The Paradoxical Role of Uric Acid in Osteoporosis
Abstract
:1. Introduction
2. The Clearance of Uric Acid (UA) in Humans
3. Osteoporosis
3.1. Normal Bone Remodeling
3.2. Coupling of Bone Stimulators and Turnover Inhibitors
3.3. The Pathogenesis of Osteoporosis
- (a)
- Increased inflammatory cytokine-associated osteolysis. It leads to excessive activity of osteoclasts and leads to more bone resorption than bone formation. This phenomenon can be seen in gouty arthritis, inflammation, and vitamin D deficiency [26].
- (b)
- Incoordination of the RANK/RANKL system. Increased RANK/RANKL signal in OC strengthens the performance of osteoclasts and brings about more bone resorption. There are many clinical scenarios such as in hyperparathyroidism and some autoimmune diseases such as rheumatoid arthritis, systemic lupus erythematosus, and estrogen deprivation (postmenopausal women) [26,27].
- (c)
- Excessive Wnt signaling inhibitors in osteoblasts. Dickkopf-1, sclerostin, and secreted frizzled related proteins lead to diminished functioning of osteoblasts via a decrease in Wnt signaling activity, then reduced bone formation. This has been proven in chronic kidney disease, glucocorticoid-induced osteoporosis, and vascular calcification-related bone loss [28].
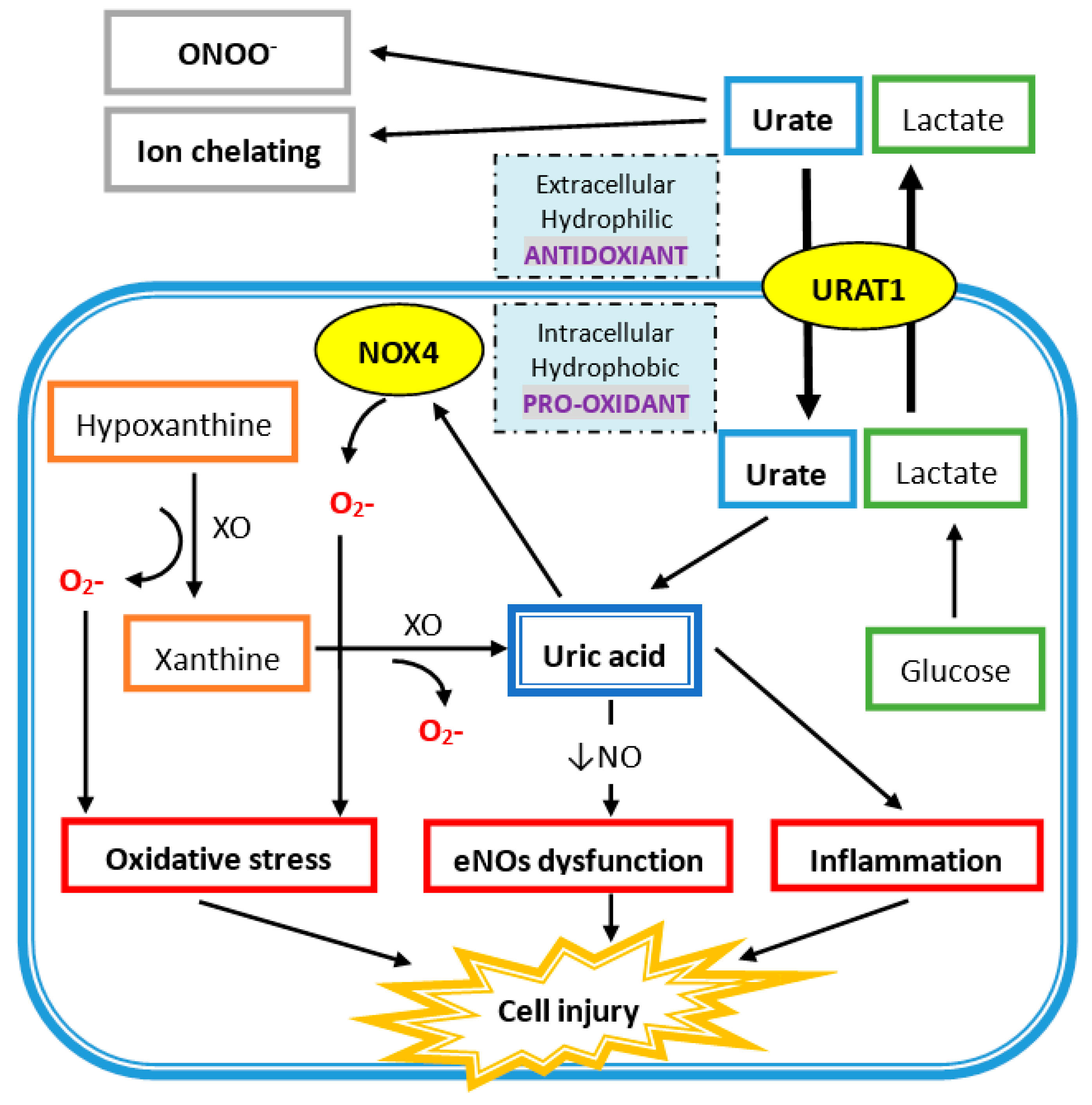
4. The Uric Acid Oxidant and Antioxidant Paradox
4.1. Antioxidant Properties of Uric Acid in Human Plasma
4.2. Intracellular Uric Acid Acts as a Pro-Oxidant to Damage Tissue
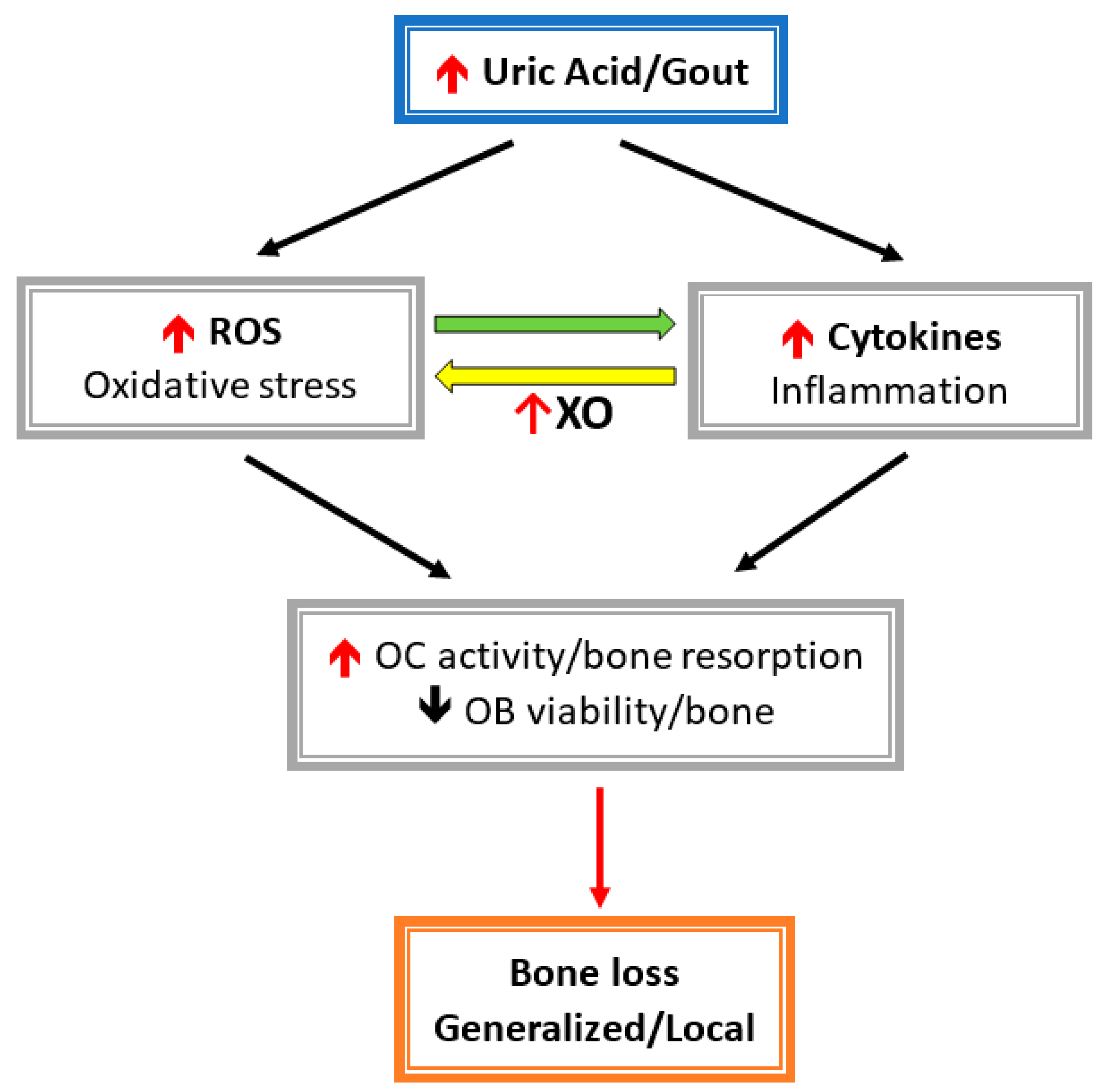
5. Pathophysiology of Uric Acid Induced Bone Loss
6. The Impact of Uric Acid on Vitamin D Metabolism
7. The Association of Uric Acid and Bone Health in Epidemiological Studies
7.1. Uric Acid as a Protective Factor in Bone Loss under Normal High Levels
7.2. Uric Acid as a Risk Factor in Bone Loss with Hyperuricemia and Gout
7.3. Potential Mechanism by which UA Disturbs Bone Remodeling
8. Magnetic Resonance Spectroscopy (MRS) for Osteoporosis
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Anton, F.M.; Garcia Puig, J.; Ramos, T.; Gonzalez, P.; Ordas, J. Sex differences in uric acid metabolism in adults: Evidence for a lack of influence of estradiol-17 beta (e2) on the renal handling of urate. Metab. Clin. Exp. 1986, 35, 343–348. [Google Scholar] [CrossRef]
- Williamson, M.A.; Snyder, L.M.; Wallach, J.B. Wallach’s Interpretation of Diagnostic Tests, 9th ed.; Wolters Kluwer/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2011; p. xvi. 1143p. [Google Scholar]
- Zhu, Y.; Pandya, B.J.; Choi, H.K. Prevalence of gout and hyperuricemia in the us general population: The national health and nutrition examination survey 2007–2008. Arthritis Rheum. 2011, 63, 3136–3141. [Google Scholar] [CrossRef]
- Campion, E.W.; Glynn, R.J.; DeLabry, L.O. Asymptomatic hyperuricemia. Risks and consequences in the normative aging study. Am. J. Med. 1987, 82, 421–426. [Google Scholar] [CrossRef]
- Hall, A.P.; Barry, P.E.; Dawber, T.R.; McNamara, P.M. Epidemiology of gout and hyperuricemia. A long-term population study. Am. J. Med. 1967, 42, 27–37. [Google Scholar] [CrossRef]
- Viggiano, D.; Gigliotti, G.; Vallone, G.; Giammarino, A.; Nigro, M.; Capasso, G. Urate-lowering agents in asymptomatic hyperuricemia: Role of urine sediment analysis and musculoskeletal ultrasound. Kidney Blood Press. Res. 2018, 43, 606–615. [Google Scholar] [CrossRef]
- Prevention and management of osteoporosis. World Health Organ. Tech. Rep. Ser. 2003, 921, 1–164.
- Griffith, J.F. Bone marrow changes in osteoporosis. In Osteoporosis and Bone Densitometry Measurements; Springer: Berlin/Heidelberg, Germany, 2013; pp. 69–85. [Google Scholar]
- Yeung, D.K.; Griffith, J.F.; Antonio, G.E.; Lee, F.K.; Woo, J.; Leung, P.C. Osteoporosis is associated with increased marrow fat content and decreased marrow fat unsaturation: A proton mr spectroscopy study. J. Magn. Reson. Imaging 2005, 22, 279–285. [Google Scholar] [CrossRef]
- Di Pietro, G.; Capuani, S.; Manenti, G.; Vinicola, V.; Fusco, A.; Baldi, J.; Scimeca, M.; Hagberg, G.; Bozzali, M.; Simonetti, G.; et al. Bone marrow lipid profiles from peripheral skeleton as potential biomarkers for osteoporosis: A 1h-mr spectroscopy study. Acad. Radiol. 2016, 23, 273–283. [Google Scholar] [CrossRef]
- Guido, G.; Scaglione, M.; Fabbri, L.; Ceglia, M.J. The “osteoporosis disease”. Clin. Cases Miner. Bone Metab. 2009, 6, 114–116. [Google Scholar]
- Charles, J.F.; Aliprantis, A.O. Osteoclasts: More than ‘bone eaters’. Trends Mol. Med. 2014, 20, 449–459. [Google Scholar] [CrossRef]
- Huls, M.; Brown, C.D.; Windass, A.S.; Sayer, R.; van den Heuvel, J.J.; Heemskerk, S.; Russel, F.G.; Masereeuw, R. The breast cancer resistance protein transporter abcg2 is expressed in the human kidney proximal tubule apical membrane. Kidney Int. 2008, 73, 220–225. [Google Scholar] [CrossRef]
- Maiuolo, J.; Oppedisano, F.; Gratteri, S.; Muscoli, C.; Mollace, V. Regulation of uric acid metabolism and excretion. Int. J. Cardiol. 2016, 213, 8–14. [Google Scholar] [CrossRef]
- Woodward, O.M.; Kottgen, A.; Coresh, J.; Boerwinkle, E.; Guggino, W.B.; Kottgen, M. Identification of a urate transporter, abcg2, with a common functional polymorphism causing gout. Proc. Natl. Acad. Sci. USA 2009, 106, 10338–10342. [Google Scholar] [CrossRef]
- Matsuo, H.; Takada, T.; Nakayama, A.; Shimizu, T.; Sakiyama, M.; Shimizu, S.; Chiba, T.; Nakashima, H.; Nakamura, T.; Takada, Y.; et al. Abcg2 dysfunction increases the risk of renal overload hyperuricemia. Nucleosides Nucleotides Nucleic Acids 2014, 33, 266–274. [Google Scholar] [CrossRef]
- Matsuo, H.; Nakayama, A.; Sakiyama, M.; Chiba, T.; Shimizu, S.; Kawamura, Y.; Nakashima, H.; Nakamura, T.; Takada, Y.; Oikawa, Y.; et al. Abcg2 dysfunction causes hyperuricemia due to both renal urate underexcretion and renal urate overload. Sci. Rep. 2014, 4, 3755. [Google Scholar] [CrossRef]
- Ichida, K.; Matsuo, H.; Takada, T.; Nakayama, A.; Murakami, K.; Shimizu, T.; Yamanashi, Y.; Kasuga, H.; Nakashima, H.; Nakamura, T.; et al. Decreased extra-renal urate excretion is a common cause of hyperuricemia. Nat. Commun. 2012, 3, 764. [Google Scholar] [CrossRef] [Green Version]
- Wallace, M.C.; Roberts, R.L.; Nanavati, P.; Miner, J.N.; Dalbeth, N.; Topless, R.; Merriman, T.R.; Stamp, L.K. Association between abcg2 rs2231142 and poor response to allopurinol: Replication and meta-analysis. Rheumatology 2018, 57, 656–660. [Google Scholar] [CrossRef]
- Cheng, S.T.; Wu, S.; Su, C.W.; Teng, M.S.; Hsu, L.A.; Ko, Y.L. Association of abcg2 rs2231142-a allele and serum uric acid levels in male and obese individuals in a han taiwanese population. J. Formos. Med. Assoc. 2017, 116, 18–23. [Google Scholar] [CrossRef]
- Kini, U.; Nandeesh, B.N. Physiology of bone formation, remodeling, and metabolism. In Radionuclide and Hybrid Bone Imaging; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Raisz, L.G. Physiology and pathophysiology of bone remodeling. Clin. Chem. 1999, 45, 1353–1358. [Google Scholar]
- Suda, T.; Takahashi, F.; Takahashi, N. Bone effects of vitamin d—Discrepancies between in vivo and in vitro studies. Arch. Biochem. Biophys. 2012, 523, 22–29. [Google Scholar] [CrossRef]
- Edwards, C.M.; Mundy, G.R. Eph receptors and ephrin signaling pathways: A role in bone homeostasis. Int. J. Med. Sci. 2008, 5, 263–272. [Google Scholar] [CrossRef]
- Matsuo, K.; Otaki, N. Bone cell interactions through eph/ephrin: Bone modeling, remodeling and associated diseases. Cell Adh. Migr. 2012, 6, 148–156. [Google Scholar] [CrossRef]
- Hou, Y.C.; Wu, C.C.; Liao, M.T.; Shyu, J.F.; Hung, C.F.; Yen, T.H.; Lu, C.L.; Lu, K.C. Role of nutritional vitamin d in osteoporosis treatment. Clin. Chim. Acta 2018, 484, 179–191. [Google Scholar] [CrossRef]
- Pivonka, P.; Zimak, J.; Smith, D.W.; Gardiner, B.S.; Dunstan, C.R.; Sims, N.A.; Martin, T.J.; Mundy, G.R. Theoretical investigation of the role of the rank-rankl-opg system in bone remodeling. J. Theor. Biol. 2010, 262, 306–316. [Google Scholar] [CrossRef]
- Khosla, S.; Hofbauer, L.C. Osteoporosis treatment: Recent developments and ongoing challenges. Lancet Diabetes Endocrinol. 2017, 5, 898–907. [Google Scholar] [CrossRef]
- Robinson, K.M.; Morre, J.T.; Beckman, J.S. Triuret: A novel product of peroxynitrite-mediated oxidation of urate. Arch. Biochem. Biophys. 2004, 423, 213–217. [Google Scholar] [CrossRef]
- Gersch, C.; Palii, S.P.; Imaram, W.; Kim, K.M.; Karumanchi, S.A.; Angerhofer, A.; Johnson, R.J.; Henderson, G.N. Reactions of peroxynitrite with uric acid: Formation of reactive intermediates, alkylated products and triuret, and in vivo production of triuret under conditions of oxidative stress. Nucleosides Nucleotides Nucleic Acids 2009, 28, 118–149. [Google Scholar] [CrossRef]
- Kuzkaya, N.; Weissmann, N.; Harrison, D.G.; Dikalov, S. Interactions of peroxynitrite with uric acid in the presence of ascorbate and thiols: Implications for uncoupling endothelial nitric oxide synthase. Biochem. Pharmacol. 2005, 70, 343–354. [Google Scholar] [CrossRef]
- Hayden, M.R.; Tyagi, S.C. Uric acid: A new look at an old risk marker for cardiovascular disease, metabolic syndrome, and type 2 diabetes mellitus: The urate redox shuttle. Nutr. Metab. 2004, 1, 10. [Google Scholar] [CrossRef]
- Hooper, D.C.; Spitsin, S.; Kean, R.B.; Champion, J.M.; Dickson, G.M.; Chaudhry, I.; Koprowski, H. Uric acid, a natural scavenger of peroxynitrite, in experimental allergic encephalomyelitis and multiple sclerosis. Proc. Natl. Acad. Sci. USA 1998, 95, 675–680. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Lu, J.M.; Yao, Q. Hyperuricemia-related diseases and xanthine oxidoreductase (xor) inhibitors: An overview. Med. Sci. Monit. 2016, 22, 2501–2512. [Google Scholar] [CrossRef]
- Fatima, T.; McKinney, C.; Major, T.J.; Stamp, L.K.; Dalbeth, N.; Iverson, C.; Merriman, T.R.; Miner, J.N. The relationship between ferritin and urate levels and risk of gout. Arthritis Res. Ther. 2018, 20, 179. [Google Scholar] [CrossRef]
- Ghio, A.J.; Ford, E.S.; Kennedy, T.P.; Hoidal, J.R. The association between serum ferritin and uric acid in humans. Free Radic. Res. 2005, 39, 337–342. [Google Scholar] [CrossRef]
- Ames, B.N.; Cathcart, R.; Schwiers, E.; Hochstein, P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: A hypothesis. Proc. Natl. Acad. Sci. USA 1981, 78, 6858–6862. [Google Scholar] [CrossRef]
- Yu, Z.F.; Bruce-Keller, A.J.; Goodman, Y.; Mattson, M.P. Uric acid protects neurons against excitotoxic and metabolic insults in cell culture, and against focal ischemic brain injury in vivo. J. Neurosci. Res. 1998, 53, 613–625. [Google Scholar] [CrossRef]
- Tsukada, K.; Hasegawa, T.; Tsutsumi, S.; Katoh, H.; Kuwano, H.; Miyazaki, T.; Yamamoto, Y. Effect of uric acid on liver injury during hemorrhagic shock. Surgery 2000, 127, 439–446. [Google Scholar] [CrossRef]
- Becker, B.; Reinholz, N.; Ozcelik, T.; Leipert, B.; Gerlach, E. Uric acid as radical scavenger and antioxidant in the heart. Pflugers Arch. 1989, 415, 127–135. [Google Scholar] [CrossRef]
- Sharaf El Din, U.A.A.; Salem, M.M.; Abdulazim, D.O. Uric acid in the pathogenesis of metabolic, renal, and cardiovascular diseases: A review. J. Adv. Res. 2017, 8, 537–548. [Google Scholar] [CrossRef]
- Fisher, A.B. Redox signaling across cell membranes. Antioxidants Redox Signal. 2009, 11, 1349–1356. [Google Scholar] [CrossRef]
- Maples, K.; Mason, R. Free radical metabolite of uric acid. J. Biol. Chem. 1988, 263, 1709–1712. [Google Scholar]
- Santos, C.X.; Anjos, E.I.; Augusto, O. Uric acid oxidation by peroxynitrite: Multiple reactions, free radical formation, and amplification of lipid oxidation. Arch. Biochem. Biophys. 1999, 372, 285–294. [Google Scholar] [CrossRef]
- Sautin, Y.Y.; Johnson, R.J. Uric acid: The oxidant-antioxidant paradox. Nucleosides Nucleotides Nucleic Acids 2008, 27, 608–619. [Google Scholar] [CrossRef]
- Bagnati, M.; Perugini, C.; Cau, C.; Bordone, R.; Albano, E.; Bellomo, G. When and why a water-soluble antioxidant becomes pro-oxidant during copper-induced low-density lipoprotein oxidation: A study using uric acid. Biochem. J. 1999, 340, 143–152. [Google Scholar] [CrossRef]
- Atashi, F.; Modarressi, A.; Pepper, M.S. The role of reactive oxygen species in mesenchymal stem cell adipogenic and osteogenic differentiation: A review. Stem Cells Dev 2015, 24, 1150–1163. [Google Scholar] [CrossRef]
- Mancini, L.; Moradi-Bidhendi, N.; Brandi, M.L.; MacIntyre, I. Nitric oxide superoxide and peroxynitrite modulate osteoclast activity. Biochem. Biophys. Res. Commun. 1998, 243, 785–790. [Google Scholar] [CrossRef]
- Airton Castro da Rocha, F.; Fernandes, A. Evidence that peroxynitrite affects human osteoblast proliferation and differentiation. J. Bone Miner. Res. 2002, 17, 434–442. [Google Scholar] [CrossRef]
- Domazetovic, V.; Marcucci, G.; Iantomasi, T.; Brandi, M.L.; Vincenzini, M.T. Oxidative stress in bone remodeling: Role of antioxidants. Clin. Cases Miner. Bone Metab. 2017, 14, 209–216. [Google Scholar] [CrossRef]
- Bai, X.C.; Lu, D.; Bai, J.; Zheng, H.; Ke, Z.Y.; Li, X.M.; Luo, S.Q. Oxidative stress inhibits osteoblastic differentiation of bone cells by erk and nf-kappab. Biochem. Biophys. Res. Commun. 2004, 314, 197–207. [Google Scholar] [CrossRef]
- Mody, N.; Parhami, F.; Sarafian, T.A.; Demer, L.L. Oxidative stress modulates osteoblastic differentiation of vascular and bone cells. Free Radic. Biol. Med. 2001, 31, 509–519. [Google Scholar] [CrossRef]
- Steinbeck, M.J.; Appel, W.H., Jr.; Verhoeven, A.J.; Karnovsky, M.J. Nadph-oxidase expression and in situ production of superoxide by osteoclasts actively resorbing bone. J. Cell Biol. 1994, 126, 765–772. [Google Scholar] [CrossRef]
- Key, L.L., Jr.; Wolf, W.C.; Gundberg, C.M.; Ries, W.L. Superoxide and bone resorption. Bone 1994, 15, 431–436. [Google Scholar]
- Garrett, I.R.; Boyce, B.F.; Oreffo, R.O.; Bonewald, L.; Poser, J.; Mundy, G.R. Oxygen-derived free radicals stimulate osteoclastic bone resorption in rodent bone in vitro and in vivo. J. Clin. Investig. 1990, 85, 632–639. [Google Scholar] [CrossRef]
- Fraser, J.H.; Helfrich, M.H.; Wallace, H.M.; Ralston, S.H. Hydrogen peroxide, but not superoxide, stimulates bone resorption in mouse calvariae. Bone 1996, 19, 223–226. [Google Scholar] [CrossRef]
- Bax, B.E.; Alam, A.T.; Banerji, B.; Bax, C.M.; Bevis, P.J.; Stevens, C.R.; Moonga, B.S.; Blake, D.R.; Zaidi, M. Stimulation of osteoclastic bone resorption by hydrogen peroxide. Biochem. Biophys. Res. Commun. 1992, 183, 153–1158. [Google Scholar] [CrossRef]
- Yokose, K.; Sato, S.; Asano, T.; Yashiro, M.; Kobayashi, H.; Watanabe, H.; Suzuki, E.; Sato, C.; Kozuru, H.; Yatsuhashi, H.; et al. Tnf-alpha potentiates uric acid-induced interleukin-1beta (il-1beta) secretion in human neutrophils. Mod. Rheumatol. 2018, 28, 513–517. [Google Scholar] [CrossRef]
- Di Giovine, F.S.; Malawista, S.E.; Thornton, E.; Duff, G.W. Urate crystals stimulate production of tumor necrosis factor alpha from human blood monocytes and synovial cells. Cytokine mrna and protein kinetics, and cellular distribution. J. Clin. Investig. 1991, 87, 1375–1381. [Google Scholar] [CrossRef]
- Chhana, A.; Pool, B.; Callon, K.E.; Tay, M.L.; Musson, D.; Naot, D.; McCarthy, G.; McGlashan, S.; Cornish, J.; Dalbeth, N. Monosodium urate crystals reduce osteocyte viability and indirectly promote a shift in osteocyte function towards a proinflammatory and proresorptive state. Arthritis Res. Ther. 2018, 20, 208. [Google Scholar] [CrossRef]
- Matsushima, K.; Morishita, K.; Yoshimura, T.; Lavu, S.; Kobayashi, Y.; Lew, W.; Appella, E.; Kung, H.F.; Leonard, E.J.; Oppenheim, J.J. Molecular cloning of a human monocyte-derived neutrophil chemotactic factor (mdncf) and the induction of mdncf mrna by interleukin 1 and tumor necrosis factor. J. Exp. Med. 1988, 167, 1883–1893. [Google Scholar] [CrossRef]
- Zhou, R.; Tardivel, A.; Thorens, B.; Choi, I.; Tschopp, J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 2010, 11, 136–140. [Google Scholar] [CrossRef]
- Martinon, F. Update on biology: Uric acid and the activation of immune and inflammatory cells. Curr. Rheumatol. Rep. 2010, 12, 135–141. [Google Scholar] [CrossRef]
- Manaka, H.; Iwakura, Y.; Lee, Y.-M.; Fujikado, N.; Yasuda, H. Il-1 plays an important role in the bone metabolism under physiological conditions. Int. Immunol. 2010, 22, 805–816. [Google Scholar]
- Udagawa, N.; Takahashi, N.; Katagiri, T.; Tamura, T.; Wada, S.; Findlay, D.M.; Martin, T.J.; Hirota, H.; Taga, T.; Kishimoto, T.; et al. Interleukin (il)-6 induction of osteoclast differentiation depends on il-6 receptors expressed on osteoblastic cells but not on osteoclast progenitors. J. Exp. Med. 1995, 182, 1461. [Google Scholar] [CrossRef]
- Ritchlin, C.T.; Haas-Smith, S.A.; Li, P.; Hicks, D.G.; Schwarz, E.M. Mechanisms of tnf-alpha- and rankl-mediated osteoclastogenesis and bone resorption in psoriatic arthritis. J. Clin. Investig. 2003, 111, 821–831. [Google Scholar] [CrossRef]
- Kobayashi, K.; Takahashi, N.; Jimi, E.; Udagawa, N.; Takami, M.; Kotake, S.; Nakagawa, N.; Kinosaki, M.; Yamaguchi, K.; Shima, N.; et al. Tumor necrosis factor alpha stimulates osteoclast differentiation by a mechanism independent of the odf/rankl-rank interaction. J. Exp. Med. 2000, 191, 275–286. [Google Scholar] [CrossRef]
- Orriss, I.R.; Arnett, T.R.; George, J.; Witham, M.D. Allopurinol and oxypurinol promote osteoblast differentiation and increase bone formation. Exp. Cell Res. 2016, 342, 166–174. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Roncal-Jimenez, C.; Lanaspa, M.; Gerard, S.; Chonchol, M.; Johnson, R.J.; Jalal, D. Uric acid suppresses 1 alpha hydroxylase in vitro and in vivo. Metab. Clin. Exp. 2014, 63, 150–160. [Google Scholar] [CrossRef]
- Vanholder, R.; Patel, S.; Hsu, C.H. Effect of uric acid on plasma levels of 1,25(oh)2d in renal failure. J. Am. Soc. Nephrol. 1993, 4, 1035–1038. [Google Scholar]
- Peng, H.; Li, H.; Li, C.; Chao, X.; Zhang, Q.; Zhang, Y. Association between vitamin d insufficiency and elevated serum uric acid among middle-aged and elderly Chinese Han women. PLoS ONE 2013, 8, e61159. [Google Scholar] [CrossRef]
- Elnokeety, M. Negative association between serum 25 hydroxy vitamin d and serum uric acid among stage 35 chronic kidneydisease patient. Ann. Med. Health Sci. Res. 2018, 8, 87–90. [Google Scholar]
- Hui, J.Y.; Choi, J.W.; Mount, D.B.; Zhu, Y.; Zhang, Y.; Choi, H.K. The independent association between parathyroid hormone levels and hyperuricemia: A national population study. Arthritis Res. Ther. 2012, 14, R56. [Google Scholar] [CrossRef]
- Yoneda, M.; Takatsuki, K.; Tomita, A. Parathyroid function and uric acid metabolism. Nihon Naibunpi Gakkai Zasshi 1983, 59, 1738–1751. [Google Scholar]
- Chin, K.-Y.; Nirwana, S.I.; Ngah, W.Z.W. Significant association between parathyroid hormone and uric acid level in men. Clin. Interv. Aging 2015, 10, 1377–1380. [Google Scholar] [CrossRef]
- Bergenfelz, A.; Bladstrom, A.; Their, M.; Nordenstrom, E.; Valdemarsson, S.; Westerdahl, J. Serum levels of uric acid and diabetes mellitus influence survival after surgery for primary hyperparathyroidism: A prospective cohort study. World J. Surg. 2007, 31, 1393–1400. [Google Scholar] [CrossRef]
- Duh, Q.Y.; Morris, R.C.; Arnaud, C.D.; Clark, O.H. Decrease in serum uric acid level following parathyroidectomy in patients with primary hyperparathyroidism. World J. Surg. 1986, 10, 729–736. [Google Scholar] [CrossRef]
- Miller, P.D.; Schwartz, E.N.; Chen, P.; Misurski, D.A.; Krege, J.H. Teriparatide in postmenopausal women with osteoporosis and mild or moderate renal impairment. Osteoporos. Int. 2007, 18, 59–68. [Google Scholar] [CrossRef]
- Hisatome, I.; Ishimura, M.; Sasaki, N.; Yamakawa, M.; Kosaka, H.; Tanaka, Y.; Kouchi, T.; Mitani, Y.; Yoshida, A.; Kotake, H.; et al. Renal handling of urate in two patients with hyperuricemia and primary hyperparathyroidism. Intern. Med. 1992, 31, 807–811. [Google Scholar] [CrossRef]
- Sugimoto, R.; Watanabe, H.; Ikegami, K.; Enoki, Y.; Imafuku, T.; Sakaguchi, Y.; Murata, M.; Nishida, K.; Miyamura, S.; Ishima, Y.; et al. Down-regulation of abcg2, a urate exporter, by parathyroid hormone enhances urate accumulation in secondary hyperparathyroidism. Kidney Int. 2017, 91, 658–670. [Google Scholar] [CrossRef]
- Fabbrini, E.; Serafini, M.; Colic Baric, I.; Hazen, S.L.; Klein, S. Effect of plasma uric acid on antioxidant capacity, oxidative stress, and insulin sensitivity in obese subjects. Diabetes 2014, 63, 976–981. [Google Scholar] [CrossRef]
- Nabipour, I.; Sambrook, P.N.; Blyth, F.M.; Janu, M.R.; Waite, L.M.; Naganathan, V.; Handelsman, D.J.; Le Couteur, D.G.; Cumming, R.G.; Seibel, M.J. Serum uric acid is associated with bone health in older men: A cross-sectional population-based study. J. Bone Miner. Res. 2011, 26, 955–964. [Google Scholar] [CrossRef]
- Ishii, S.; Miyao, M.; Mizuno, Y.; Tanaka-Ishikawa, M.; Akishita, M.; Ouchi, Y. Association between serum uric acid and lumbar spine bone mineral density in peri- and postmenopausal Japanese women. Osteoporos. Int. 2014, 25, 1099–1105. [Google Scholar] [CrossRef]
- Makovey, J.; Macara, M.; Chen, J.S.; Hayward, C.S.; March, L.; Seibel, M.J.; Sambrook, P.N. Serum uric acid plays a protective role for bone loss in peri- and postmenopausal women: A longitudinal study. Bone 2013, 52, 400–406. [Google Scholar] [CrossRef]
- Lane, N.E.; Parimi, N.; Lui, L.-Y.; Wise, B.L.; Yao, W.; Lay, Y.-A.E.; Cawthon, P.M.; Orwoll, E.; Osteoporotic Fractures in Men Study, G. Association of serum uric acid and incident nonspine fractures in elderly men: The osteoporotic fractures in men (mros) study. J. Bone Miner. Res. 2014, 29, 1701–1707. [Google Scholar] [CrossRef]
- Finck, H.; Hart, A.R.; Jennings, A.; Welch, A.A. Is there a role for vitamin c in preventing osteoporosis and fractures? A review of the potential underlying mechanisms and current epidemiological evidence. Nutr. Res. Rev. 2014, 27, 268–283. [Google Scholar] [CrossRef]
- Kim, M.H.; Lee, H.-J. Osteoporosis, vitamin c intake, and physical activity in Korean adults aged 50 years and over. J. Phys. Ther. Sci. 2016, 28, 725–730. [Google Scholar] [CrossRef]
- Chai, S.C.; Wei, C.I.; Brummel-Smith, K.; Arjmandi, B.H. The role of vitamin e in reversing bone loss. Aging Clin. Exp. Res. 2008, 20, 521–527. [Google Scholar] [CrossRef]
- Naina Mohamed, I.; Borhanuddin, B.; Shuid, A.N.; Mohd Fozi, N.F. Vitamin e and bone structural changes: An evidence-based review. Evid.-Based Complementary Altern. Med. 2012, 2012, 250584. [Google Scholar] [CrossRef]
- Shi, W.Q.; Liu, J.; Cao, Y.; Zhu, Y.Y.; Guan, K.; Chen, Y.M. Association of dietary and serum vitamin e with bone mineral density in middle-aged and elderly Chinese adults: A cross-sectional study. Br. J. Nutr. 2016, 115, 113–120. [Google Scholar] [CrossRef]
- Behfar, A.; Sadeghi, N.; Oveisi, M.R.; Jannat, B.; Hajimahmoodi, M.; Jamshidi, A.; Behzad, M.; Rastegary, P. The plasma antioxidant activity of superoxide dismutase enzyme in osteoporosis. Acta Med. Iran. 2008, 46, 441–446. [Google Scholar]
- Cherubini, A.; Polidori, M.C.; Catani, M.; Pierandrei, M.; Barabani, M.; Mecocci, P.; Senin, U.; Maggio, D.; Pacifici, R. Marked decrease in plasma antioxidants in aged osteoporotic women: Results of a cross-sectional study. J. Clin. Endocrinol. Metab. 2003, 88, 1523–1527. [Google Scholar]
- Avitabile, M.; Campagna, N.E.; Magri, G.A.; Vinci, M.; Sciacca, G.; Alia, G.; Ferro, A. Correlation between serum glutathione reductases and bone densitometry values. Bollettino della Societa Italiana di Biologia Sperimentale 1991, 67, 931–937. [Google Scholar]
- Glantzounis, G.K.; Tsimoyiannis, E.C.; Kappas, A.M.; Galaris, D.A. Uric acid and oxidative stress. Curr. Pharm. Des. 2005, 11, 4145–4151. [Google Scholar] [CrossRef]
- Stocker, R.; Keaney, J.F., Jr. Role of oxidative modifications in atherosclerosis. Physiol. Rev. 2004, 84, 1381–1478. [Google Scholar] [CrossRef]
- Mehta, T.; Bůžková, P.; Sarnak, M.J.; Chonchol, M.; Cauley, J.A.; Wallace, E.; Fink, H.A.; Robbins, J.; Jalal, D. Serum urate levels and the risk of hip fractures: Data from the cardiovascular health study. Metab. Clin. Exp. 2015, 64, 438–446. [Google Scholar] [CrossRef]
- Paik, J.M.; Kim, S.C.; Feskanich, D.; Choi, H.K.; Solomon, D.H.; Curhan, G.C. Gout and risk of fracture in women: A prospective cohort study. Arthritis Rheumatol. 2017, 69, 422–428. [Google Scholar] [CrossRef]
- Ikeda, K.; Ogata, E. The effect of vitamin d on osteoblasts and osteoclasts. Curr. Opin. Orthop. 1999, 10, 339–343. [Google Scholar] [CrossRef]
- Van Driel, M.; van Leeuwen, J.P.T.M. Vitamin d endocrine system and osteoblasts. BoneKEy Rep. 2014, 3, 493. [Google Scholar] [CrossRef]
- Lu, C.L.; Shyu, J.F.; Wu, C.C.; Hung, C.F.; Liao, M.T.; Liu, W.C.; Zheng, C.M.; Hou, Y.C.; Lin, Y.F.; Lu, K.C. Association of anabolic effect of calcitriol with osteoclast-derived wnt 10b secretion. Nutrients 2018, 10, 1164. [Google Scholar] [CrossRef]
- Larijani, B.; Hossein-Nezhad, A.; Feizabad, E.; Maghbooli, Z.; Adibi, H.; Ramezani, M.; Taheri, E. Vitamin d deficiency, bone turnover markers and causative factors among adolescents: A cross-sectional study. J. Diabetes Metab. Disord. 2016, 15, 46. [Google Scholar] [CrossRef]
- Thiering, E.; Brüske, I.; Kratzsch, J.; Hofbauer, L.C.; Berdel, D.; von Berg, A.; Lehmann, I.; Hoffmann, B.; Bauer, C.P.; Koletzko, S.; et al. Associations between serum 25-hydroxyvitamin d and bone turnover markers in a population based sample of german children. Scie. Rep. 2015, 5, 18138. [Google Scholar] [CrossRef]
- Nahas-Neto, J.; Cangussu, L.M.; Orsatti, C.L.; Bueloni-Dias, F.N.; Poloni, P.F.; Schmitt, E.B.; Nahas, E.A.P. Effect of isolated vitamin d supplementation on bone turnover markers in younger postmenopausal women: A randomized, double-blind, placebo-controlled trial. Osteoporos. Int. 2018, 29, 1125–1133. [Google Scholar] [CrossRef]
- Lips, P. Vitamin d deficiency and secondary hyperparathyroidism in the elderly: Consequences for bone loss and fractures and therapeutic implications. Endocr. Rev. 2001, 22, 477–501. [Google Scholar] [CrossRef]
- Serhan, E.; Holland, M.R. Relationship of hypovitaminosis d and secondary hyperparathyroidism with bone mineral density among uk resident indo-asians. Ann. Rheum. Dis. 2002, 61, 456–458. [Google Scholar] [CrossRef]
- Ooms, M.E.; Lips, P.; Roos, J.C.; van der Vijgh, W.J.F.; Popp-Snijders, C.; Bezemer, P.D.; Bouter, L.M. Vitamin d status and sex hormone binding globulin: Determinants of bone turnover and bone mineral density in elderly women. J. Bone Miner. Res. 1995, 10, 1177–1184. [Google Scholar] [CrossRef]
- Khaw, K.T.; Sneyd, M.J.; Compston, J. Bone density parathyroid hormone and 25-hydroxyvitamin d concentrations in middle aged women. BMJ 1992, 305, 273–277. [Google Scholar] [CrossRef]
- Steingrimsdottir, L.; Halldorsson, T.I.; Siggeirsdottir, K.; Cotch, M.F.; Einarsdottir, B.O.; Eiriksdottir, G.; Sigurdsson, S.; Launer, L.J.; Harris, T.B.; Gudnason, V.; et al. Hip fractures and bone mineral density in the elderly–importance of serum 25-hydroxyvitamin d. PLoS ONE 2014, 9, e91122. [Google Scholar] [CrossRef]
- Barbour, K.E.; Houston, D.K.; Cummings, S.R.; Boudreau, R.; Prasad, T.; Sheu, Y.; Bauer, D.C.; Tooze, J.A.; Kritchevsky, S.B.; Tylavsky, F.A.; et al. Calciotropic hormones and the risk of hip and nonspine fractures in older adults: The health abc study. J. Bone Miner. Res. 2012, 27, 1177–1185. [Google Scholar] [CrossRef]
- Lv, Q.-B.; Gao, X.; Liu, X.; Shao, Z.-X.; Xu, Q.-H.; Tang, L.; Chi, Y.-L.; Wu, A.-M. The serum 25-hydroxyvitamin d levels and hip fracture risk: A meta-analysis of prospective cohort studies. Oncotarget 2017, 8, 39849–39858. [Google Scholar] [CrossRef]
- Tzeng, H.-E.; Lin, C.-C.; Wang, I.K.; Huang, P.-H.; Tsai, C.-H. Gout increases risk of fracture: A nationwide population-based cohort study. Medicine 2016, 95, e4669. [Google Scholar] [CrossRef]
- Kok, V.C.; Horng, J.T.; Wang, M.N.; Chen, Z.Y.; Kuo, J.T.; Hung, G.D. Gout as a risk factor for osteoporosis: Epidemiologic evidence from a population-based longitudinal study involving 108,060 individuals. Osteoporos. Int. 2018, 29, 973–985. [Google Scholar] [CrossRef]
- Sultan, A.A.; Whittle, R.; Muller, S.; Roddy, E.; Mallen, C.D.; Bucknall, M.; Helliwell, T.; Hider, S.; Paskins, Z. Risk of fragility fracture among patients with gout and the effect of urate-lowering therapy. CMAJ 2018, 190, E581–E587. [Google Scholar] [CrossRef] [Green Version]
- Basu, U.; Goodbrand, J.; McMurdo, M.E.T.; Donnan, P.T.; McGilchrist, M.; Frost, H.; George, J.; Witham, M.D. Association between allopurinol use and hip fracture in older patients. Bone 2016, 84, 189–193. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, A.V. Marrow fat and bone: Review of clinical findings. Front. Endocrinol. 2015, 6, 40. [Google Scholar] [CrossRef]
- Yeung, D.K.; Lam, S.L.; Griffith, J.F.; Chan, A.B.; Chen, Z.; Tsang, P.H.; Leung, P.C. Analysis of bone marrow fatty acid composition using high-resolution proton nmr spectroscopy. Chem. Phys. Lipids 2008, 151, 103–109. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, K.-M.; Lu, C.-L.; Hung, K.-C.; Wu, P.-C.; Pan, C.-F.; Wu, C.-J.; Syu, R.-S.; Chen, J.-S.; Hsiao, P.-J.; Lu, K.-C. The Paradoxical Role of Uric Acid in Osteoporosis. Nutrients 2019, 11, 2111. https://doi.org/10.3390/nu11092111
Lin K-M, Lu C-L, Hung K-C, Wu P-C, Pan C-F, Wu C-J, Syu R-S, Chen J-S, Hsiao P-J, Lu K-C. The Paradoxical Role of Uric Acid in Osteoporosis. Nutrients. 2019; 11(9):2111. https://doi.org/10.3390/nu11092111
Chicago/Turabian StyleLin, Kun-Mo, Chien-Lin Lu, Kuo-Chin Hung, Pei-Chen Wu, Chi-Feng Pan, Chih-Jen Wu, Ren-Si Syu, Jin-Shuen Chen, Po-Jen Hsiao, and Kuo-Cheng Lu. 2019. "The Paradoxical Role of Uric Acid in Osteoporosis" Nutrients 11, no. 9: 2111. https://doi.org/10.3390/nu11092111




