The Inhibitory Potential of Selected Essential Oils on Fusarium spp. Growth and Mycotoxins Biosynthesis in Maize Seeds
Abstract
:1. Introduction
2. Results
2.1. The Effect of EOs on the Growth of Fusarium spp. in Maize Samples
2.2. The Effect of EOs on Maize Germination
2.3. ZEA Content in EO-Treated Maize Samples after Inoculation with Fusarium spp.
2.4. DON Content in EO-Treated Maize Samples after Inoculation with Fusarium spp.
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Fusarium Strains
4.3. Standards, Chemicals, and Reagents
4.4. EO Characteristics and Preparation
4.5. Effect of EOs on Growth of Fusarium and Mycotoxins Biosynthesis
4.6. Effect of EOs on Maize Seed Germination
4.7. Chemical Analysis
4.7.1. ERG
4.7.2. Mycotoxins
4.7.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Smyth, C.; Kurz, W.A.; Rampley, G.; Lemprière, T.C.; Schwab, O. Climate change mitigation potential of local use of harvest residues for bioenergy in Canada. Glob. Chang. Biol. Bioenergy 2017, 9, 817–832. [Google Scholar] [CrossRef]
- McMullen, M.; Bergstrom, G.; de Wolf, E.; Dill-Macky, R.; Hershman, D.; Shaner, G.; Van Sanford, D.A. Unified effort to fight an enemy of wheat and barley: Fusarium head blight. Plant Dis. 2012, 96, 1712–1728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, H.; Yang, L.; Kang, J.; Wang, Q.; Wang, X.; Fang, Z.; Liu, Y.; Zhuang, M.; Zhang, Y.; Lin, Y.; et al. Development of InDel markers linked to Fusarium wilt resistance in cabbage. Mol. Breed. 2013, 32, 961–967. [Google Scholar] [CrossRef]
- Borrego-Benjumea, A.; Basallote-Ureba, M.J.; Melero-Vara, J.M.; Abbasi, P.A. Characterization of Fusarium isolates from asparagus fields in southwestern Ontario and influence of soil organic amendments on Fusarium crown and root rot. Phytopathology 2014, 104, 403–415. [Google Scholar] [CrossRef] [Green Version]
- Haapalainen, M.; Latvala, S.; Kuivainen, E.; Qiu, Y.; Segerstedt, M.; Hannukkala, A.O. Fusarium oxysporum, F. proliferatum and F. redolens associated with basal rot of onion in Finland. Plant Pathol. 2016, 65, 1310–1320. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.T.X.; Dehne, H.W.; Steiner, U. Histopathological assessment of the infection of maize leaves by Fusarium graminearum, F. proliferatum and F. verticillioides. Fungal Biol. 2016, 120, 1094–1104. [Google Scholar] [CrossRef]
- Scherm, B.; Balmas, V.; Spanu, F.; Pani, G.; Delogu, G.; Pasquali, M.; Migheli, Q. Fusarium culmorum: Causal agent of foot and root rot and head blight on wheat. Mol. Plant Pathol. 2013, 14, 323–341. [Google Scholar] [CrossRef]
- Van der Lee, T.; Zhang, H.; van Diepeningen, A.; Waalwijk, C. Biogeography of Fusarium graminearum species complex and chemotypes: A review. Food Addit. Contam. A 2015, 32, 453–460. [Google Scholar] [CrossRef] [Green Version]
- Pasquali, M.; Beyer, M.; Logrieco, A.; Audenaert, K.; Balmas, V.; Basler, R.; Boutigny, A.L.; Chrpová, J.; Czembor, E.; Gagkaeva, T.; et al. A European database of Fusarium graminearum and F. culmorum trichothecene genotypes. Front. Microbiol. 2016, 7, 406. [Google Scholar] [CrossRef] [Green Version]
- Pinton, P.; Oswald, I.P. Effect of deoxynivalenol and other type B trichothecenes on the intestine: A review. Toxins 2014, 6, 1615–1643. [Google Scholar] [CrossRef]
- Gajęcka, M.; Waśkiewicz, A.; Zielonka, Ł.; Goliński, P.; Rykaczewska, A.; Lisieska-Żołnierczyk, S.; Gajęcki, M.T. Mycotoxin levels in the digestive tissues of immature gilts exposed to zearalenone and deoxynivalenol. Toxicon 2018, 153, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Minervini, F.; Dell’Aquila, M.E. Zearalenone and reproductive function in farm animals. Int. J. Mol. Sci. 2008, 9, 2570–2584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Burns, K.A.; Arao, Y.; Luh, C.J.; Korach, K.S. Differential estrogenic actions of endocrine-disrupting chemicals bisphenol a, bisphenol af and zearalenone through estrogen receptor α and β In Vitro. Environ. Health Perspect. 2012, 120, 1029–1035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Ponte, E.M.; Garda-Buffon, J.; Badiale-Furlong, E. Deoxynivalenol and nivalenol in commercial wheat grain related to Fusarium head blight epidemics in southern Brazil. Food Chem. 2012, 132, 1087–1091. [Google Scholar] [CrossRef] [Green Version]
- Rossi, V.; Scandolara, A.; Battilano, P. Effect of environmental conditions on spore production by Fusarium verticillioides, the causal agent of maize ear rot. Eur. J. Plant Pathol. 2009, 123, 159–169. [Google Scholar] [CrossRef]
- Wachowska, U.; Packa, D.; Wiwart, M. Microbial inhibition of Fusarium pathogens and biological modification of trichothecenes in cereal grains. Toxins 2017, 9, 408. [Google Scholar] [CrossRef] [Green Version]
- Köhl, J.; Kolnaar, R.; Ravensberg, W.J. Mode of action of microbial biological control agents against plant diseases: Relevance beyond efficacy. Front. Plant Sci. 2019, 10, 845. [Google Scholar] [CrossRef] [Green Version]
- Franco, T.S.; Garcia, S.; Hirooka, E.Y.; Ono, Y.S.; dos Santos, J.S. Lactic acid bacteria in the inhibition of Fusarium graminearum and deoxynivalenol detoxification. J. Appl. Microbiol. 2011, 111, 739–748. [Google Scholar] [CrossRef]
- Gwiazdowska, D.; Czaczyk, K.; Filipiak, M.; Gwiazdowski, R. Effects of Propionibacterium on the growth and mycotoxins production by some species of Fusarium and Alternaria. Pol. J. Microbiol. 2008, 57, 205–212. [Google Scholar]
- Armando, M.R.; Dogi, C.A.; Poloni, V.; Rosa, C.A.R.; Dalcero, A.M.; Cavaglieri, L.R. In Vitro study on the effect of Saccharomyces cerevisiae strains on growth and mycotoxin production by Aspergillus carbonarius and Fusarium graminearum. Int. J. Food Microbiol. 2013, 161, 182–188. [Google Scholar] [CrossRef]
- Krzyśko-Łupicka, T.; Walkowiak, W.; Białoń, M. Comparison of the fungistatic activity of selected essential oils relative to Fusarium graminearum isolates. Molecules 2019, 24, 311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tumen, I.; Hafizoglu, H.; Kilic, A.; Dönmez, I.E.; Sivrikaya, H.; Reunanen, M. Yields and constituents of essential oil from cones of Pinaceae spp. natively grown in Turkey. Molecules 2010, 15, 5797–5806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandey, A.K.; Kumar, P.; Singh, P.; Tripathi, N.N.; Bajpai, V.B. Essential oils: Sources of antimicrobials and food preservatives. Front. Microbiol. 2017, 7, 2161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaouali, Y.; Bouzaine, T.; Boussaid, M. Essential oils composition in two Rosmarinus officinalis L. varieties and incidence for antimicrobial and antioxidant activities. Food Chem. Toxicol. 2010, 48, 3144–3152. [Google Scholar] [CrossRef] [PubMed]
- Solórzano-Santos, F.; Miranda-Novales, M.G. Essential oils from aromatic herbs as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Rubiolo, P.; Sgorbini, B.; Liberto, E.; Cordero, C.; Bicchi, C. Essential oils and volatiles: Sample preparation and analysis. Flavour Fragr. J. 2010, 25, 282–290. [Google Scholar] [CrossRef]
- Daugavietis, M.; Spalvis, K. The development of technology for obtaining essential oils from Scots pine tree foliage. For. Wood Technol. 2014, 87, 51–54. [Google Scholar]
- Zabka, M.; Pavela, R.; Slezakova, L. Antifungal effect of Pimenta dioica essential oil against dangerous pathogenic and toxinogenic fungi. Ind. Crop Prod. 2009, 30, 250–253. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Mohamady, M.A.; Fernández-López, J.; Abd El-Razik, K.A.; Omer, E.A.; Pérez-Alvarez, J.A.; Sendra, E. In Vitro antioxidant and antibacterial activities of essentials oils obtained from Egyptian aromatic plants. Food Control 2011, 22, 1715–1722. [Google Scholar] [CrossRef]
- Marin, S.; Velluti, A.; Ramos, A.J.; Sanchis, V. Effect of essential oils on zearalenone and deoxynivalenol production by Fusarium graminearum in non-sterilized maize grain. Food Microbiol. 2004, 21, 313–318. [Google Scholar] [CrossRef]
- Velluti, A.; Sanchis, V.; Ramos, A.J.; Turon, C.; Marin, S. Impact of essential oils on growth rate, zearalenone and deoxynivalenol production by Fusarium graminearum under different temperature and water activity conditions in maize grain. J. Appl. Microbiol. 2004, 96, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Kalagatur, N.K.; Mudili, V.; Siddaiah, C.; Gupta, V.K.; Natarajan, G.; Sreepathi, M.H.; Vardhan, B.H.; Putcha, V.L.R. Antagonistic activity of Ocimum sanctum L. essential oil on growth and zearalenone production by Fusarium graminearum in maize grains. Front. Microbiol. 2015, 6, 892. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Zhou, H.; Zhou, W.; Hu, L.; Chen, J.; Shi, Z. The fungicidal activity of thymol against Fusarium graminearum via inducing lipid peroxidation and disrupting ergosterol biosynthesis. Molecules 2016, 21, 770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gill, T.A.; Li, J.; Saenger, M.; Scofield, S.R. Thymol-based submicron emulsions exhibit antifungal activity against Fusarium graminearum and inhibit Fusarium head blight in wheat. J. Appl. Microbiol. 2016, 121, 1103–1116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Long, L.; Zhang, F.; Chen, Q.; Chen, C.; Yu, X.; Liu, Q.; Bao, J.; Long, Z. Antifungal activity, main active components and mechanism of Curcuma longa extract against Fusarium graminearum. PLoS ONE 2018, 13, e0194284. [Google Scholar] [CrossRef] [Green Version]
- Sellamani, M.; Kalagatur, N.K.; Siddaiah, C.; Mudili, V.; Krishna, K.; Natarajan, G.; Venkata, L. Antifungal and zearalenone inhibitory activity of Pediococcus pentosaceus isolated from dairy products on Fusarium graminearum. Front. Microbiol. 2016, 7, 890. [Google Scholar] [CrossRef]
- Dambolena, J.S.; López, A.G.; Meriles, J.M.; Rubinstein, H.R.; Zygaldo, J.A. Inhibitory effect of 10 natural phenolic compounds on Fusarium verticillioides. A structure-property-activity relationship study. Food Control 2012, 28, 163–170. [Google Scholar] [CrossRef]
- Da Silva Bomfim, N.; Nakassugi, L.P.; Oliveira, J.F.P.; Kohiyama, C.Y.; Mossini, S.A.G.; Grespan, R.; Nerilo, S.B.; Mallmann, C.A.; Filho, B.A.A.; Machinski, M., Jr. Antifungal activity and inhibition of fumonisin production by Rosmarinus officinalis L. essential oil in Fusarium verticillioides (Sacc.) Nirenberg. Food Chem. 2015, 166, 330–336. [Google Scholar] [CrossRef] [Green Version]
- Yoruk, E.; Sefer, O.; Sezer, A.S.; Konukcu, Z.; Develi, E.S. Investigation of the effects of eugenol on Fusarium culmorum. Iğdır Univ. J. Inst. Sci. Tech. 2018, 8, 215–221. [Google Scholar] [CrossRef]
- Naeini, A.; Ziglari, T.; Shokri, H.; Khosravi, A.R. Assessment of growth-inhibiting effect of some plant essential oils on different Fusarium isolates. J. Mycol. Méd. 2010, 20, 174–178. [Google Scholar] [CrossRef]
- Grata, K. Sensitivity of Fusarium solani isolated from asparagus on essential oils. Ecol. Chem. Eng. A 2016, 23, 453–464. [Google Scholar] [CrossRef]
- Kumar, K.N.; Venkataramana, M.; Allen, J.A.; Chandranayaka, S.; Murali, H.S.; Batra, H.V. Role of Curcuma longa L. essential oil in controlling the growth and zearalenone production of Fusarium graminearum. LWT Food Sci. Technol. 2016, 69, 522–528. [Google Scholar] [CrossRef]
- Estrada-Cano, C.; Castro, M.A.A.; Muñoz-Castellanos, L.; García-Triana, N.A.-O.A.; Hernández-Ochoa, L. Antifungal activity of microcapsulated clove (Eugenia caryophyllata) and Mexican oregano (Lippia berlandieri) essential oils against Fusarium oxysporum. J. Microb. Biochem. Technol. 2017, 9, 1. [Google Scholar] [CrossRef] [Green Version]
- Perczak, A.; Gwiazdowska, D.; Marchwińska, K.; Juś, K.; Gwiazdowski, R.; Waśkiewicz, A. Antifungal activity of selected essential oils against Fusarium culmorum and F. graminearum and their secondary metabolites in wheat seeds. Arch. Microbiol. 2019, 201, 1085–1097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zulak, K.G.; Cox, B.A.; Tucker, M.A.; Oliver, R.P.; Lopez-Ruiz, F.J. Improved detection and monitoring of fungicide resistance in Blumeria graminis f. sp. hordei with high-throughput genotype quantification by digital PCR. Front. Microbiol. 2018, 9, 706. [Google Scholar] [CrossRef] [PubMed]
- Cabo, M.L.; Braber, A.F.; Koenraad, P.M. Apparent antifungal activity of several lactic acid bacteria against Penicillium discolor is due to acetic acid in the medium. J. Food Prot. 2002, 65, 1309–1316. [Google Scholar] [CrossRef] [PubMed]
- Lavermicocca, P.; Valerio, F.; Evidente, A.; Lazzaroni, S.; Corsetti, A.; Gobbetti, M. Purification and characterization of novel antifungal compounds from the sourdough Lactobacillus plantarum strain 21B. Appl. Environ. Microbiol. 2000, 66, 4084–4090. [Google Scholar] [CrossRef] [Green Version]
- Dalié, D.; Pinson-Gadais, L.; Atanasova-Penichon, V.; Marchegay, G.; Barreau, C.; Deschamps, A.; Richard-Forget, F. Impact of Pediococcus pentosaceus strain L006 and its metabolites on fumonisin biosynthesis by Fusarium verticillioides. Food Control 2012, 23, 405–411. [Google Scholar] [CrossRef]
- Juodeikiene, G.; Bartkiene, E.; Cernauskas, D.; Cizeikiene, D.; Zadeike, D.; Lele, V.; Bartkevics, V. Antifungal activity of lactic acid bacteria and their application for Fusarium mycotoxin reduction in malting wheat grains. LWT 2018, 89, 307–314. [Google Scholar] [CrossRef]
- Singh, P.; Pandey, A.K. Prospective of essential oils of the genus Mentha as biopesticides: A review. Front. Plant Sci. 2018, 8, 1296. [Google Scholar] [CrossRef]
- Matusinsky, P.; Zouhar, M.; Pavela, R.; Novy, P. Antifungal effect of five essential oils against important pathogenic fungi of cereals. Ind. Crop Prod. 2015, 67, 208–215. [Google Scholar] [CrossRef]
- Ferreira, F.M.D.; Hirooka, E.Y.; Ferreira, F.D.; Silva, M.V.; Mossini, S.A.G.; Machinski, M., Jr. Effect of Zingiber officinale Roscoe essential oil in fungus control and deoxynivalenol production of Fusarium graminearum Schwabe In Vitro. Food Addit. Contam. A 2018, 35, 2168–2174. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto-Ribeiro, M.M.G.; Grespan, R.; Kohiyama, C.Y.; Ferreira, F.D.; Mossini, S.A.G.; Silva, E.L.; Filho, B.A.A.; Mikcha, J.M.G.; Junior, M.M. Effect of Zingiber officinale essential oil on Fusarium verticillioides and fumonisin production. Food Chem. 2013, 141, 3147–3152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalagatur, N.K.; Ghosh, O.S.N.; Sundararaj, N.; Mudili, V. Antifungal activity of chitosan nanoparticles encapsulated with Cymbopogon martini essential oil on plant pathogenic fungi Fusarium graminearum. Front. Pharmacol. 2018, 9, 610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalagatur, N.K.; Mudili, V.; Kamasani, J.R.; Siddaiah, C. Discrete and combined effects of Ylang-Ylang (Cananga odorata) essential oil and gamma irradiation on growth and mycotoxins production by Fusarium graminearum in maize. Food Control 2018, 94, 276–283. [Google Scholar] [CrossRef]
- Dambolena, J.S.; Zunino, M.P.; López, A.G.; Rubinstein, H.R.; Zygadlo, J.A.; Mwangi, J.W.; Thoithi, G.N.; Kibwage, I.O.; Mwalukumbi, J.M.; Kariuki, S.T. Essential oils composition of Ocimum basilicum L. and Ocimum gratissimum L. from Kenya and their inhibitory effects on growth and fumonisin production by Fusarium verticillioides. Innov. Food Sci. Emerg. Technol. 2010, 11, 410–414. [Google Scholar] [CrossRef]
- Ávila-Sosa, R.; Palou, E.; Jiménez, M.T.; Nerváez-Moorillón, G.; Navarro, A.D.; López-Malo, A. Antifungal activity by vapor contact essential oils added to amaranth, chitosan, or tarch edible films. Int. J. Food Microbiol. 2012, 153, 66–72. [Google Scholar] [CrossRef]
- Ochoa-Velascoa, C.E.; Navarro-Cruza, A.R.; Vera-López, O.; Paloub, E.; Avila-Sosa, R. Growth modeling to control (In Vitro) Fusarium verticillioides and Rhizopus stolonifer with thymol and carvacrol. Rev. Argent. Microbiol. 2018, 50, 70–74. [Google Scholar] [CrossRef]
- Soylu, S.; Yigitbas, H.; Soylu, E.M.; Kurt, Ş. Antifungal effects of essential oils from oregano and fennel on Sclerotinia sclerotiorum. J. Appl. Microbiol. 2007, 103, 1021–1030. [Google Scholar] [CrossRef]
- Xing, F.; Hua, H.; Selvaraj, J.N.; Zhao, Y.; Zhou, L.; Liu, X.; Liu, Y. Growth inhibition and morphological alterations of Fusarium verticillioides by cinnamon oil and cinnamaldehyde. Food Control 2014, 46, 343–350. [Google Scholar] [CrossRef]
- Jing, L.; Lei, Z.; Li, L.; Xie, R.; Xi, W.; Guan, Y.; Sumner, L.W.; Zhou, Z. Antifungal activity of Citrus essential oils. J. Agric. Food Chem. 2014, 62, 3011–3033. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Ban, X.; Huang, B.O.; He, J.; Tian, J.; Zeng, H.; Chen, Y.; Wang, Y. Interference and mechanism of dill seed essential oil and contribution of carvone and limonene in preventing sclerotia rot of rapeseed. PLoS ONE 2015, 10, e0131733. [Google Scholar] [CrossRef] [Green Version]
- Dias, A.L.B.; Sousa, W.C.; Batista, H.R.F.; Alves, C.C.F.; Souchie, E.L.; Silva, F.G.; Miranda, M.L.D. Chemical composition and In Vitro inhibitory effects of essential oils from fruit peel of three Citrus species and limonene on mycelial growth of Sclerotinia sclerotiorum. Braz. J. Biol. 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, R.-Y.; Wu, X.-M.; Yin, X.-H.; Long, Y.-H.; Li, M. Naturally produced citral can significantly inhibit normal physiology and induce cytotoxicity on Magnaporthe grisea. Pest. Biochem. Physiol. 2015, 118, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Jayashree, T.; Subramanyam, C. Antiaflatoxigenic activity of eugenol is due to inhibition of lipid peroxidation. Lett. Appl. Microbiol. 1999, 28, 179–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mimica-Dukić, N.; Kujundžić, S.; Soković, M.; Couladis, M. Essential oil composition and antifungal activity of Foeniculum vulgare Mill. obtained by different distillation conditions. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2003, 17, 368–371. [Google Scholar] [CrossRef]
- Marichali, A.; Hosni, K.; Dallali, S.; Ouerghemmi, S.; Hadjltaief, H.B.; Benzarti, S.; Kerkeni, A.; Sebei, H. Allelopathic effects of Carum carvi L. essential oil on germination of wheat, maize, flax and canary grass. Allelopath. J. 2014, 34, 81–94. [Google Scholar] [CrossRef]
- Ibáñez, M.; Blázquez, M. Phytotoxicity of essential oils on selected weeds: Potential hazard on food crops. Plants 2018, 7, 79. [Google Scholar] [CrossRef] [Green Version]
- Azirak, S.; Karaman, S. Allelopathic effect of some essential oils and components on germination of weed species. Acta Agric. Scand. Sect. B Soil Plant Sci. 2008, 58, 88–92. [Google Scholar] [CrossRef]
- De Martino, L.; Mancini, E.; de Almeida, L.F.R.; De Feo, V. The antigerminative activity of twenty-seven monoterpenes. Molecules 2010, 15, 6630–6637. [Google Scholar] [CrossRef] [Green Version]
- De Luna-López, M.C.; Valdivia-Flores, A.G.; Jaramillo-Juárez, F.; Reyes, J.L.; Ortiz-Martínez, R.; Quezada-Tristán, T. Association between Aspergillus flavus colonization and aflatoxins production in immature grains of maize genotypes. J. Food Eng. 2013, 3, 688–698. [Google Scholar]
- International Seed Testing Association (ISTA). Seed Testing International ISTA. ISTA News Bull. 2013, 145, 5–8. [Google Scholar]
- Waśkiewicz, A.; Morkunas, I.; Bednarski, W.; Mai, V.C.; Formela, M.; Beszterda, M.; Wiśniewska, H.; Goliński, P. Deoxynivalenol and oxidative stress indicators in winter wheat inoculated with Fusarium graminearum. Toxins 2014, 6, 575–591. [Google Scholar] [CrossRef] [PubMed]
- Moghimipour, E.; Ramezani, Z.; Handali, S. Solid lipid nanoparticles as a delivery system for Zataria multiflora essential oil: Formulation and characterization. Curr. Drug Deliv. 2013, 10, 151–157. [Google Scholar] [CrossRef]
- Mohammadi, A.; Hashemi, M.; Hosseini, S.M. Chitosan nanoparticles loaded with Cinnamomum zeylanicum essential oil enhance the shelf life of cucumber during cold storage. Postharvest Biol. Tec. 2015, 110, 203–213. [Google Scholar] [CrossRef]
- Pandey, A.K.; Palni, U.T.; Tripathi, N.N. Evaluation of Clausena pentaphylla (Roxb.) DC oil as fungitoxicant against storage mycoflora of pigeon pea seeds. J. Sci. Food Agric. 2013, 93, 1680–1686. [Google Scholar] [CrossRef]
- Pandey, A.K.; Singh, P.; Palni, U.T.; Tripathi, N.N. Application of Chenopodium ambrosioides Linn. essential oil as botanical fungicide for the management of fungal deterioration in pulse. Biol. Agric. Hortic. 2013, 29, 197–208. [Google Scholar] [CrossRef]
- Sonker, N.; Pandey, A.K.; Singh, P. Efficiency of Artemisia nilagirica (Clarke) Pamp essential oil as a mycotoxicant against postharvest mycobiota of table grapes. J. Sci. Food Agric. 2015, 95, 1932–1939. [Google Scholar] [CrossRef]


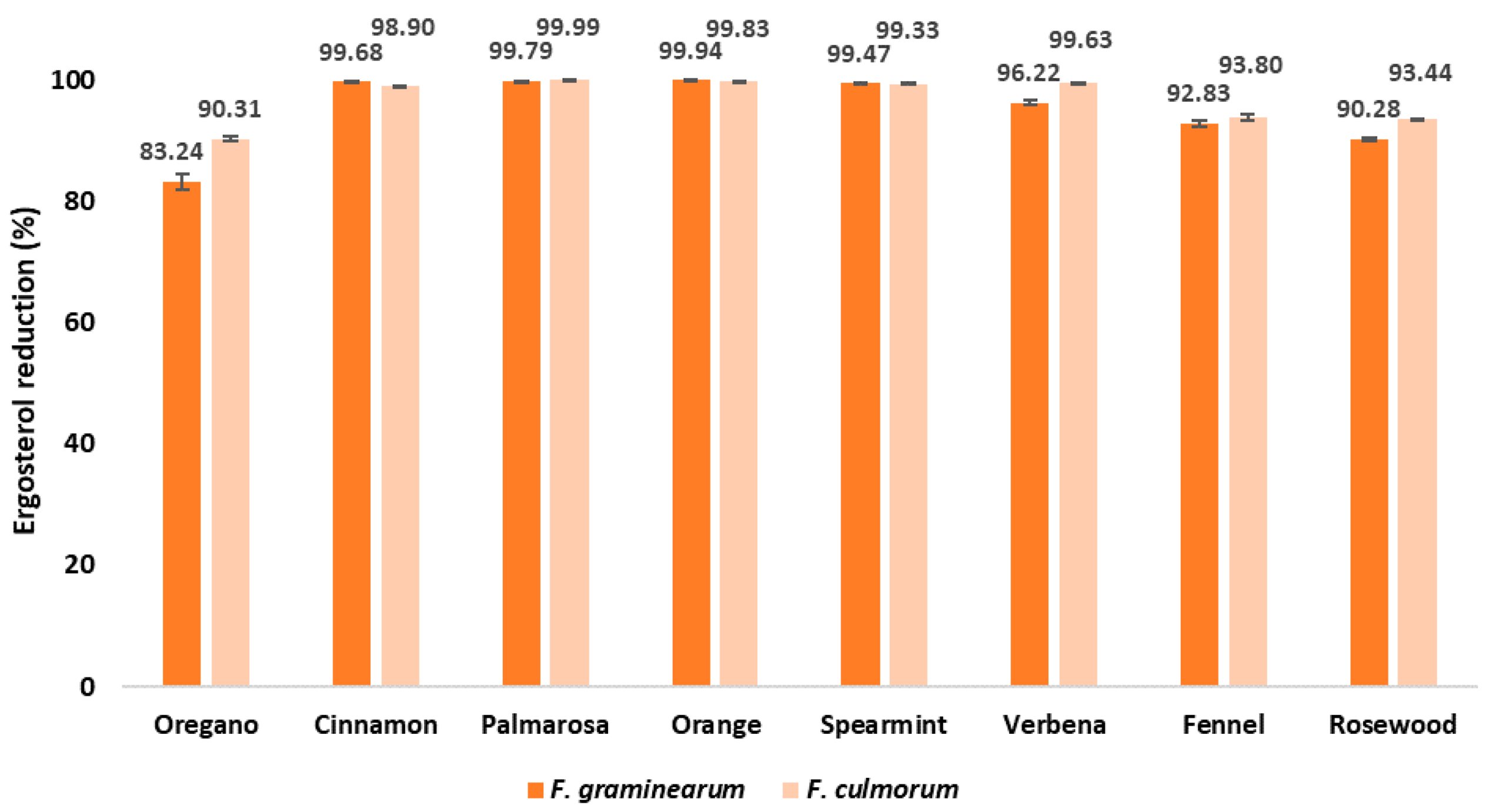
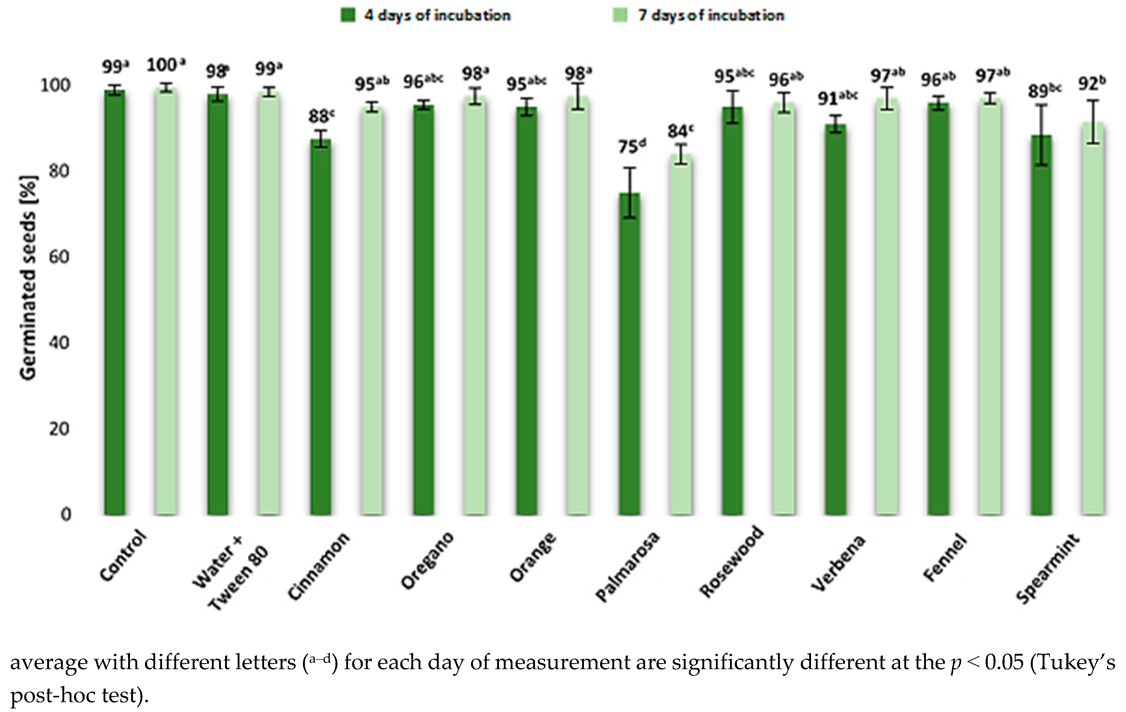
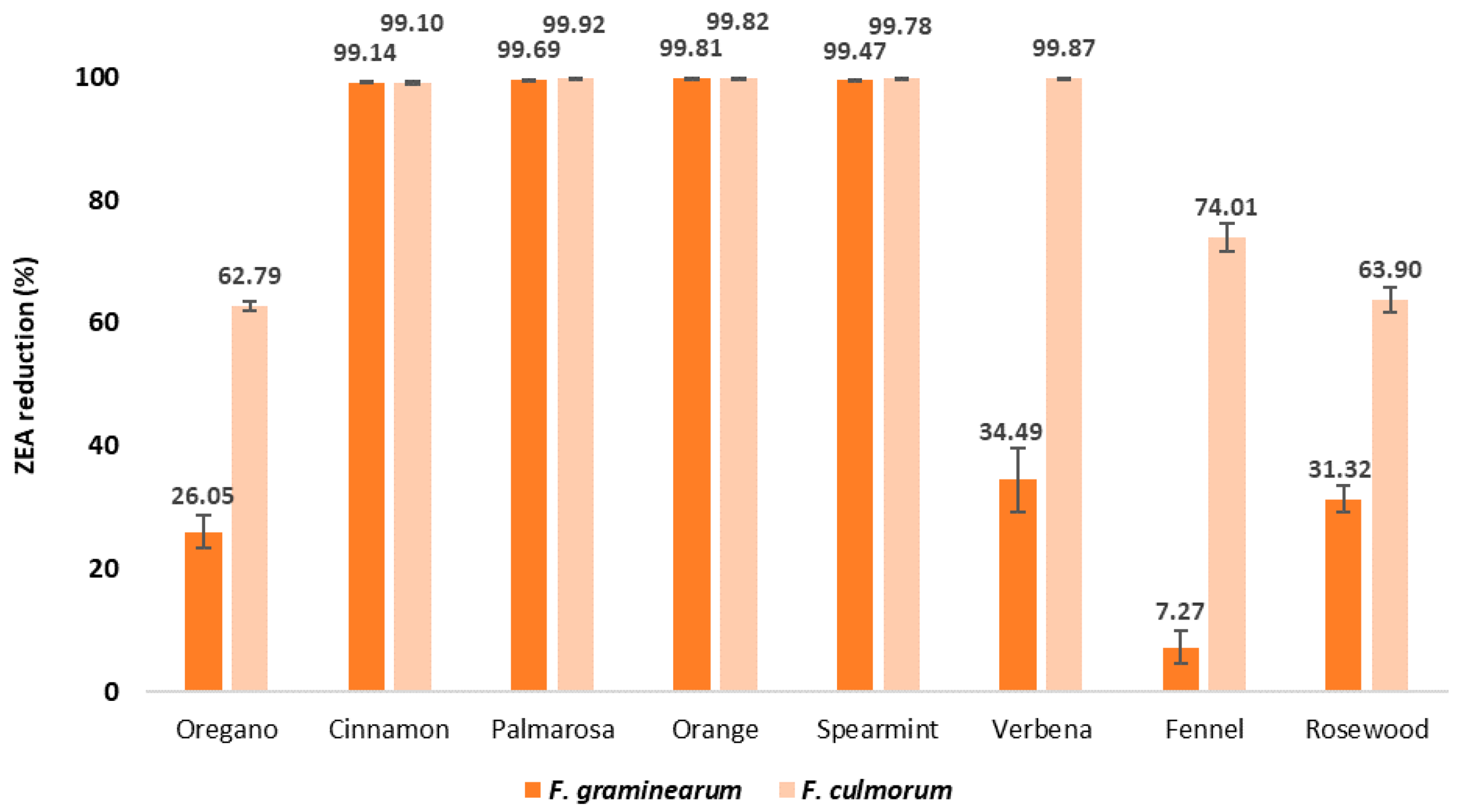
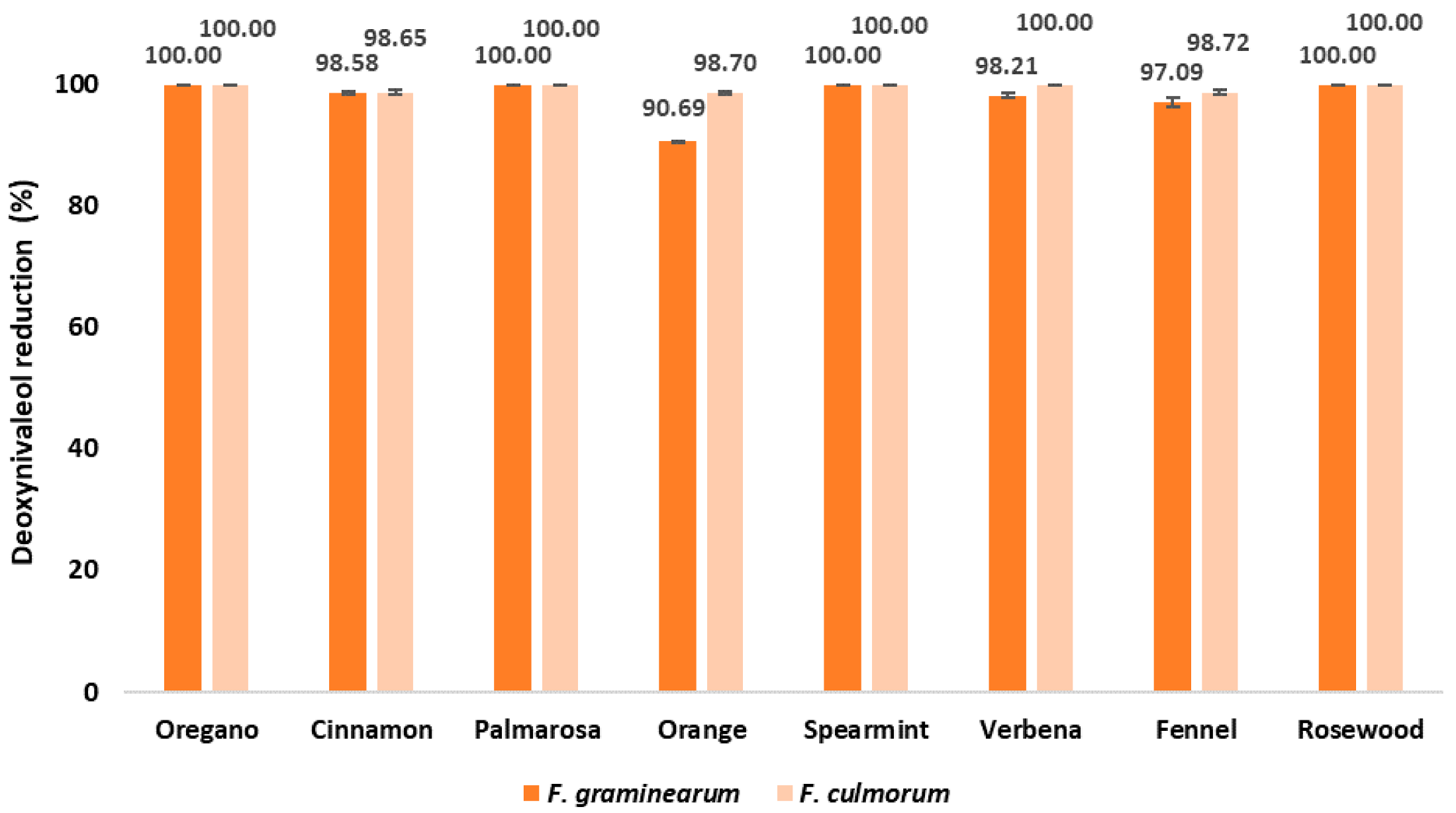
| Ergosterol ERG Content in Maize Grain (μg/g) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Oregano | Cinnamon | Palmarose | Orange | Spearmint | Verbena | Fennel | Rosewood | |
| F. graminearum | 2044.79 a ± 98.38 | 342.72 b ± 24.85 | 6.55 f ± 1.51 | 4.30 f ± 0.45 | 1.13 g ± 0.13 | 10.76 f ± 2.52 | 77.26 e ± 7.37 | 146.63 d ± 12.29 | 198.70 c ± 4.88 |
| F. culmorum | 3049.84 a ± 93.47 | 295.56 b ± 11.46 | 33.69 d ± 2.23 | 0.44 h ± 0.11 | 5.10 g ± 0.62 | 20.38 e ± 2.11 | 11.25 f ± 0.60 | 189.09 c ± 14.91 | 200.08 c ± 3.44 |
| ZEA Content in Maize Grain (μg/g) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Oregano | Cinnamon | Palmarose | Orange | Spearmint | Verbena | Fennel | Rosewood | |
| F. graminearum | 87.15 a ± 1.89 | 64.45 c ± 2.39 | 0.75 d ± 0.15 | 0.27 e ± 0.01 | 0.16 f ± 0.00 | 0.46 d ± 0.07 | 57.09 c ± 4.59 | 80.81 b ± 2.36 | 59.85 c ± 1.86 |
| F. culmorum | 175.85 a ± 7.59 | 65.43 b ± 1.15 | 1.59 d,e,f ± 0.61 | 0.14 g ± 0.01 | 0.32 e,f ± 0.03 | 0.39 d,e,f ± 0.10 | 0.23 f ± 0.03 | 45.08 c ± 3.94 | 63.48 b ± 3.57 |
| DON Content in Maize Grain (μg/g) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Oregano | Cinnamon | Palmarose | Orange | Spearmint | Verbena | Fennel | Rosewood | |
| F. graminearum | 1.79 a ± 0.01 | nd | 0.03 c ± 0.00 | nd | 0.17 b ± 0.00 | nd | 0.03 c ± 0.01 | 0.05 c ± 0.01 | nd |
| F. culmorum | 1.60 a ± 0.63 | nd | 0.02 b ± 0.01 | nd | 0.02 b ± 0.00 | nd | nd | 0.02 b ± 0.01 | nd |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perczak, A.; Gwiazdowska, D.; Gwiazdowski, R.; Juś, K.; Marchwińska, K.; Waśkiewicz, A. The Inhibitory Potential of Selected Essential Oils on Fusarium spp. Growth and Mycotoxins Biosynthesis in Maize Seeds. Pathogens 2020, 9, 23. https://doi.org/10.3390/pathogens9010023
Perczak A, Gwiazdowska D, Gwiazdowski R, Juś K, Marchwińska K, Waśkiewicz A. The Inhibitory Potential of Selected Essential Oils on Fusarium spp. Growth and Mycotoxins Biosynthesis in Maize Seeds. Pathogens. 2020; 9(1):23. https://doi.org/10.3390/pathogens9010023
Chicago/Turabian StylePerczak, Adam, Daniela Gwiazdowska, Romuald Gwiazdowski, Krzysztof Juś, Katarzyna Marchwińska, and Agnieszka Waśkiewicz. 2020. "The Inhibitory Potential of Selected Essential Oils on Fusarium spp. Growth and Mycotoxins Biosynthesis in Maize Seeds" Pathogens 9, no. 1: 23. https://doi.org/10.3390/pathogens9010023




