Bifunctional Carbon Dots—Magnetic and Fluorescent Hybrid Nanoparticles for Diagnostic Applications
Abstract
:1. Introduction
2. Experimental
2.1. Synthetic Procedure
2.2. Characterization Technique
3. Results and Discussion
3.1. Structural Characterization
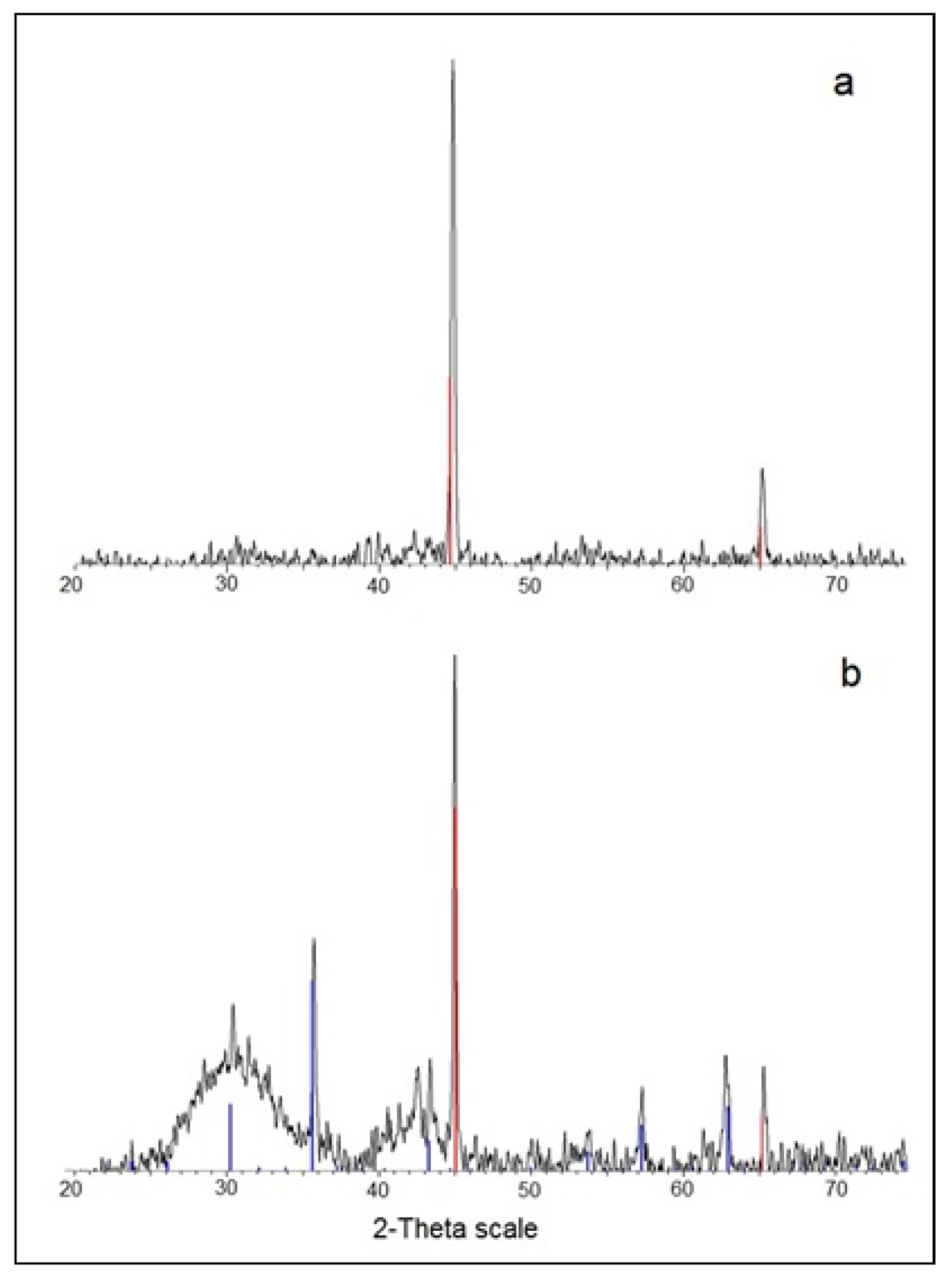
3.1.1. XRD
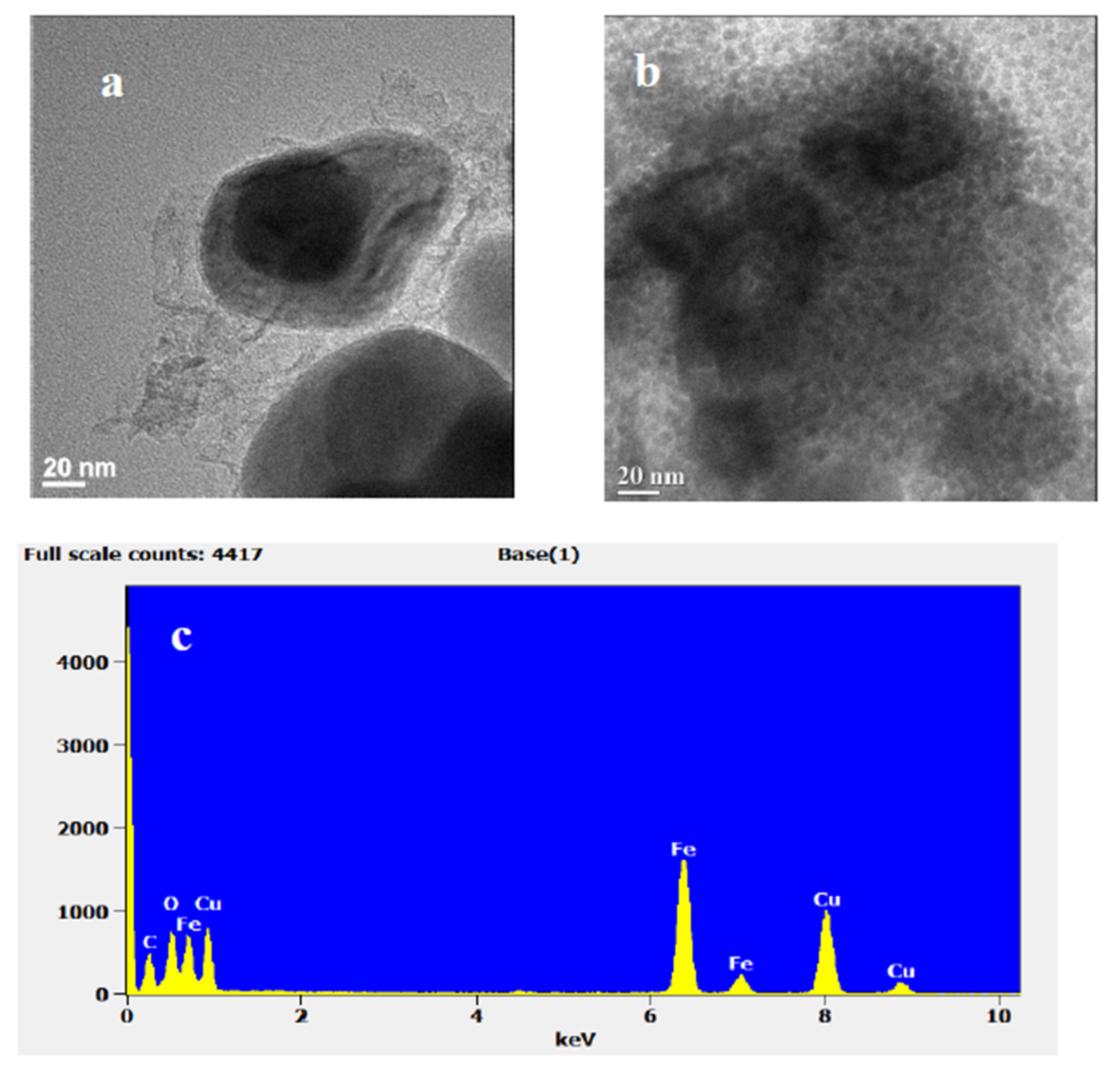
3.1.2. Morphology and Composition
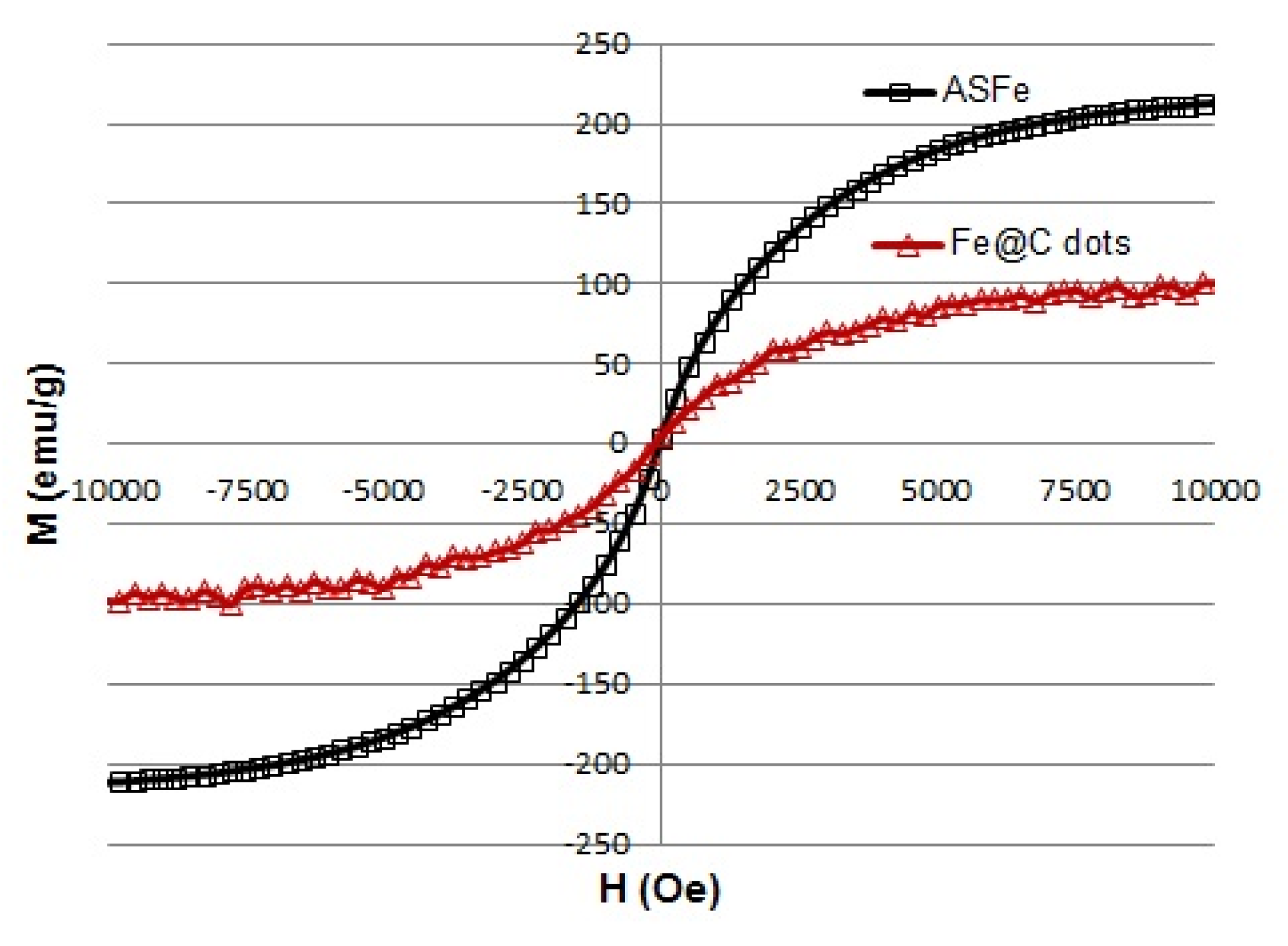
3.1.3. Magnetic Properties
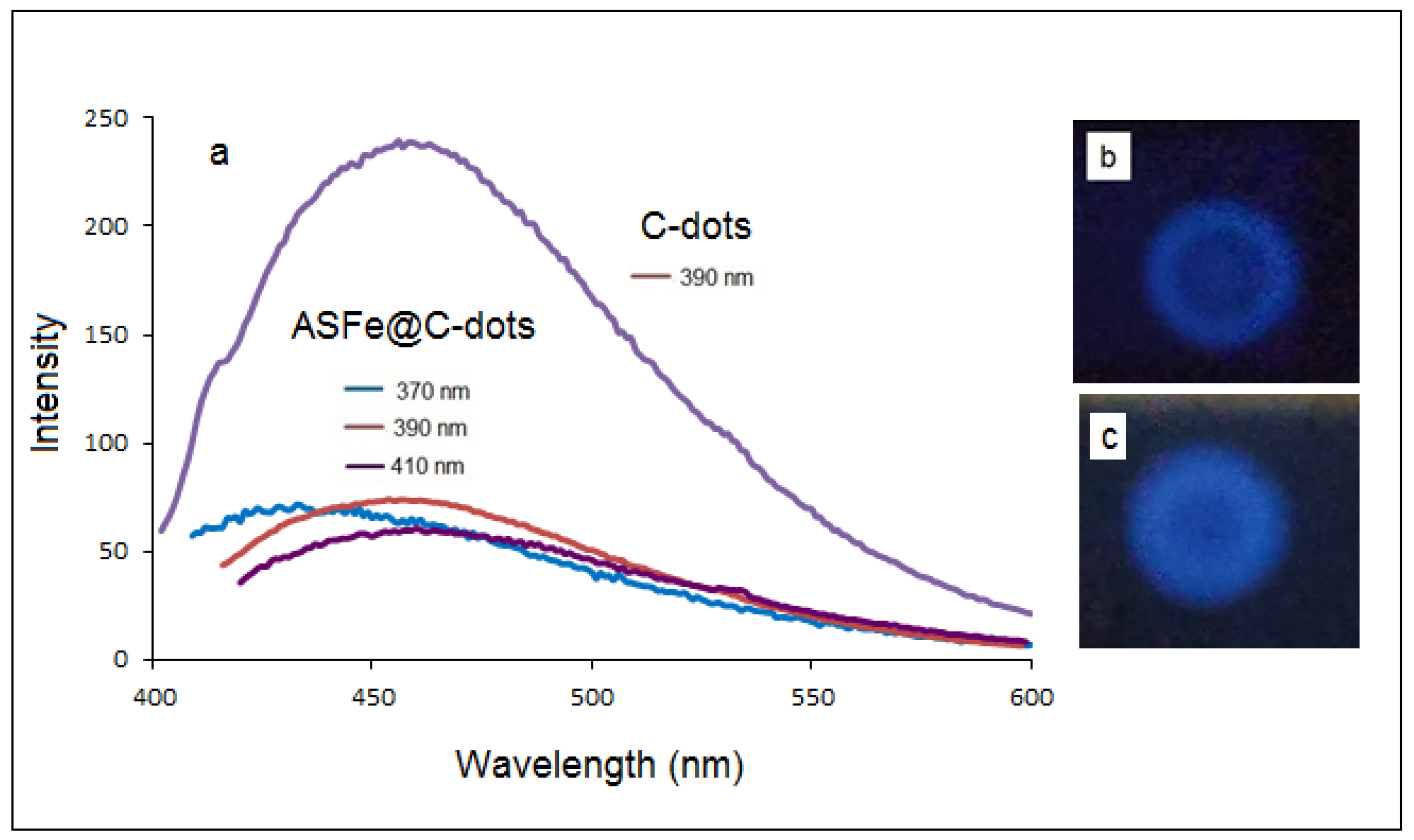
3.2. Photoluminescence (PL) Properties of Fe@C-Dots
3.3. FTIR Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fan, D.M.; Shi, H.R.; Chen, Z.M.; Wu, Q.H.; Liu, H.N.; Zhang, R.T. Early detection of ovarian carcinoma by proteome profiling based on magnetic bead separation and atrix-assisted laser desorption/ionization time of flight mass spectrometry. Afr. J. Microbiol. Res. 2010, 4, 940–951. [Google Scholar]
- Figuerola, A.; Di Corato, R.; Manna, L.; Pellegrino, T. From iron oxide nanoparticles towards advanced iron-based inorganic materials designed for biomedical applications. Pharmacol. Res. 2010, 62, 126–143. [Google Scholar] [CrossRef]
- Cabada, T.F.; Ramos-Gómez, M. A Novel Contrast Agent Based on Magnetic Nanoparticles for Cholesterol Detection as Alzheimer’s Disease Biomarker. Nanoscale Res. Lett. 2019, 14, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Yang, X.; Wei, Y.; Yuan, Q. Applications of DNA Nanotechnology in Synthesis and Assembly of Inorganic Nanomaterials. Chin. J. Chem. 2016, 34, 291–298. [Google Scholar] [CrossRef]
- Tran, N.; Webster, T.J.T. Magnetic nanoparticles: Biomedical applications and challenges. J. Mater. Chem. 2010, 20, 8760–8767. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, Y.; Xu, C. Magnetic Nanoparticles for Biomedical Applications: From Diagnosis to Treatment to Regeneration. In Engineering in Translational Medicine; Cai, W., Ed.; Springer: London, UK, 2013; Chapter 21; pp. 567–583. [Google Scholar]
- Gonzalez, C.C.; Pérez, C.A.M.; Martínez-Martínez, A.; Armendáriz, I.O.; Tapia, O.Z.; Martel-Estrada, S.; García-Casillas, P.E.; Martí Nez Pé Rez, C.A. Development of Antibody-Coated Magnetite Nanoparticles for Biomarker Immobilization. J. Nanomater. 2014, 2014, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Baker, S.N.; Baker, G.A. Luminescent Carbon Nanodots: Emergent Nanolights. Angew. Chem. Int. Ed. 2010, 49, 6726–6744. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Das, P.; Bag, S.; Laha, D.; Pramanik, P. Synthesis, functionalization and bioimaging applications of highly fluorescent carbon nanoparticles. Nanoscale 2011, 3, 1533–1540. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Kang, Z.; Liu, Y.; Lee, S.-T. Carbon nanodots: Synthesis, properties and applications. J. Mater. Chem. 2012, 22, 24230–24253. [Google Scholar] [CrossRef]
- Wang, H.; Shen, J.; Li, Y.; Wei, Z.; Cao, G.; Gai, Z.; Hong, K.; Banerjee, P.; Zhou, S. Magnetic iron oxide–fluorescent carbon dots integrated nanoparticles for dual-modal imaging, near-infrared light-responsive drug carrier and photothermal therapy. Biomater. Sci. 2014, 2, 915–923. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, P.; Bunker, C.E.; Teisl, L.R.; Reibold, M.; Yan, S.; Qian, H.; He, D.; Sun, Y.-P. Preparation and optical properties of magnetic carbon/iron oxide hybrid dots. RSC Adv. 2017, 7, 41304–41310. [Google Scholar] [CrossRef] [Green Version]
- Bhaisare, M.L.; Gedda, G.; Khan, M.S.; Wu, H. Fluorimetric detection of pathogenic bacteria using magnetic carbon dots. Anal. Chim. Acta 2016, 920, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, A.; Jones, S.; Pedraza, F.; Vangara, A.; Sweet, C.; Williams, M.S.; Ruppa-Kasani, V.; Risher, S.E.; Sardar, D.; Ray, P.C. Fluorescent, Magnetic Multifunctional Carbon Dots for Selective Separation, Identification, and Eradication of Drug-Resistant Superbugs. ACS Omega 2017, 2, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, X.; Wang, Y.; Lin, Y.; Zhou, J. Loading controlled magnetic carbon dots for microwave-assisted solid-phase extraction: Preparation, extraction evaluation and applications in environmental aqueous samples. J. Sep. Sci. 2018, 41, 3622–3630. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, B.; Sarma, D.; Jain, S.; Sarma, T.K. One-pot magnetic iron oxide-carbon nanodot composite—Catalyzed cyclooxidative aqueous tandem synthesis of ouinazolinones in the presence of tert-butyl hydroperoxide. ACS Omega 2018, 3, 13711–13719. [Google Scholar] [CrossRef]
- Han, B.; Peng, T.; Yu, M.; Chi, C.; Li, Y.; Hu, X.; He, G. One-pot synthesis of highly fluorescent Fe2+-doped carbon dots for a dual-emissive nanohybrid for the detection of zinc ions and histidine. New J. Chem. 2018, 42, 13651–13659. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, Y.; Zheng, L.; Wu, L.; Zhou, Z.; Chen, J.; Chen, W.; Zhao, H. Biocompatible iron(II)-doped carbon dots as T1-weighted magnetic resonance contrast agents and fluorescence imaging probes. Microchim. Acta 2019, 186, 492. [Google Scholar] [CrossRef]
- Zhuo, S.; Guan, Y.; Li, H.; Fang, J.; Zhang, P.; Du, J.; Zhu, C.-Q. Facile fabrication of fluorescent Fe-doped carbon quantum dots for dopamine sensing and bioimaging application. Analyst 2019, 144, 656–662. [Google Scholar] [CrossRef]
- Huang, S.; Yang, E.; Yao, J.; Chu, X.; Liu, Y.; Zhang, Y.; Xiao, Q. Nitrogen, Cobalt Co-doped Fluorescent Magnetic Carbon Dots as Ratiometric Fluorescent Probes for Cholesterol and Uric Acid in Human Blood Serum. ACS Omega 2019, 4, 9333–9342. [Google Scholar] [CrossRef] [Green Version]
- Nikitenko, S.I.; Koltypin, Y.; Palchik, O.; Felner, I.; Xu, X.N.; Gedanken, A. Synthesis of Highly Magnetic, Air-Stable Iron-Iron Carbide Nanocrystalline Particles by Using Power Ultrasound. Angew. Chem. 2001, 113, 4579–4581. [Google Scholar] [CrossRef]
- Kumar, V.B.; Porat, Z.; Gedanken, A. Facile one-step sonochemical synthesis of ultrafine and stable fluorescent C-dots. Ultrason. Sonochem. 2016, 28, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, W.S.; Chan, K.L. FTIR Spectroscopic Study of Poly(Ethylene Glycol)–Nifedipine Dispersion Stability in Different Relative Humidities. J. Pharm. Sci. 2015, 104, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Coates, J. Interpretation of infrared spectra, a practical approach. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; John Wiley & Sons New Jersey: Somerset, NJ, USA, 2006; pp. 11–23. [Google Scholar]
- Shameli, K.; Bin Ahmad, M.; Jazayeri, S.D.; Sedaghat, S.; Shabanzadeh, P.; Jahangirian, H.; Mahdavi, M.; Abdollahi, Y. Synthesis and Characterization of Polyethylene Glycol Mediated Silver Nanoparticles by the Green Method. Int. J. Mol. Sci. 2012, 13, 6639–6650. [Google Scholar] [CrossRef] [Green Version]
- Gotić, M.; Musić, S. Mössbauer, FT-IR and FE SEM investigation of iron oxides precipitated from FeSO4 solutions. J. Mol. Struct. 2007, 834, 445–453. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, N.; Li, J.; Gong, H.; An, T.; Zhao, F.; Ma, H. Thermal behavior of nitrocellulose-based superthermites: Effects of nano-Fe2O3 with three morphologies. RSC Adv. 2017, 7, 23583–23590. [Google Scholar] [CrossRef] [Green Version]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perelshtein, I.; Perkas, N.; Rahimipour, S.; Gedanken, A. Bifunctional Carbon Dots—Magnetic and Fluorescent Hybrid Nanoparticles for Diagnostic Applications. Nanomaterials 2020, 10, 1384. https://doi.org/10.3390/nano10071384
Perelshtein I, Perkas N, Rahimipour S, Gedanken A. Bifunctional Carbon Dots—Magnetic and Fluorescent Hybrid Nanoparticles for Diagnostic Applications. Nanomaterials. 2020; 10(7):1384. https://doi.org/10.3390/nano10071384
Chicago/Turabian StylePerelshtein, Ilana, Nina Perkas, Shai Rahimipour, and Aharon Gedanken. 2020. "Bifunctional Carbon Dots—Magnetic and Fluorescent Hybrid Nanoparticles for Diagnostic Applications" Nanomaterials 10, no. 7: 1384. https://doi.org/10.3390/nano10071384



