Biosurfactant-and-Bioemulsifier Produced by a Promising Cunninghamella echinulata Isolated from Caatinga Soil in the Northeast of Brazil
Abstract
:1. Introduction
2. Results and Discussion
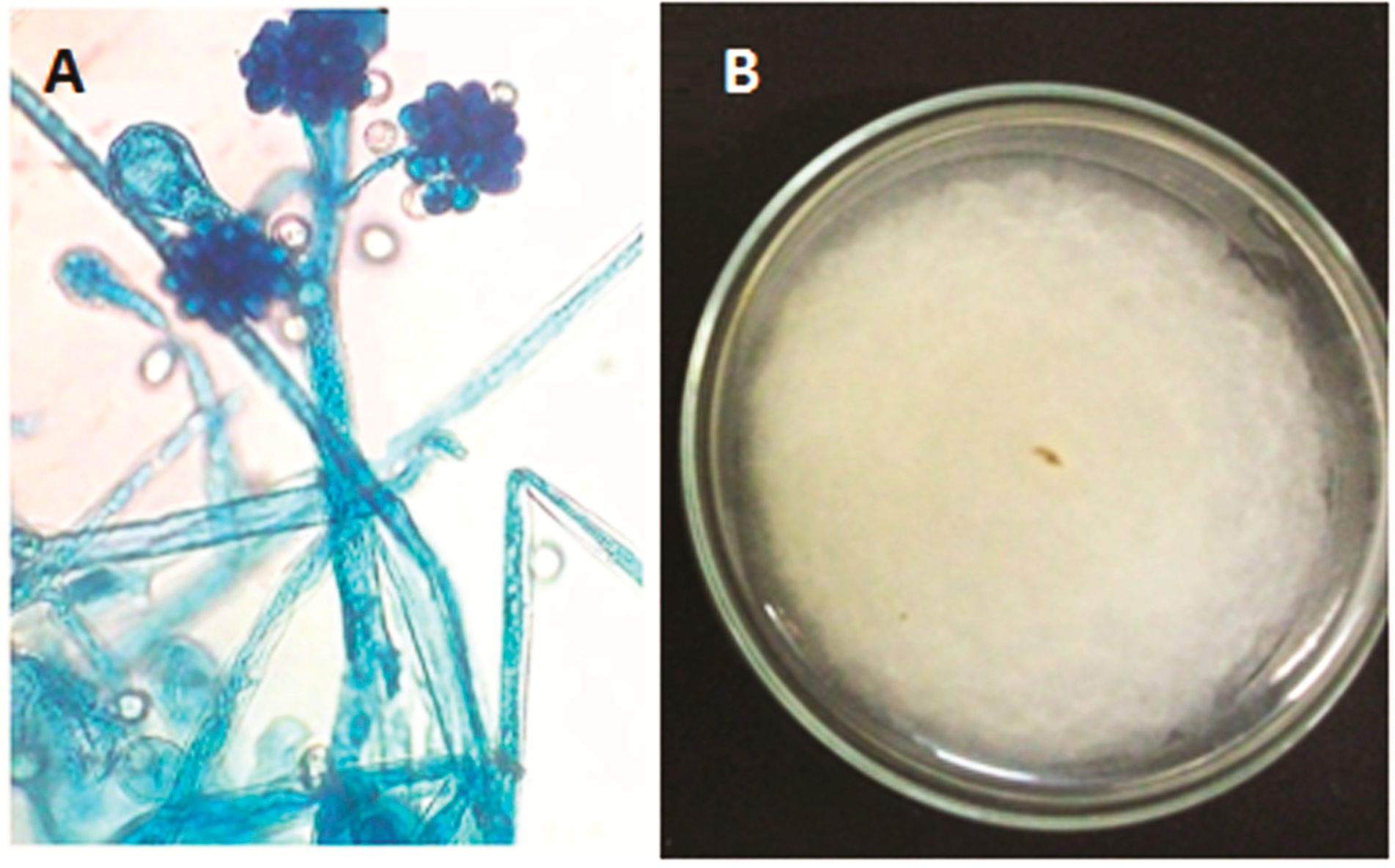
2.1. Description of the Isolate of Cunninghamella echinulata
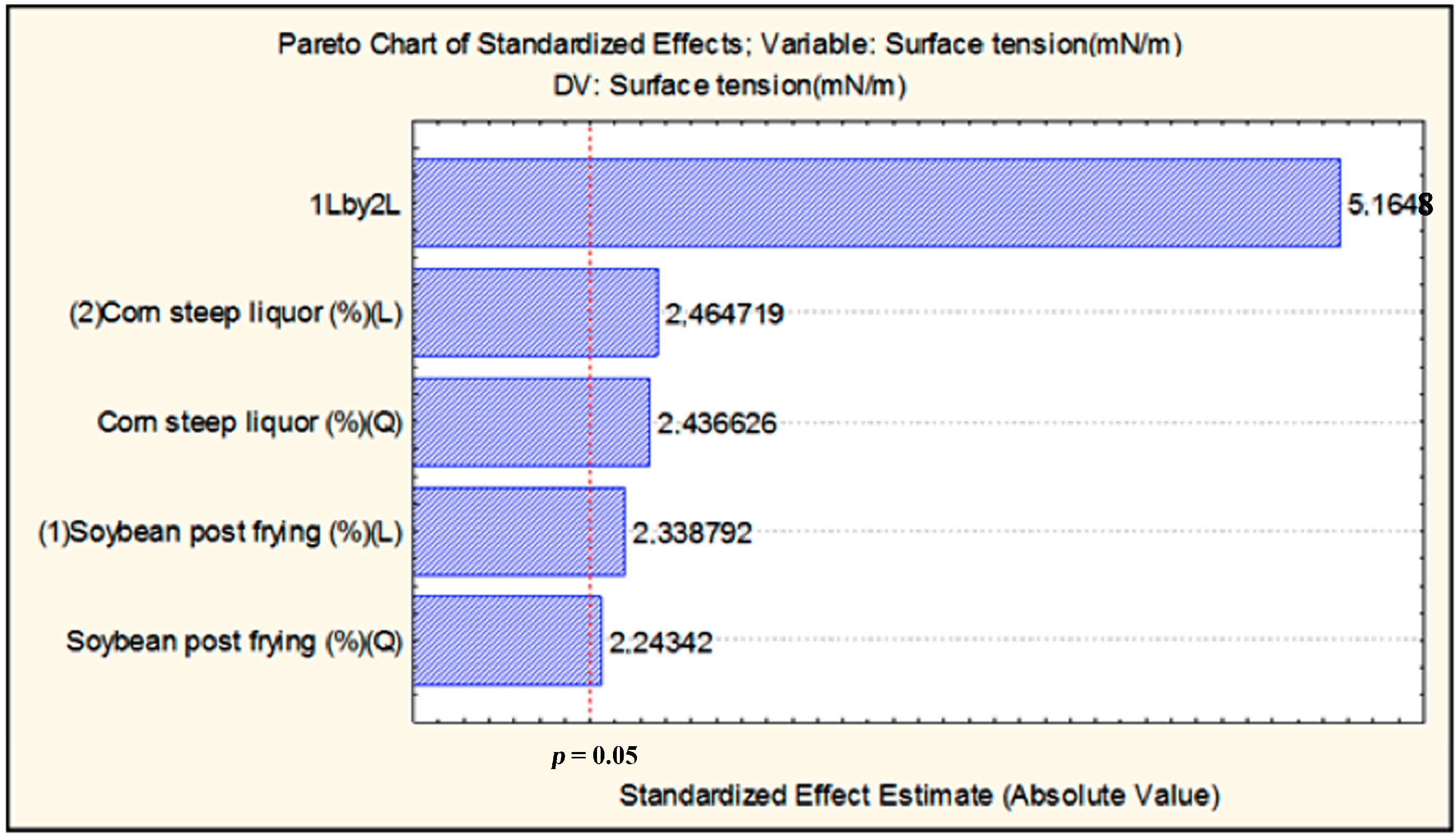
2.2. Time-Course of Growth, Carbohydrate Consumption, pH, and Biosurfactant Production
| Time (h) | Biomass (g·L−1) | pH | Carbohydrate Consumption (g·L−1) | * Biosurfactant Surface Tension (mN/m) |
|---|---|---|---|---|
| 24 | 15.0 | 5.0 | 2.30 | 42.9 |
| 48 | 30.0 | 5.7 | 1.72 | 41.0 |
| 72 | 31.0 | 7.1 | 0.17 | 37.0 |
| 96 | 32.0 | 7.1 | 0.07 | 36.0 |
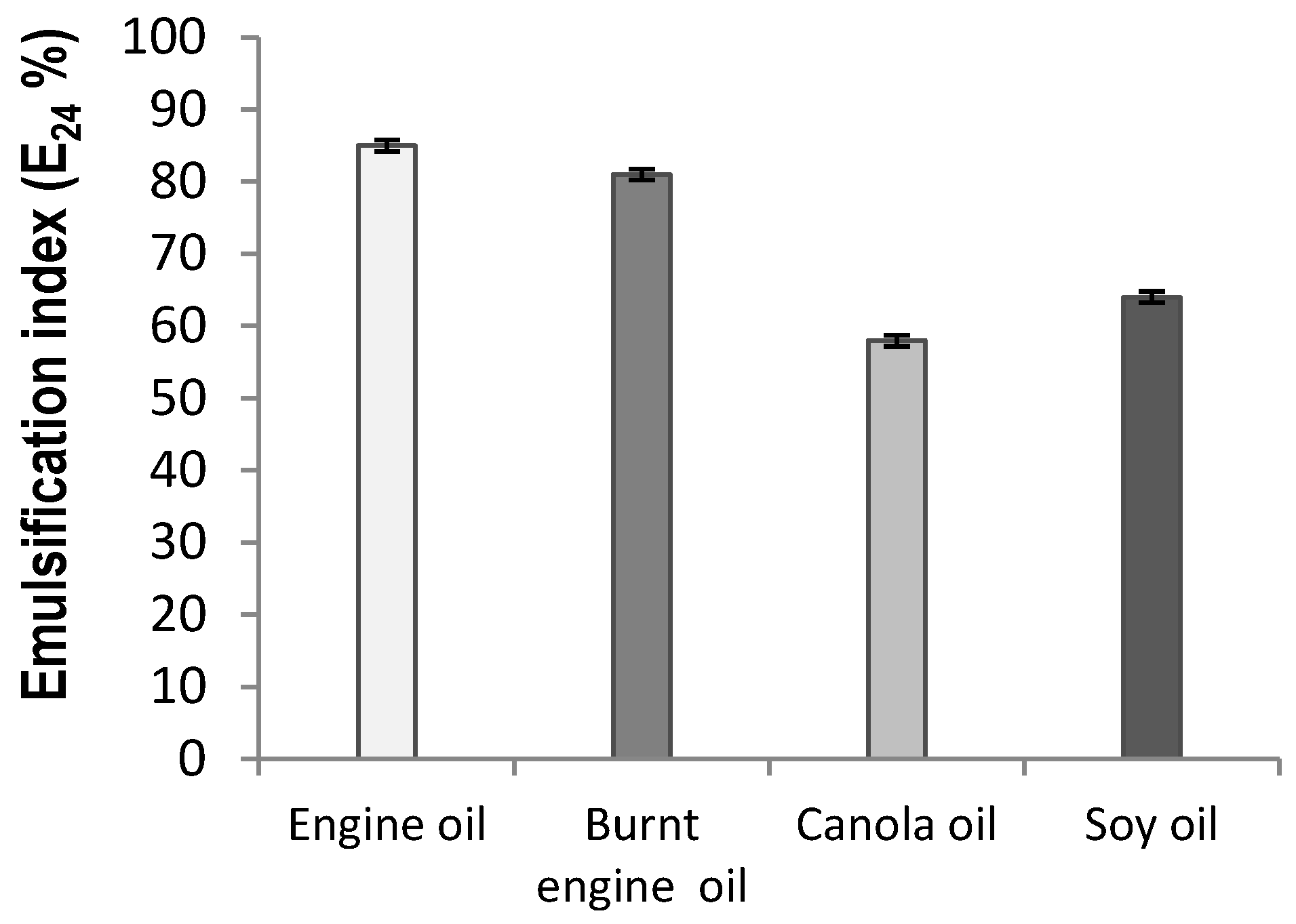
2.3. Emulsification Characteristic of the Biosurfactant
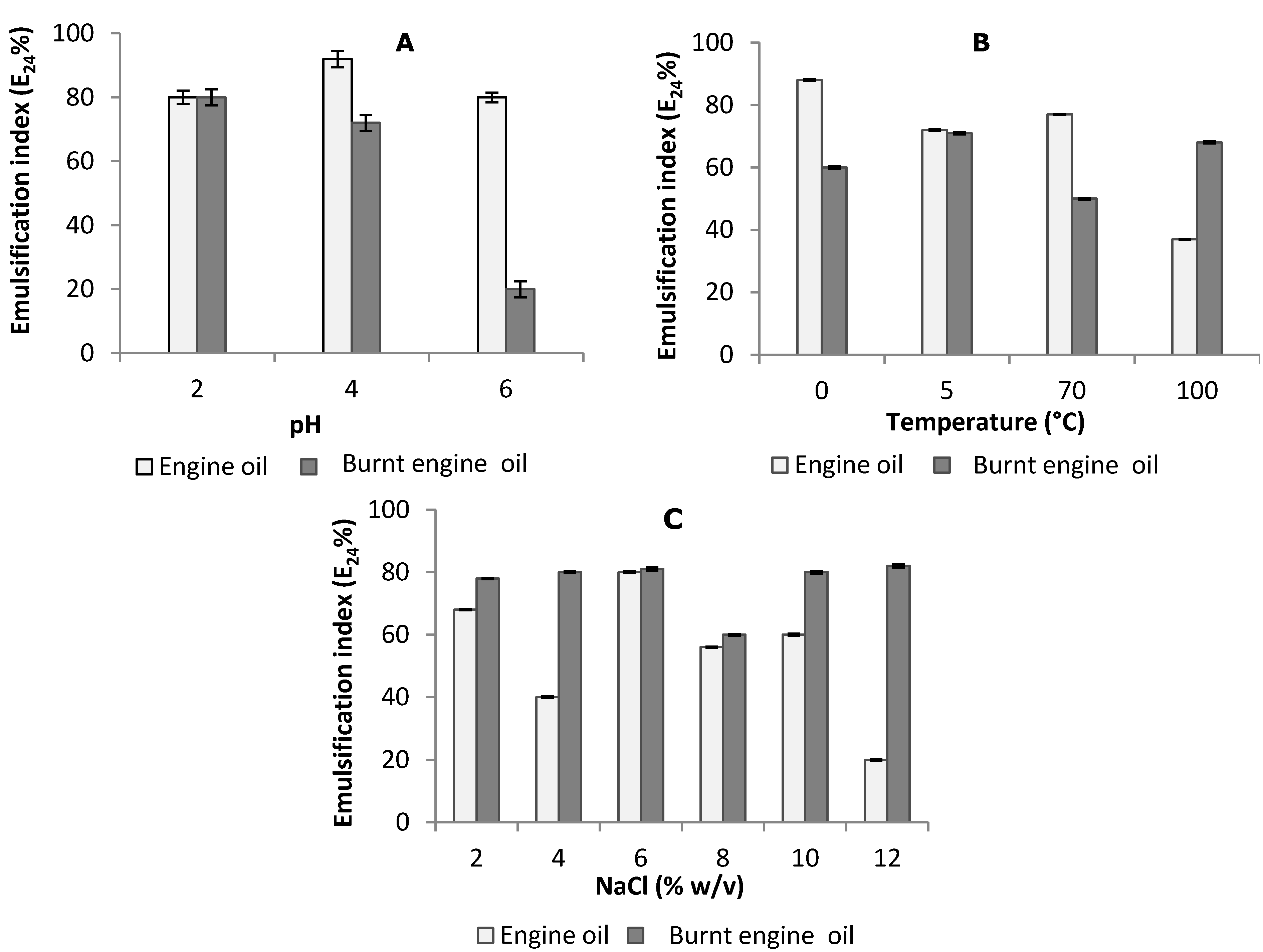
2.4. Stability Studies

2.5. Yield, CMC, Ionic Charge and Preliminary Chemical Composition of Biosurfactant
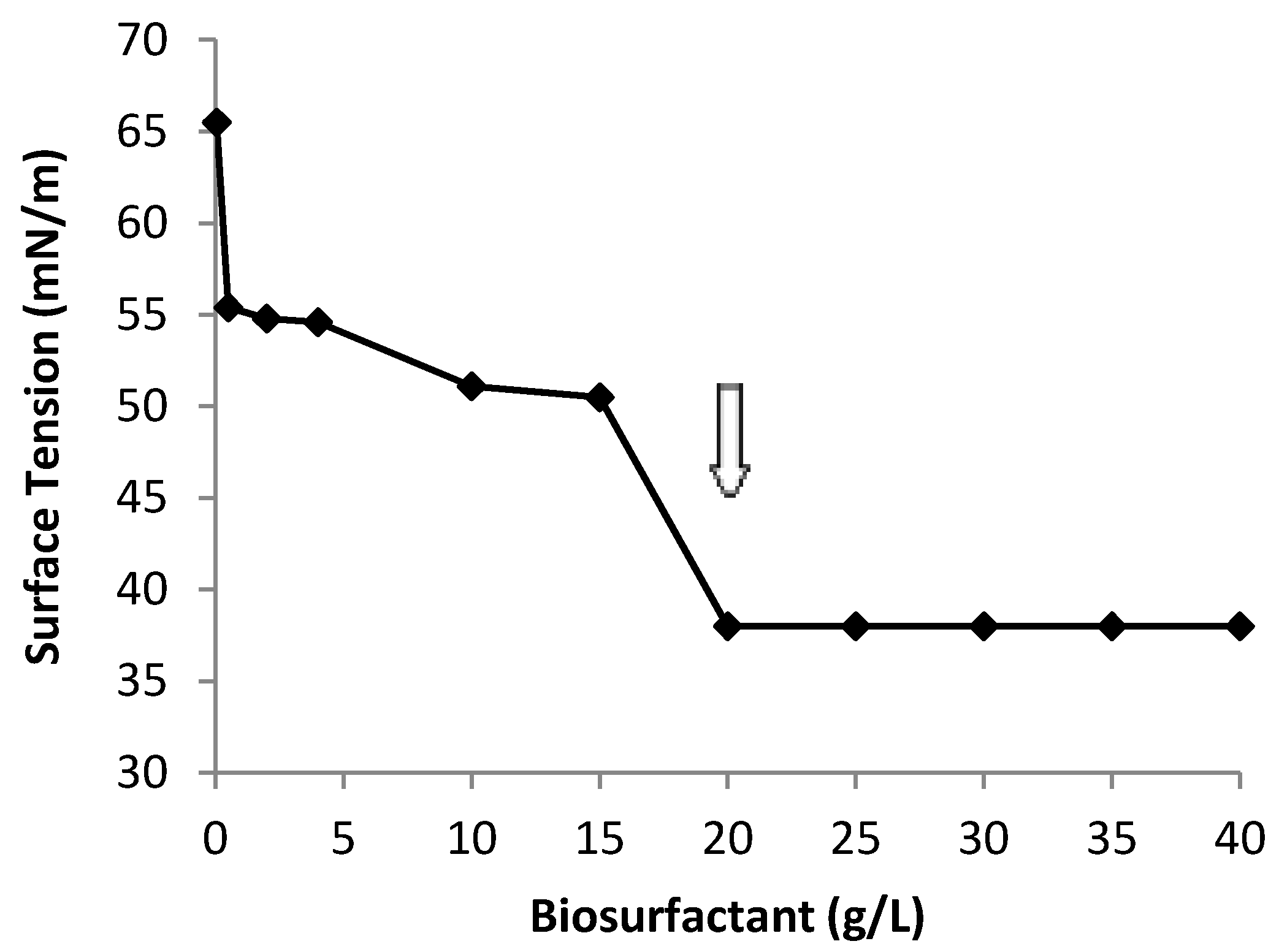
2.6. Oil Displacement Area-ODA
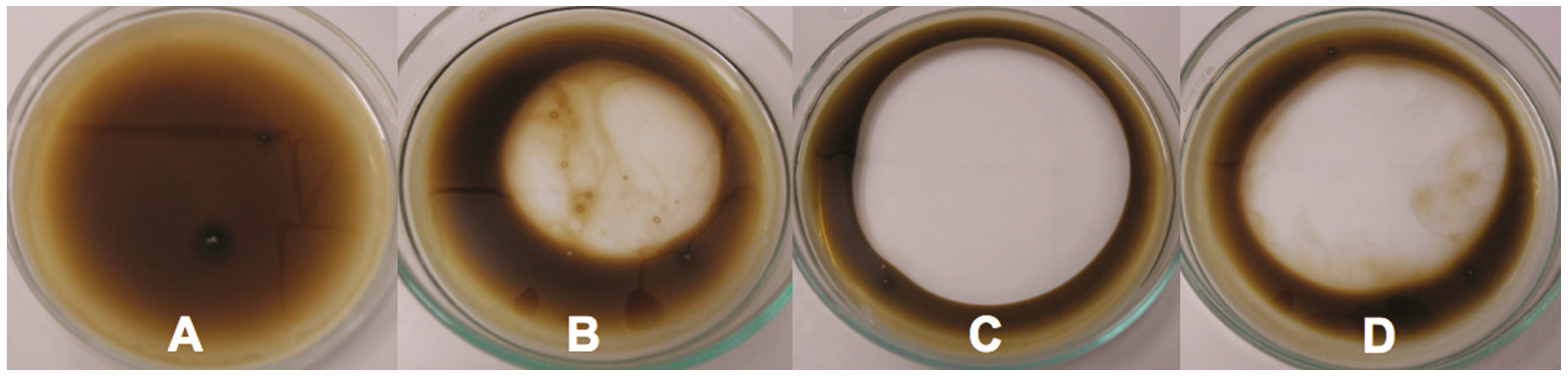
2.7. Effect of the Biosurfactant on Viscosity
| Hydrophobic Substrates | Viscosity without Biosurfactant | Viscosity with Biosurfactant | ||
|---|---|---|---|---|
| (cP) | (%) | (cP) | (%) | |
| Engine oil | 736.6 | 59.9 | 179.0 | 72.5 |
| Burnt engine oil | 148.9 | 48.3 | 210.7 | 87.9 |
| Diesel | 154.1 | 25.1 | 43.8 | 35.7 |
| Biodiesel | 36.0 | 29.3 | 51.3 | 41.9 |
| Canola oil | 374.0 | 60.8 | 110.9 | 78.5 |
| Corn oil | 47.6 | 38.8 | 404.0 | 66.1 |
| Soybean oil | 472.8 | 38.2 | 970.1 | 38.9 |
| Soybean waste oil | 380.1 | 61.9 | 556.3 | 45.3 |
| Castor oil | 187.9 | 75.1 | 185.4 | 61.6 |
| Sunflower oil | 355.5 | 57.6 | 493.3 | 40.9 |
| Palm oil | 403.0 | 67.7 | 536.3 | 45.1 |
| Rice oil | 459.9 | 74.8 | 461.1 | 75.2 |
| Mineral oil | 27.0 | 22.0 | 102.2 | 83.3 |
| Water(Control) | 0.9 | 0.9 | 1.3 | 1.1 |
3. Experimental Section
3.1. Isolation, Identification and Preservation of the Microorganism
3.2. Agroindustrial Substrates
3.3. Culture Conditions and Biosurfactant Production
3.4. Kinetics of Growth and Biosurfactant Production
3.5. Determining the pH
3.6. Determining the Surface Tension
3.7. Determining the Emulsification Index and Activity
3.8. Determining the Critical Micelle Concentration (CMC)
3.9. Determining the Stability of the Biosurfactant
3.10. Isolation of the Biosurfactant
3.11. Characterization of the Biosurfactant
3.12. Ionic Charge and Viscosity
3.13. Oil Spread Test (Oil Displacement Area-ODA)
3.14. Determining Oil Viscosity
3.15. Statistical Analysis
| Parameters | (−1.68) | (−1) | 0 | (+1) | (+1.68) |
|---|---|---|---|---|---|
| Soybean oil waste (SOW) (%) | 0.62 | 3.00 | 6.50 | 10.00 | 12.38 |
| Corn steep liquor(CSL) (%) | 2.64 | 4.00 | 6.00 | 8.00 | 9.36 |
4. Conclusions
Supplementary Materials
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kitamoto, D.; Morita, T.; Fukuoka, T.; Konishi, M.; Imura, T. Self-assembling properties of glycolipid biosurfactants and their potential applications. Curr. Opin. Colloid Interface Sci. 2009, 14, 315–328. [Google Scholar] [CrossRef]
- Bhardwaj, G.; Cameotra, S.S.; Chopra, H.K. Biosurfactants from Fungi: A Review. J. Pet. Environ. Biotechnol. 2013, 4, 160–166. [Google Scholar]
- Felse, P.A.; Shah, V.; Chan, J.; Rao, K.J.; Gross, R.A. Sophorolipid biosynthesis by Candida bombicola from industrial fatty acid residues. Enzym. Microb. Technol. 2007, 40, 316–323. [Google Scholar] [CrossRef]
- Deshpande, M.; Daniels, L. Evaluation of sophorolipid biosurfactant production by Candida bombicola using animal fat. Bioresour. Technol. 1995, 54, 143–150. [Google Scholar] [CrossRef]
- Casas, J.A.; Garcia de Lara, S.; Garcia-Ochoa, F. Optimization of a synthetic medium for Candida bombicola growth using factorial design of experiments. Enzym. Microb. Technol. 1997, 21, 221–229. [Google Scholar] [CrossRef]
- Williams, K. Biosurfactants for cosmetic applications: Overcoming production challenges. MMG 445 Basic Biotechnol. 2009, 5, 78–83. [Google Scholar]
- Sarubbo, L.A.; Farias, C.B.; Campos-Takaki, G.M. Co-utilization of canola oil and glucose on the production of a surfactant by Candida lipolytica. Curr. Microbiol. 2008, 54, 68–73. [Google Scholar]
- Rufino, R.D.; Sarubbo, L.A.; Campos-Takaki, G.M. Enhancement of stability of biosurfactant produced by Candida lipolytica using industrial residue as substrate. World J. Microbiol. Biotechnol. 2007, 23, 729–734. [Google Scholar] [CrossRef]
- Rufino, R.D.; Luna, J.M.; Sarubbo, L.A.; Rodrigues, L.R.M.; Texeira, J.A.C.; Campos-Takaki, G.M. Antimicrobial and anti-adhesive potential of a biosurfactant Rufisan produced by Candida lipolytica UCP 0988. Colloids Surf. B 2011, 84, 1–5. [Google Scholar] [CrossRef]
- Luna, J.M.; Rufino, R.D.; Albuquerque, C.D.C.; Sarubbo, L.A.; Campos-Takaki, G.M. Economic Optimized Medium for Tensio-Active Agent Production by Candida sphaerica UCP0995 and Application in the Removal of Hydrophobic Contaminant from Sand. Int. J. Mol. Sci. 2011, 12, 2463–2476. [Google Scholar] [CrossRef]
- Luna, J.M.; Rufino, R.D.; Sarubbo, L.A.; Campos-Takaki, G.M. Characterisation, surface properties and biological activity of a biosurfactant produced from industrial waste by Candida sphaerica UCP0995 for application in the petroleum industry. Colloids Surf. B 2013, 102, 202–209. [Google Scholar] [CrossRef]
- Thanomsub, B.; Watcharachaipong, T.; Chotelersak, K.; Arunrattiyakorn, P.; Nitoda, T.; Kanzaki, H. Monoacylglycerols: Glycolipid biosurfactants produced by a thermotolerant yeast, Candida ishiwadae. J. Appl. Microbiol. 2004, 96, 588–592. [Google Scholar] [CrossRef]
- Konishi, M.; Fukuoka, T.; Morita, T.; Imura, T.; Kitamoto, D. Production of new types of sophorolipids by Candida batistae. J. Oleo Sci. 2008, 57, 359–369. [Google Scholar] [CrossRef]
- Kiran, G.S.; Hema, T.A.; Gandhimathi, R.; Selvin, J.; Thomas, T.A.; Rajeetha Ravji, T.; Natarajaseenivasan, K. Optimization and production of a biosurfactant from the sponge-associated marine fungus Aspergillus ustus MSF3. Colloids Surf. B 2009, 73, 250–256. [Google Scholar] [CrossRef]
- Alejandro, C.S.; Humberto, H.S.; María, J.F. Production of glycolipids with antimicrobial activity by Ustilago maydis FBD12 in submerged culture. Afr. J. Microbiol. Res. 2011, 5, 2512–2523. [Google Scholar]
- Chandran, P.; Das, N. Biosurfactant production and diesel oil degradation by yeast species Trichosporon asahii isolated from petroleum hydrocarbon contaminated soil. Int. J. Eng. Sci. Technol. 2010, 2, 6942–6953. [Google Scholar]
- Chandankere, R.; Yao, J.; Choi, M.M.F.; Masakorala, K.; Chan, Y. An efficient biosurfactant-producing and crude-oil emulsifying bacterium Bacillus methylotrophicus USTBa isolated from petroleum reservoir. Biochem. Eng. J. 2013, 74, 46–53. [Google Scholar] [CrossRef]
- Gudiña, R.; Rangarajan, V.; Sen, R.; Rodrigues, L.R. Potential therapeutic applications of biosurfactants. Trends Pharmacol. Sci. 2013, 34, 667–675. [Google Scholar] [CrossRef] [Green Version]
- Donio, M.B.S.; Ronica, S.F.A.; Thanga Viji, V.; Velmurugan, S.; Adlin Jenifer, J.; Michaelbabu, M.; Citarasu, T. Isolation and characterization of halophilic Bacillus sp. BS3 able to produce pharmacologically important biosurfactants. Asian Pac. J. Trop. Med. 2013, 6, 873–886. [Google Scholar]
- Rodrigues, L.R.; Teixeira, J.A.; Mei, H.C.; Oliveira, R. Physicochemical and functional characterization of a biosurfactant produced by Lactococcus lactis 53. Colloids Surf. B 2006, 49, 79–86. [Google Scholar] [CrossRef] [Green Version]
- Janek, T.; Łukaszewicza, M.; Krasowska, A. Identification and characterization of biosurfactants produced by the Arctic bacterium Pseudomonas putida BD2. Colloids Surf. B 2013, 110, 379–386. [Google Scholar] [CrossRef]
- Pacwa-Plociniczak, M.; Plaza, G.A.; Piotrowska-Seget, Z.; Cameotra, S.S. Review Environmental Applications of Biosurfactants: Recent Advances. Int. J. Mol. Sci. 2011, 12, 633–654. [Google Scholar]
- Ron, E.Z.; Rosenberg, E. Biosurfactants and oil bioremediation. Curr. Opin. Biotechnol. 2002, 13, 249–252. [Google Scholar] [CrossRef]
- Mukherjee, S.; Das, P.; Sen, R. Towards commercial production of microbial surfactants. Trends Biotechnol. 2006, 24, 509–515. [Google Scholar] [CrossRef]
- Makkar, R.S.; Cameotra, S.S.; Banat, I.M. Advances in utilization of renewable substrates for biosurfactant production. AMB Express 2011, 1, 5–24. [Google Scholar] [CrossRef]
- Solaiman, D.; Ashby, R.; Zerkowski, J.; Foglia, T. Simplified soy molasses-based medium for reduced-cost production of sophorolipids by Candida bombicola. Biotechnol. Lett. 2007, 29, 1341–1347. [Google Scholar] [CrossRef]
- Batrakov, S.G.; Konova, I.V.; Sheichenko, V.I.; Galanina, L.A. Glycolipids of the filamentous fungus Absidia corymbifera F-295. Chem. Phys. Lipids 2003, 123, 157–164. [Google Scholar] [CrossRef]
- Banat, I.M.; Makkar, R.S.; Cameotra, S.S. Potential commercial applications of microbial surfactants. Appl. Microbiol. Biotechnol. 2000, 53, 495–508. [Google Scholar] [CrossRef]
- Nitschke, M.; Costa, S.G. Biosurfactants in food industry. Trends Food Sci. Technol. 2007, 18, 252–259. [Google Scholar] [CrossRef]
- Banat, I.M.; Franzetti, A.; Gandolfi, I.; Bestetti, G.; Martinotti, M.G.; Fracchia, L.; Smyth, T.J.; Marchant, R. Microbial biosurfactants production, applications and future potential. Appl. Microbiol. Biotechnol. 2010, 87, 427–444. [Google Scholar]
- Rocha e Silva, N.M.P.; Rufino, R.D.; Luna, J.M.; Santos, V.A.; Sarubbo, L.A. Screening of Pseudomonas species for biosurfactant production using low-cost substrates. Biocatal. Agric. Biotechnol. 2014, 3, 132–139. [Google Scholar]
- Santos, S.C.; Fernandez, L.G.; Rossi-Alva, J.C.; Roque, M.R.A. Evaluation of substrates from renewable resources in biosurfactants production by Pseudomonas strains. Afr. J. Biotechnol. 2010, 9, 5704–5711. [Google Scholar]
- Zheng, R.Y.; Chen, G.Q. Cunninghamella echinulata (Thaxt.) Thaxt. ex Blakeslee var. echinulata and var. verticillata (Paine) comb. nov. Mycosystema 1996, 8, 1–13. [Google Scholar]
- Domsch, K.H.; Gams, W.; Anderson, T.-H. Compendium of Soil Fungi; Academic Press: London, UK, 1980. [Google Scholar]
- Zheng, R.Y.; Chen, G.Q. A monograph of Cunninghamella. Mycotaxon 2001, 80, 1–75. [Google Scholar]
- Rufino, R.D.; Luna, J.M.; Campos-Takaki, G.M.; Sarubbo, L.A. Characterization and properties of the biosurfactant produced by Candida lipolytica UCP 0988. Electron. J. Biotechnol. 2013, 17, 34–38. [Google Scholar]
- Qazi, M.A.; Subhan, M.; Nighat, F.; Ali, M.I.; Ahmed, S. Role of biosurfactant produced by Fusarium sp. BS-8 in enhanced oil recovery (EOR) through sand Pack column. Int. J. Biosci. Biochem. Bioinform. 2013, 3, 6–10. [Google Scholar]
- Sarubbo, L.A.; de Luna, G.M.; de Campos-Takaki, G.M. Production and stability studies of the bioemulsifier obtained from a new strain of Candida glabrata UCP 1002. Electron. J. Biotechnol. 2006, 9, 400–406. [Google Scholar]
- Amiriyan, A.; Mazaheri Assadi, M.; Sajadian, V.A.; Noohi, A.A. Bioemulsan production by Iranian oil reservoirs microorganism. Iran. J. Environ. Health Sci. Eng. 2004, 1, 28–35. [Google Scholar]
- Fontes, G.C.; Amaral, P.F.F.; Coelho, M.A.Z. Biosurfactant production by yeasts. Quím. Nova 2008, 31, 2091–2099. [Google Scholar]
- Morita, T.; Ishibashi, Y.; Fukuoka, T.; Imura, T.; Sakai, T.; Abe, M.; Kitamoto, D. Production of glycolipid biosurfactants, mannosylerythritol lipids, by a smut fungus, Ustilago scitaminea NBRC 32730. Biosci. Biotechnol. Biochem. 2009, 73, 788–792. [Google Scholar] [CrossRef]
- Cavalero, D.A.; Cooper, D.G. The effect of medium composition on the structure and physical state of sophorolipids produced by Candida bombicola ATCC 22214. J. Biotechnol. 2003, 103, 31–41. [Google Scholar] [CrossRef]
- Diab, A.; Din, S.G.E. Production and characterization of biosurfactants produced by Bacillus spp. and Pseudomonas spp. isolated from the rhizosphere soil of an egyptian salt marsh plant. Nat. Sci. 2013, 11, 103. [Google Scholar]
- Deleu, M.; Paquot, M. From renewable vegetables resources to microorganisms: New trends in surfactants. Comptes Randus Chim. 2004, 7, 641–646. [Google Scholar] [CrossRef]
- Satpute, S.K.; Banpurkar, A.G.; Dhakephalkar, P.K.; Banat, I.M.; Chopade, B.A. Methods for investigating biosurfactants and bioemulsifiers: A review. Crit. Rev. Biotechnol. 2010, 30, 127–144. [Google Scholar] [CrossRef]
- Youssef, N.H.; Duca, K.E.; Nagle, D.P.; Savager, K.N.; Kapp, R.M.; Mclnemey, M.J. Comparison of methods to detect biosurfactant production by diverse microorganism. J. Microbiol. Methods 2004, 56, 339–346. [Google Scholar]
- Pornsunthorntawee, O.; Wongpanit, P.; Chavadej, S.; Abe, M.; Rujiravanit, R. Structural and physicochemical characterization of crude biosurfactant produced by Pseudomonas aeruginosa SP4 isolated from petroleum-contaminated soil. Bioresour. Technol. 2008, 99, 1589–1595. [Google Scholar]
- Whang, L.M.; Liu, P.W.G.; Ma, C.C.; Cheng, S.S. Application of biosurfactant, rhamnolipid, and surfactin, for enhanced biodegradation of diesel-contaminated water and soil. J. Hazard. Mater. 2008, 151, 155–163. [Google Scholar] [CrossRef]
- Mulligan, C.N. Environmental applications for biosurfactants. Environ. Pollut. 2005, 133, 183–198. [Google Scholar]
- Ghurye, G.L.; Vipulanandan, C.; Willson, R.C. A practical approach to biossurfactant production using nonaseptic fermentation of mixed cultures. Biotechnol. Bioeng. 1994, 44, 661–666. [Google Scholar] [CrossRef]
- Shavandi, M.; Mohebali, G.; Haddadib, A.; Shakaramia, H.; Nuhic, A. Emulsification potential of a newly isolated biosurfactant-producing bacterium, Rhodococcus sp. strain TA6. Colloids Surf. B 2011, 82, 477–482. [Google Scholar] [CrossRef]
- Kim, H.S.; Jeon, J.W.; Lee, H.W.; Park, Y.I.; Seo, W.T.; Oh, H.-M.; Katsuragi, T.; Tani, Y.; Yoon, B.-D. Extracellular production of a glycolipid biosurfactant, mannosylerythritol lipid, from Candida antarctica. Biotechnol. Lett. 2002, 24, 225–229. [Google Scholar] [CrossRef]
- Patil, J.R.; Chopade, B.A. Studies on bioemulsifier production by Acinetobacter strains isolated from healthy human skin. J. Appl. Microbiol. 2001, 91, 290–298. [Google Scholar] [CrossRef]
- Ali Diab, A.; El Din, S.G. Application of the biosurfactants produced by Bacillus spp. (SH 20 and SH 26) and Pseudomonas aeruginosa SH 29 isolated from the rhizosphere soil of an Egyptian salt marsh plant for the cleaning of oil-contaminated vessels and enhancing the biodegradation of oily sludge. Afr. J. Environ. Sci. Technol. 2013, 7, 671–679. [Google Scholar]
- Banat, I.M. Biosurfactants production and possible uses in microbial enhanced oil recovery and oil pollution remediation: A review. Bioresour. Technol. 1995, 51, 1–12. [Google Scholar] [CrossRef]
- Sen, R. Biotechnology in petroleum recovery: The microbial EOR. Prog. Energy Combust. 2008, 34, 714–724. [Google Scholar] [CrossRef]
- Kuyukina, M.S.; Ivshina, I.B.; Makarov, S.O.; Litvinenko, L.V.; Cunningham, C.J.; Philip, J.C. Effect of biosurfactants on crude oil desorption and mobilization in a soil system. Environ. Int. 2005, 31, 155–161. [Google Scholar]
- Cooper, D.G.; Goldenberg, B.G. Surface-active agents from two Bacillus species. Appl. Environ. Microbiol. 1987, 53, 224–229. [Google Scholar]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K. Colorimetric method for determination of sugar and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Manocha, M.S.; San-Blas, G.; Centeno, S. Lipid composition of paracciodioids brasilienses: Possible correlation with virulence of different strains. J. Gen. Microbiol. 1980, 177, 147–154. [Google Scholar]
- Meylheuc, T.; van Oss, C.J.; Bellon-Fontaine, M.N. Adsorption of biosurfactants on solid surface and consequences regarding the bioahesion of Listeria monocytogenes LO28. J. Appl. Microbiol. 2001, 91, 822–832. [Google Scholar] [CrossRef]
- Morikawa, M.; Daido, H.; Takao, T.; Murata, S. A new lipopeptide biosurfactant produced by Arthrobacter sp. strain MIS38. J. Bacteriol. 1993, 175, 6459–6466. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Andrade Silva, N.R.; Luna, M.A.C.; Santiago, A.L.C.M.A.; Franco, L.O.; Silva, G.K.B.; De Souza, P.M.; Okada, K.; Albuquerque, C.D.C.; Silva, C.A.A.d.; Campos-Takaki, G.M. Biosurfactant-and-Bioemulsifier Produced by a Promising Cunninghamella echinulata Isolated from Caatinga Soil in the Northeast of Brazil. Int. J. Mol. Sci. 2014, 15, 15377-15395. https://doi.org/10.3390/ijms150915377
Andrade Silva NR, Luna MAC, Santiago ALCMA, Franco LO, Silva GKB, De Souza PM, Okada K, Albuquerque CDC, Silva CAAd, Campos-Takaki GM. Biosurfactant-and-Bioemulsifier Produced by a Promising Cunninghamella echinulata Isolated from Caatinga Soil in the Northeast of Brazil. International Journal of Molecular Sciences. 2014; 15(9):15377-15395. https://doi.org/10.3390/ijms150915377
Chicago/Turabian StyleAndrade Silva, Nadielly R., Marcos A. C. Luna, André L. C. M. A. Santiago, Luciana O. Franco, Grayce K. B. Silva, Patrícia M. De Souza, Kaoru Okada, Clarissa D. C. Albuquerque, Carlos A. Alves da Silva, and Galba M. Campos-Takaki. 2014. "Biosurfactant-and-Bioemulsifier Produced by a Promising Cunninghamella echinulata Isolated from Caatinga Soil in the Northeast of Brazil" International Journal of Molecular Sciences 15, no. 9: 15377-15395. https://doi.org/10.3390/ijms150915377




