vaccines in rhesus macaque models of HIV infection. Proc. Natl. Acad. Sci. USA 2015, 112, E992–E999. [Google Scholar] [CrossRef] [Green Version]
- Staprans, S.I.; Barry, A.P.; Silvestri, G.; Safrit, J.T.; Kozyr, N.; Sumpter, B.; Nguyen, H.; McClure, H.; Montefiori, D.; Cohen, J.I.; et al. Enhanced SIV replication and accelerated progression to AIDS in macaques primed to mount a CD4 T cell response to the SIV envelope protein. Proc. Natl. Acad. Sci. USA 2004, 101, 13026–13031. [Google Scholar] [CrossRef] [Green Version]
- Temchura, V.; Tenbusch, M. The two faces of vaccine-induced immune response: Protection or increased risk of HIV infection? Virol. Sin. 2014, 29, 7–9. [Google Scholar] [CrossRef] [PubMed]
- Tenbusch, M.; Ignatius, R.; Temchura, V.; Nabi, G.; Tippler, B.; Stewart-Jones, G.; Salazar, A.M.; Sauermann, U.; Stahl-Hennig, C.; Überla, K. Risk of Immunodeficiency Virus Infection May Increase with Vaccine-Induced Immune Response. J. Virol. 2012, 86, 10533–10539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elsayed, H.; Nabi, G.; McKinstry, W.J.; Khoo, K.K.; Mak, J.; Salazar, A.M.; Tenbusch, M.; Temchura, V.; Überla, K. Intrastructural Help: Harnessing T Helper Cells Induced by Licensed Vaccines for Improvement of HIV Env Antibody Responses to Virus-Like Particle Vaccines. J. Virol. 2018, 92, e00141-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grun, J.L.; Maurer, P.H. Different T helper cell subsets elicited in mice utilizing two different adjuvant vehicles: The role of endogenous interleukin 1 in proliferative responses. Cell. Immunol. 1989, 121, 134–145. [Google Scholar] [CrossRef]
- Corbett, E.L.; Watt, C.J.; Walker, N.; Maher, D.; Williams, B.G.; Raviglione, M.C.; Dye, C. The growing burden of tuberculosis: Global trends and interactions with the HIV epidemic. Arch. Intern. Med. 2003, 163, 1009–1021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harries, A.D.; Hargreaves, N.J.; Kemp, J.; Jindani, A.; Enarson, A.D.; Maher, D.; Salaniponi, F.M. Deaths from tuberculosis in sub-Saharan African countries with a high prevalence of HIV-1. Lancet 2001, 357, 1519–1523. [Google Scholar] [CrossRef]
- Desel, C.; Werninghaus, K.; Ritter, M.; Jozefowski, K.; Wenzel, J.; Russkamp, N.; Schleicher, U.; Christensen, D.; Wirtz, S.; Kirschning, C.; et al. The Mincle-Activating Adjuvant TDB Induces MyD88-Dependent Th1 and Th17 Responses through IL-1R Signaling. PLoS ONE 2013, 8, e53531. [Google Scholar] [CrossRef] [Green Version]
- Lindenstrøm, T.; Agger, E.M.; Korsholm, K.S.; Darrah, P.A.; Aagaard, C.; Seder, R.A.; Rosenkrands, I.; Andersen, P. Tuberculosis Subunit Vaccination Provides Long-Term Protective Immunity Characterized by Multifunctional CD4 Memory T Cells. J. Immunol. 2009, 182, 8047–8055. [Google Scholar] [CrossRef]
- Van Dissel, J.T.; Joosten, S.A.; Hoff, S.T.; Soonawala, D.; Prins, C.; Hokey, A.D.; O’Dee, D.M.; Graves, A.J.; Thierry-Carstensen, B.; Andreasen, L.V.; et al. A novel liposomal adjuvant system, CAF01, promotes long-lived Mycobacterium tuberculosis-specific T-cell responses in human. Vaccine 2014, 32, 7098–7107. [Google Scholar] [CrossRef] [Green Version]
- Werninghaus, K.; Babiak, A.; Groß, O.; Hölscher, C.; Dietrich, H.; Agger, E.M.; Mages, J.; Mocsai, A.; Schoenen, H.; Finger, K.; et al. Adjuvanticity of a synthetic cord factor analogue for subunit Mycobacterium tuberculosis vaccination requires FcRgamma-Syk-Card9-dependent innate immune activation. J. Exp. Med. 2009, 206, 89–97. [Google Scholar]
- Müller, B.; Daecke, J.; Fackler, O.T.; Dittmar, M.T.; Zentgraf, H.; Kräusslich, H.G. Construction and Characterization of a Fluorescently Labeled Infectious Human Immunodeficiency Virus Type 1 Derivative. J. Virol. 2004, 78, 10803–10813. [Google Scholar]
- Temchura, V.; Kalinin, S.; Nabi, G.; Tippler, B.; Niezold, T.; Überla, K. Divergence of Primary Cognate B- and T-Cell Proliferative Responses to Subcutaneous and Intravenous Immunization with Virus-Like Particles. Viruses 2014, 6, 3334–3347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fraser, C.C.; Altreuter, D.H.; Ilyinskii, P.; Pittet, L.; Lamothe, R.A.; Keegan, M.; Johnston, L.; Kishimoto, T.K. Generation of a universal CD4 memory T cell recall peptide effective in humans, mice and non-human primates. Vaccine 2014, 32, 2896–2903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chesebro, B.; Wehrly, K.; Nishio, J.; Perryman, S. Macrophage-tropic human immunodeficiency virus isolates from different patients exhibit unusual V3 envelope sequence homogeneity in comparison with T-cell-tropic isolates: Definition of critical amino acids involved in cell tropism. J. Virol. 1992, 66, 6547–6554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davidsen, J.; Rosenkrands, I.; Christensen, D.; Vangala, A.; Kirby, D.; Perrie, Y.; Agger, E.M.; Andersen, P. Characterization of cationic liposomes based on dimethyldioctadecylammonium and synthetic cord factor from M. tuberculosis (trehalose 6,6′-dibehenate)—A novel adjuvant inducing both strong CMI and antibody responses. Biochim. Biophys. Acta. 2005, 1718, 22–31. [Google Scholar] [CrossRef] [Green Version]
- Tannig, P.; Peter, A.S.; Lapuente, D.; Klessing, S.; Damm, D.; Tenbusch, M.; Überla, K.; Temchura, V. Modulation of Vaccine-Induced HIV-1-Specific Immune Responses by Co-Electroporation of PD-L1 Encoding DNA. Vaccines 2020, 8, 27. [Google Scholar] [CrossRef] [Green Version]
- Escolano, A.; Steichen, J.M.; Dosenovic, P.; Kulp, D.W.; Golijanin, J.; Sok, D.; Freund, N.T.; Gitlin, A.D.; Oliveira, T.Y.; Araki, T.; et al. Sequential Immunization Elicits Broadly Neutralizing Anti-HIV-1 Antibodies in Ig Knockin Mice. Cell 2016, 166, 1445–1458.e12. [Google Scholar] [CrossRef] [Green Version]
- Binley, J.M.; Lybarger, E.A.; Crooks, E.T.; Seaman, M.S.; Gray, E.S.; Davis, K.L.; Decker, J.M.; Wycuff, D.; Harris, L.; Hawkins, N.; et al. Profiling the Specificity of Neutralizing Antibodies in a Large Panel of Plasmas from Patients Chronically Infected with Human Immunodeficiency Virus Type 1 Subtypes B and C. J. Virol. 2008, 82, 11651–11668. [Google Scholar] [CrossRef] [Green Version]
- Khalife, J.; Guy, B.; Capron, M.; Kieny, M.-P.; Ameisen, J.-C.; Montagnier, L.; Lecocq, J.-P.; Capron, A. Isotypic Restriction of the Antibody Response to Human Immunodeficiency Virus. AIDS Res. Hum. Retrovir. 1988, 4, 3–9. [Google Scholar] [CrossRef]
- Tomaras, G.D.; Haynes, B.F. HIV-1-specific antibody responses during acute and chronic HIV-1 infection. Curr. Opin. HIV AIDS 2009, 4, 373–379. [Google Scholar] [CrossRef] [Green Version]
- Ackerman, M.E.; Mikhailova, A.; Brown, E.P.; Dowell, K.G.; Walker, B.D.; Bailey-Kellogg, C.; Suscovich, T.J.; Alter, G. Polyfunctional HIV-Specific Antibody Responses Are Associated with Spontaneous HIV Control. PLoS Pathog. 2016, 12, e1005315. [Google Scholar] [CrossRef] [PubMed]
- Chung, A.W.; Ghebremichael, M.; Robinson, H.; Brown, E.; Choi, I.; Lane, S.; Dugast, A.-S.; Schoen, M.K.; Rolland, M.; Suscovich, T.J.; et al. Polyfunctional Fc-Effector Profiles Mediated by IgG Subclass Selection Distinguish RV144 and VAX003 Vaccines. Sci. Transl. Med. 2014, 6, 228ra38. [Google Scholar] [CrossRef]
- Excler, J.-L.; Ake, J.; Robb, M.L.; Kim, J.H.; Plotkin, S.A. Nonneutralizing Functional Antibodies: A New “Old” Paradigm for HIV Vaccines. Clin. Vaccine Immunol. 2014, 21, 1023–1036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.H.; Excler, J.-L.; Michael, N.L. Lessons from the RV144 Thai Phase III HIV-1 Vaccine Trial and the Search for Correlates of Protection. Annu. Rev. Med. 2015, 66, 423–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tay, M.Z.; Liu, P.; Williams, L.D.; McRaven, M.D.; Sawant, S.; Gurley, T.C.; Xu, T.T.; Dennison, S.M.; Liao, H.-X.; Chenine, A.-L.; et al. Antibody-Mediated Internalization of Infectious HIV-1 Virions Differs among Antibody Isotypes and Subclasses. PLoS Pathog. 2016, 12, e1005817. [Google Scholar] [CrossRef] [Green Version]
- Yates, N.L.; Liao, H.-X.; Fong, Y.; DeCamp, A.; Vandergrift, N.A.; Williams, W.T.; Alam, S.M.; Ferrari, G.; Yang, Z.-Y.; Seaton, K.E.; et al. Vaccine-Induced Env V1-V2 IgG3 Correlates with Lower HIV-1 Infection Risk and Declines Soon After Vaccination. Sci. Transl. Med. 2014, 6, 228ra39. [Google Scholar] [CrossRef] [Green Version]
- Asbach, B.; Wagner, R. Particle-based delivery of the HIV envelope protein. Curr. Opin. HIV AIDS 2017, 12, 265–271. [Google Scholar] [CrossRef]
- Bachmann, M.F.; Jennings, G.T. Vaccine delivery: A matter of size, geometry, kinetics and molecular patterns. Nat. Rev. Immunol. 2010, 10, 787–796. [Google Scholar] [CrossRef]
- Chroboczek, J.; Szurgot, I.; Szolajska, E. Virus-like particles as vaccine. Acta Biochim. Pol. 2014, 61, 531–539. [Google Scholar] [CrossRef]
- Krueger, C.C.; Thoms, F.; Keller, E.; Vogel, M.; Bachmann, M.F. Virus-Specific Secondary Plasma Cells Produce Elevated Levels of High-Avidity Antibodies but Are Functionally Short Lived. Front. Immunol. 2019, 10, 1831. [Google Scholar] [CrossRef] [Green Version]
- Coutelier, J.-P.; Van Der Logt, J.T.M.; Heessen, F.W.A.; Warnier, G.; Van Snick, J. IgG2a restriction of murine antibodies elicited by viral infections. J. Exp. Med. 1987, 165, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Fossati-Jimack, L.; Ioan-Facsinay, A.; Reininger, L.; Chicheportiche, Y.; Watanabe, N.; Saito, T.; Hofhuis, F.M.A.; Gessner, J.E.; Schiller, C.; Schmidt, R.E.; et al. Markedly Different Pathogenicity of Four Immunoglobulin G Isotype-Switch Variants of an Antierythrocyte Autoantibody Is Based on Their Capacity to Interact in Vivo with the Low-Affinity Fcγ Receptor III. J. Exp. Med. 2000, 191, 1293–1302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markine-Goriaynoff, D.; Coutelier, J.-P. Increased Efficacy of the Immunoglobulin G2a Subclass in Antibody-Mediated Protection against Lactate Dehydrogenase-Elevating Virus-Induced Polioencephalomyelitis Revealed with Switch Mutants. J. Virol. 2002, 76, 432–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nimmerjahn, F. Divergent Immunoglobulin G Subclass Activity Through Selective Fc Receptor Binding. Science 2005, 310, 1510–1512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snapper, C.M.; Paul, E.W. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science 1987, 236, 944–947. [Google Scholar] [CrossRef]
- Mitsdoerffer, M.; Lee, Y.; Jäger, A.; Kim, H.-J.; Korn, T.; Kolls, J.K.; Cantor, H.; Bettelli, E.; Kuchroo, V.K. Proinflammatory T helper type 17 cells are effective B-cell helpers. Proc. Natl. Acad. Sci. USA 2010, 107, 14292–14297. [Google Scholar] [CrossRef] [Green Version]
- Stebegg, M.; Kumar, S.; Silva-Cayetano, A.; Fonseca, V.R.; Linterman, M.A.; Graca, L. Regulation of the Germinal Center Response. Front. Immunol. 2018, 9, 2469. [Google Scholar] [CrossRef] [Green Version]
- Vono, M.; Eberhardt, C.S.; Mohr, E.; Auderset, F.; Christensen, D.; Schmolke, M.; Coler, R.; Meinke, A.; Andersen, P.; Lambert, P.-H.; et al. Overcoming the Neonatal Limitations of Inducing Germinal Centers through Liposome-Based Adjuvants Including C-Type Lectin Agonists Trehalose Dibehenate or Curdlan. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Donnelly, J.J.; Wahren, B.; Liu, M.A. DNA Vaccines: Progress and Challenges. J. Immunol. 2005, 175, 633–639. [Google Scholar] [CrossRef]
- Ng, T.W.; Wirchnianski, A.S.; Wec, A.Z.; Fels, J.M.; Johndrow, C.T.; Saunders, K.O.; Liao, H.-X.; Chan, J.; Jacobs, W.R., Jr.; Chandran, K.; et al. Exploiting Pre-Existing CD4+ T Cell Help from Bacille Calmette–Guérin Vaccination to Improve Antiviral Antibody Responses. J. Immunol. 2020, 205, 425–437. [Google Scholar] [CrossRef]
- Coffman, R.L.; Sher, A.; Seder, R.A. Vaccine Adjuvants: Putting Innate Immunity to Work. Immunity 2010, 33, 492–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Didierlaurent, A.M.; Morel, S.; Lockman, L.; Giannini, S.L.; Bisteau, M.; Carlsen, H.; Kielland, A.; Vosters, O.; Vanderheyde, N.; Schiavetti, F.; et al. AS04, an Aluminum Salt- and TLR4 Agonist-Based Adjuvant System, Induces a Transient Localized Innate Immune Response Leading to Enhanced Adaptive Immunity. J. Immunol. 2009, 183, 6186–6197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eng, N.F.; Bhardwaj, N.; Mulligan, R.; Diaz-Mitoma, F. The potential of 1018 ISS adjuvant in hepatitis B vaccines: HEPLISAV™ review. Hum. Vaccines Immunother. 2013, 9, 1661–1672. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Horner, A.A.; Takabayashi, K.; Nguyen, M.D.; Huang, E.; Cinman, N.; Raz, E. Immunostimulatory DNA pre-priming: A novel approach for prolonged Th1-biased immunity. Cell Immunol. 1999, 198, 69–75. [Google Scholar] [CrossRef]
- Barnowski, C.; Kadzioch, N.P.; Damm, D.; Yan, H.; Temchura, V. Advantages and Limitations of Integrated Flagellin Adjuvants for HIV-Based Nanoparticle B-Cell Vaccines. Pharmaceutics 2019, 11, 204. [Google Scholar] [CrossRef] [Green Version]
- Woodruff, M.C.; Kim, E.H.; Luo, W.; Pulendran, B. B Cell Competition for Restricted T Cell Help Suppresses Rare-Epitope Responses. Cell Rep. 2018, 25, 321–327.e3. [Google Scholar] [CrossRef] [Green Version]
- Bachmann, M.F.; Rohrer, U.H.; Kündig, T.M.; Burki, K.; Hengartner, H.; Zinkernagel, R.M. The influence of antigen organization on B cell responsiveness. Science 1993, 262, 1448–1451. [Google Scholar] [CrossRef]
- Jennings, G.T.; Bachmann, M.F. The coming of age of virus-like particle vaccines. Biol. Chem. 2008, 389, 521–536. [Google Scholar] [CrossRef]
- Justewicz, D.M.; Doherty, P.C.; Webster, R.G. The B-cell response in lymphoid tissue of mice immunized with various antigenic forms of the influenza virus hemagglutinin. J. Virol. 1995, 69, 5414–5421. [Google Scholar] [CrossRef] [Green Version]
- Suleiman, E.; Damm, D.; Batzoni, M.; Temchura, V.; Wagner, A.; Überla, K.; Uhl, K. Electrostatically Driven Encapsulation of Hydrophilic, Non-Conformational Peptide Epitopes into Liposomes. Pharmaceutics 2019, 11, 619. [Google Scholar] [CrossRef] [Green Version]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klessing, S.; Temchura, V.; Tannig, P.; Peter, A.S.; Christensen, D.; Lang, R.; Überla, K. CD4+ T Cells Induced by Tuberculosis Subunit Vaccine H1 Can Improve the HIV-1 Env Humoral Response by Intrastructural Help. Vaccines 2020, 8, 604. https://doi.org/10.3390/vaccines8040604
Klessing S, Temchura V, Tannig P, Peter AS, Christensen D, Lang R, Überla K. CD4+ T Cells Induced by Tuberculosis Subunit Vaccine H1 Can Improve the HIV-1 Env Humoral Response by Intrastructural Help. Vaccines. 2020; 8(4):604. https://doi.org/10.3390/vaccines8040604
Chicago/Turabian StyleKlessing, Stephan, Vladimir Temchura, Pierre Tannig, Antonia Sophia Peter, Dennis Christensen, Roland Lang, and Klaus Überla. 2020. "CD4+ T Cells Induced by Tuberculosis Subunit Vaccine H1 Can Improve the HIV-1 Env Humoral Response by Intrastructural Help" Vaccines 8, no. 4: 604. https://doi.org/10.3390/vaccines8040604




