Mast Cells, Stress, Fear and Autism Spectrum Disorder
Abstract
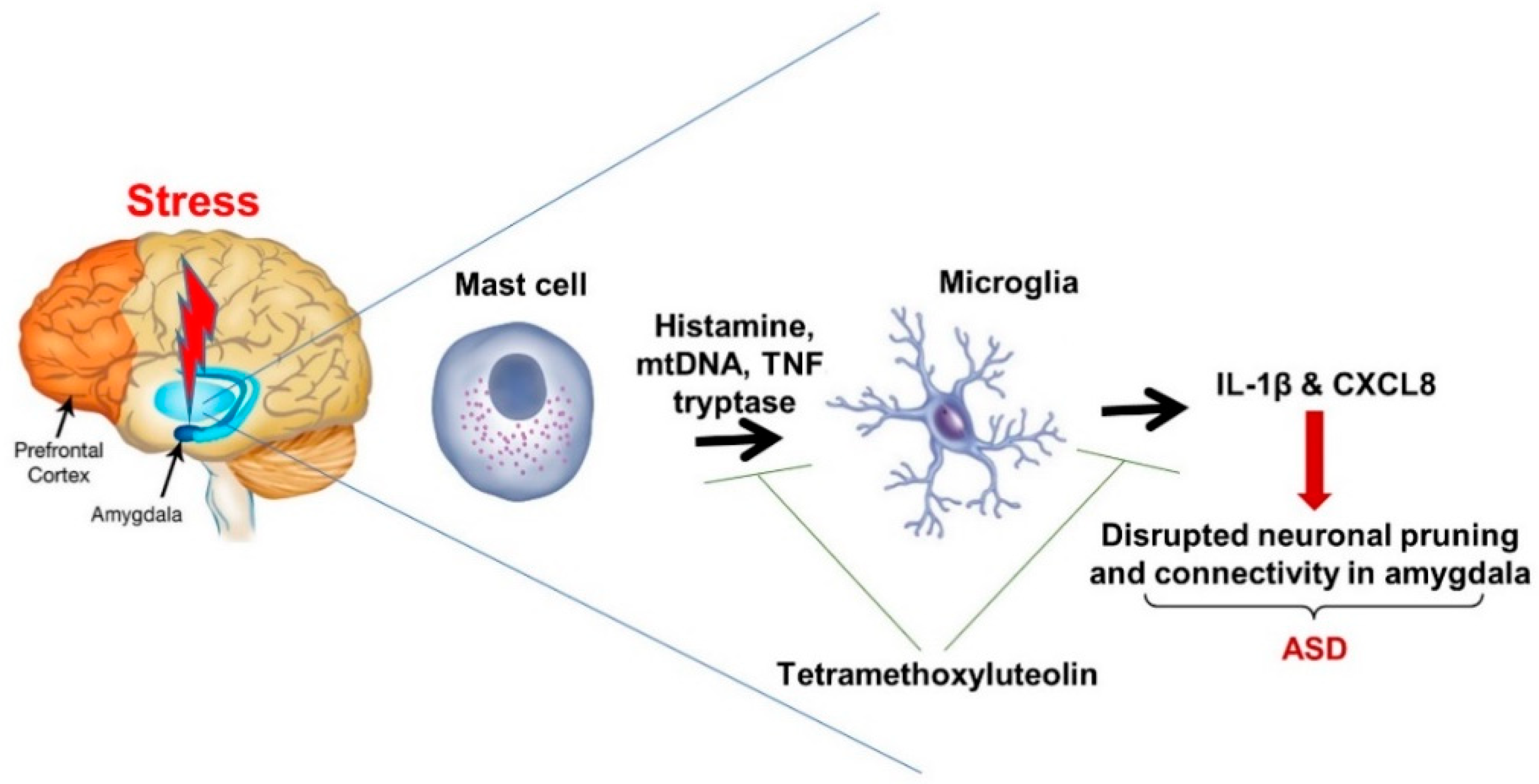
:1. Introduction
2. Mast Cells and Neuroinflammation
3. Stress and the Fear Response
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| BBB | blood–brain barrier |
| BLA | basolateral nucleus of amygdala |
| CRH | corticotropin-releasing hormone |
| HPA | hypothalamic-pituitary-adrenal |
| mtDNA | mitochondrial DNA |
| NT | neurotensin |
| PANS | pediatric acute neuropsychiatric syndrome |
| SP | substance P |
References
- Fombonne, E. Epidemiology of pervasive developmental disorders. Pediatr. Res. 2009, 65, 591. [Google Scholar] [CrossRef] [PubMed]
- McPartland, J.; Volkmar, F.R. Autism and related disorders. Handb. Clin. Neurol. 2012, 106, 407–418. [Google Scholar] [PubMed] [Green Version]
- Lai, M.C.; Lombardo, M.V.; Baron-Cohen, S. Autism. Lancet 2014, 383, 896–910. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. CDC Estimates 1 in 59 Children has been Identified with Autism Spectrum Disorder. 2018. Available online: https://www.cdc.gov/features/new-autism-data/index.html (accessed on 27 April 2018).
- Leigh, J.P.; Du, J. Brief Report: Forecasting the Economic Burden of Autism in 2015 and 2025 in the United States. J Autism. Dev. Disord. 2015, 12, 4135–4139. [Google Scholar] [CrossRef] [PubMed]
- Durkin, M.S.; Maenner, M.J.; Baio, J.; Christensen, D.; Daniels, J.; Fitzgerald, R.; Imm, P.; Lee, L.C.; Schieve, L.A.; Van Naarden, B.K.; et al. Autism Spectrum Disorder Among US Children (2002-2010): Socioeconomic, Racial, and Ethnic Disparities. Am. J. Public Health 2017, 107, 1818–1826. [Google Scholar] [CrossRef]
- Ruggeri, B.; Sarkans, U.; Schumann, G.; Persico, A.M. Biomarkers in autism spectrum disorder: The old and the new. Psychopharmacology 2014, 231, 1201–1216. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Doyle, R.; Francis, K.; Conti, P.; Kalogeromitros, D. Novel therapeutic targets for autism. Trends Pharmacol. Sci. 2008, 29, 375–382. [Google Scholar] [CrossRef]
- Bauman, M.L. Medical comorbidities in autism: Challenges to diagnosis and treatment. Neurotherapeutics 2010, 7, 320–327. [Google Scholar] [CrossRef]
- Eissa, N.; Al-Houqani, M.; Sadeq, A.; Ojha, S.K.; Sasse, A.; Sadek, B. Current Enlightenment About Etiology and Pharmacological Treatment of Autism Spectrum Disorder. Front. Neurosci. 2018, 12, 304. [Google Scholar] [CrossRef] [Green Version]
- Abrahams, B.S.; Geschwind, D.H. Advances in autism genetics: On the threshold of a new neurobiology. Nat. Rev. Genet. 2008, 9, 341–355. [Google Scholar] [CrossRef]
- Geschwind, D.H.; State, M.W. Gene hunting in autism spectrum disorder: On the path to precision medicine. Lancet. Neurol. 2015, 11, 1109–1120. [Google Scholar] [CrossRef]
- Willsey, A.J.; State, M.W. Autism spectrum disorders: From genes to neurobiology. Curr. Opin. Neurobiol. 2015, 30, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Loke, Y.J.; Hannan, A.J.; Craig, J.M. The Role of Epigenetic Change in Autism Spectrum Disorders. Front. Neurol. 2015, 6, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eshraghi, A.A.; Liu, G.; Kay, S.S.; Eshraghi, R.S.; Mittal, J.; Moshiree, B.; Mittal, R. Epigenetics and Autism Spectrum Disorder: Is There a Correlation? Front. Cell. Neurosci. 2018, 12, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siu, M.T.; Weksberg, R. Epigenetics of Autism Spectrum Disorder. Adv. Exp. Med. Biol. 2017, 978, 63–90. [Google Scholar] [PubMed]
- Rossignol, D.A.; Frye, R.E. A review of research trends in physiological abnormalities in autism spectrum disorders: Immune dysregulation, inflammation, oxidative stress, mitochondrial dysfunction and environmental toxicant exposures. Mol. Psychiatry 2012, 17, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Onore, C.; Careaga, M.; Ashwood, P. The role of immune dysfunction in the pathophysiology of autism. Brain. Behav. Immun. 2012, 26, 383–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estes, M.L.; McAllister, A.K. Immune mediators in the brain and peripheral tissues in autism spectrum disorder. Nat. Rev. Neurosci. 2015, 16, 469–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauman, M.D.; Iosif, A.M.; Smith, S.E.; Bregere, C.; Amaral, D.G.; Patterson, P.H. Activation of the maternal immune system during pregnancy alters behavioral development of rhesus monkey offspring. Biol. Psychiatry 2014, 75, 332–341. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Asadi, S.; Panagiotidou, S.; Weng, Z. The “missing link” in autoimmunity and autism: Extracellular mitochondrial components secreted from activated live mast cells. Autoimmun. Rev. 2013, 12, 1136–1142. [Google Scholar] [CrossRef]
- Gesundheit, B.; Rosenzweig, J.P.; Naor, D.; Lerer, B.; Zachor, D.A.; Prochazka, V.; Melamed, M.; Kristt, D.A.; Steinberg, A.; Shulman, C.; et al. Immunological and autoimmune considerations of Autism Spectrum Disorders. J. Autoimmun. 2013, 44, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C.; Asadi, S.; Patel, A.B. Focal brain inflammation and autism. J. Neuroinflamm. 2013, 10, 46. [Google Scholar] [CrossRef] [PubMed]
- Angelidou, A.; Asadi, S.; Alysandratos, K.D.; Karagkouni, A.; Kourembanas, S.; Theoharides, T.C. Perinatal stress, brain inflammation and risk of autism-Review and proposal. BMC Pediatr. 2012, 12, 89. [Google Scholar] [CrossRef] [PubMed]
- Hagberg, H.; Gressens, P.; Mallard, C. Inflammation during fetal and neonatal life: Implications for neurologic and neuropsychiatric disease in children and adults. Ann. Neurol. 2012, 71, 444–457. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.A.; Thomsen, C. The role of the innate immune system in psychiatric disorders. Mol. Cell. Neurosci. 2013, 53, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Chavarria, A.; Alcocer-Varela, J. Is damage in central nervous system due to inflammation? Autoimmun. Rev. 2004, 3, 251–360. [Google Scholar] [CrossRef]
- Le Belle, J.E.; Sperry, J.; Ngo, A.; Ghochani, Y.; Laks, D.R.; Lopez-Aranda, M.; Silva, A.J.; Kornblum, H.I. Maternal inflammation contributes to brain overgrowth and autism-associated behaviors through altered redox signaling in stem and progenitor cells. Stem. Cell. Rep. 2014, 3, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, A.W.; Jyonouchi, H.; Comi, A.M.; Connors, S.L.; Milstien, S.; Varsou, A.; Heyes, M.P. Cerebrospinal fluid and serum markers of inflammation in autism. Pediatr. Neurol. 2005, 33, 195–201. [Google Scholar] [CrossRef]
- Li, X.; Chauhan, A.; Sheikh, A.M.; Patil, S.; Chauhan, V.; Li, X.M.; Ji, L.; Brown, T.; Malik, M. Elevated immune response in the brain of autistic patients. J. Neuroimmunol. 2009, 207, 111–116. [Google Scholar] [CrossRef] [Green Version]
- Tsilioni, I.; Taliou, A.; Francis, K.; Theoharides, T.C. Children with Autism Spectrum Disorders, who improved with a luteolin containing dietary formulation, show reduced serum levels of TNF and IL-6. Transl. Psychiatry 2015, 5, 647. [Google Scholar] [CrossRef]
- Rodriguez, J.I.; Kern, J.K. Evidence of microglial activation in autism and its possible role in brain underconnectivity. Neuron. Glia. Biol. 2011, 7, 205–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, S.; Ellis, S.E.; Ashar, F.N.; Moes, A.; Bader, J.S.; Zhan, J.; West, A.B.; Arking, D.E. Transcriptome analysis reveals dysregulation of innate immune response genes and neuronal activity-dependent genes in autism. Nat. Commun. 2014, 5, 5748. [Google Scholar] [CrossRef] [PubMed]
- Koyama, R.; Ikegaya, Y. Microglia in the pathogenesis of autism spectrum disorders. Neurosci. Res. 2015, 100, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Takano, T. Role of Microglia in Autism: Recent Advances. Dev. Neurosci. 2015, 37, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Ransohoff, R.M.; Brown, M.A. Innate immunity in the central nervous system. J. Clin. Investig. 2012, 122, 1164–1171. [Google Scholar] [CrossRef] [PubMed]
- Aguzzi, A.; Barres, B.A.; Bennett, M.L. Microglia: Scapegoat, saboteur, or something else? Science 2013, 339, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.B.; Tsilioni, I.; Leeman, S.E.; Theoharides, T.C. Neurotensin stimulates sortilin and mTOR in human microglia inhibitable by methoxyluteolin, a potential therapeutic target for autism. Proc. Natl. Acad. Sci. USA 2016, 113, 7049–7058. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C.; Tsilioni, I.; Patel, A.B.; Doyle, R. Atopic diseases and inflammation of the brain in the pathogenesis of autism spectrum disorders. Transl. Psychiatry 2016, 6, e844. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C.; Valent, P.; Akin, C. Mast Cells, Mastocytosis, and Related Disorders. N Engl. J. Med. 2015, 373, 163–172. [Google Scholar] [CrossRef]
- Lyall, K.; Van, W.J.; Ashwood, P.; Hertz-Picciotto, I. Asthma and Allergies in Children With Autism Spectrum Disorders: Results From the CHARGE Study. Autism. Res. 2015, 8, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Liao, T.C.; Lien, Y.T.; Wang, S.; Huang, S.L.; Chen, C.Y. Comorbidity of Atopic Disorders with Autism Spectrum Disorder and Attention Deficit/Hyperactivity Disord. J. Pediatr. 2016, 171, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Kotey, S.; Ertel, K.; Whitcomb, B. Co-occurrence of autism and asthma in a nationally-representative sample of children in the United States. J. Autism. Dev. Disord. 2014, 44, 3083–3088. [Google Scholar] [CrossRef] [PubMed]
- Billeci, L.; Tonacci, A.; Tartarisco, G.; Ruta, L.; Pioggia, G.; Gangemi, S. Association Between Atopic Dermatitis and Autism Spectrum Disorders: A Systematic Review. Am. J. Clin. Dermatol. 2015, 16, 371–388. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C. Autism spectrum disorders and mastocytosis. Int. J. Immunopathol. Pharmacol. 2009, 22, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Galli, S.J.; Borregaard, N.; Wynn, T.A. Phenotypic and functional plasticity of cells of innate immunity: Macrophages, mast cells and neutrophils. Nat. Immunol. 2011, 12, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C.; Stewart, J.M.; Panagiotidou, S.; Melamed, I. Mast cells, brain inflammation and autism. Eur. J. Pharmacol. 2015, 778, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Lambracht-Hall, M.; Konstantinidou, A.D.; Theoharides, T.C. Serotonin release from rat brain mast cells in vitro. Neuroscience 1990, 39, 199–207. [Google Scholar] [CrossRef]
- Edvinsson, L.; Cervos-Navarro, J.; Larsson, L.I.; Owman, C.; Ronnberg, A.L. Regional distribution of mast cells containing histamine, dopamine or 5-hydroxytryptamine in the mammalian brain. Neurology 1977, 27, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, I.; Inoue, Y.; Shimada, T.; Aikawa, T. Brain mast cells act as an immune gate to the hypothalamic-pituitary-adrenal axis in dogs. J. Exp. Med. 2001, 194, 71–78. [Google Scholar] [CrossRef]
- Goldschmidt, R.C.; Hough, L.B.; Glick, S.D.; Padawer, J. Mast cells in rat thalamus: Nuclear localization, sex difference and left-right asymmetry. Brain Res. 1984, 323, 209–217. [Google Scholar] [CrossRef]
- Taiwo, O.B.; Kovacs, K.J.; Sun, Y.; Larson, A.A. Unilateral spinal nerve ligation leads to an asymmetrical distribution of mast cells in the thalamus of female but not male mice. Pain 2005, 114, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Marathias, K.; Lambracht-Hall, M.; Savala, J.; Theoharides, T.C. Endogenous regulation of rat brain mast cell serotonin release. Int. Arch. Allergy Appl. Immunol. 1991, 95, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Letourneau, R.; Rozniecki, J.J.; Wang, L.; Theoharides, T.C. Definitive characterization of rat hypothalamic mast cells. Neuroscience 1996, 73, 889–902. [Google Scholar] [CrossRef]
- Theoharides, T.C. Neuroendocrinology of mast cells: Challenges and controversies. Exp. Dermatol. 2017, 26, 751–759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theoharides, T.C.; Konstantinidou, A. Corticotropin-releasing hormone and the blood-brain-barrier. Front. Biosci. 2007, 12, 1615–1628. [Google Scholar] [CrossRef] [PubMed]
- Esposito, P.; Chandler, N.; Kandere-Grzybowska, K.; Basu, S.; Jacobson, S.; Connolly, R.; Tutor, D.; Theoharides, T.C. Corticotropin-releasing hormone (CRH) and brain mast cells regulate blood-brain-barrier permeability induced by acute stress. J. Pharmacol. Exp. Ther. 2002, 303, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C. Mast cells: The immune gate to the brain. Life Sci. 1990, 46, 607–617. [Google Scholar] [CrossRef]
- Ribatti, D. The crucial role of mast cells in blood-brain barrier alterations. Exp. Cell. Res. 2015, 338, 119–125. [Google Scholar] [CrossRef]
- Mukai, K.; Tsai, M.; Saito, H.; Galli, S.J. Mast cells as sources of cytokines, chemokines, and growth factors. Immunol. Rev. 2018, 282, 121–150. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Kalogeromitros, D. The critical role of mast cells in allergy and inflammation. Ann. N. Y. Acad. Sci. 2006, 1088, 78–99. [Google Scholar] [CrossRef]
- Wernersson, S.; Pejler, G. Mast cell secretory granules: Armed for battle. Nat. Rev. Immunol. 2014, 14, 478–494. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Bin, N.R.; Sugita, S. Diverse exocytic pathways for mast cell mediators. Biochem. Soc. Trans. 2018, 46, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C.; Kempuraj, D.; Tagen, M.; Conti, P.; Kalogeromitros, D. Differential release of mast cell mediators and the pathogenesis of inflammation. Immunol. Rev. 2007, 217, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Gordon, J.R.; Galli, S.J. Mast cells as a source of both preformed and immunologically inducible TNF-a/cachectin. Nature 1990, 346, 274–276. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Alysandratos, K.D.; Angelidou, A.; Asadi, S.; Sismanopoulos, N.; Delivanis, D.A.; Weng, Z.; Miniati, A.; Vasiadi, M.; Katsarou-Katsari, A.; et al. Human mast cell degranulation and preformed TNF secretion require mitochondrial translocation to exocytosis sites: Relevance to atopic dermatitis. J. Allergy Clin. Immunol. 2011, 127, 1522–1531. [Google Scholar] [CrossRef] [PubMed]
- Conti, P.; Caraffa, A.; Kritas, S.K.; Ronconi, G.; Lessiani, G.; Toniato, E.; Theoharides, T.C. Mast cell, pro-inflammatory and anti-inflammatory: Jekyll and Hyde, the story continues. J. Biol. Regul. Homeost. Agents 2017, 312, 63–67. [Google Scholar]
- Noordenbos, T.; Blijdorp, I.; Chen, S.; Stap, J.; Mul, E.; Canete, J.D.; Lubberts, E.; Yeremenko, N.; Baeten, D. Human mast cells capture, store, and release bioactive, exogenous IL-17A. J. Leukoc. Biol. 2016, 100, 453–462. [Google Scholar] [CrossRef] [Green Version]
- Al-Ayadhi, L.Y.; Mostafa, G.A. Elevated serum levels of interleukin-17A in children with autism. J. Neuroinflamm. 2012, 9, 158. [Google Scholar] [CrossRef]
- Akintunde, M.E.; Rose, M.; Krakowiak, P.; Heuer, L.; Ashwood, P.; Hansen, R.; Hertz-Picciotto, I.; Van, W.J. Increased production of IL-17 in children with autism spectrum disorders and co-morbid asthma. J. Neuroimmunol. 2015, 286, 33–41. [Google Scholar] [CrossRef] [Green Version]
- Theoharides, T.C. Danger Signals and Inflammation. Clin. Ther. 2016, 38, 996–999. [Google Scholar] [CrossRef]
- Zhang, B.; Asadi, S.; Weng, Z.; Sismanopoulos, N.; Theoharides, T.C. Stimulated human mast cells secrete mitochondrial components that have autocrine and paracrine inflammatory actions. PLoS ONE 2012, 7, e49767. [Google Scholar] [CrossRef] [PubMed]
- Collins, L.V.; Hajizadeh, S.; Holme, E.; Jonsson, I.M.; Tarkowski, A. Endogenously oxidized mitochondrial DNA induces in vivo and in vitro inflammatory responses. J. Leukoc. Biol. 2004, 75, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Marques, P.E.; Amaral, S.S.; Pires, D.A.; Nogueira, L.L.; Soriani, F.M.; Lima, B.H.; Lopes, G.A.; Russo, R.C.; Avila, T.V.; Melgaco, J.G.; et al. Chemokines and mitochondrial products activate neutrophils to amplify organ injury during mouse acute liver failure. Hepatology 2012, 56, 1971–1982. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Sursal, T.; Adibnia, Y.; Zhao, C.; Zheng, Y.; Li, H.; Otterbein, L.E.; Hauser, C.J.; Itagaki, K. Mitochondrial DAMPs increase endothelial permeability through neutrophil dependent and independent pathways. PLoS ONE 2013, 8, 59989. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Angelidou, A.; Alysandratos, K.D.; Vasiadi, M.; Francis, K.; Asadi, S.; Theoharides, A.; Sideri, K.; Lykouras, L.; Kalogeromitros, D.; et al. Mitochondrial DNA and anti-mitochondrial antibodies in serum of autistic children. J. Neuroinflamm. 2010, 7, 80. [Google Scholar] [CrossRef] [PubMed]
- Marone, G.; Galli, S.J.; Kitamura, Y. Probing the roles of mast cells and basophils in natural and acquired immunity, physiology and disease. Trends Immunol. 2002, 23, 425–427. [Google Scholar] [CrossRef]
- Beaven, M.A. Our perception of the mast cell from Paul Ehrlich to now. Eur. J. Immunol. 2009, 39, 11–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galli, S.J.; Tsai, M.; Piliponsky, A.M. The development of allergic inflammation. Nature 2008, 454, 445–454. [Google Scholar] [CrossRef] [Green Version]
- Toniato, E.; Frydas, I.; Robuffo, I.; Ronconi, G.; Caraffa, A.; Kritas, S.K.; Conti, P. Activation and inhibition of adaptive immune response mediated by mast cells. J. Biol. Regul. Homeost. Agents 2017, 31, 543–548. [Google Scholar]
- Gong, J.; Yang, N.S.; Croft, M.; Weng, I.C.; Sun, L.; Liu, F.T.; Chen, S.S. The antigen presentation function of bone marrow-derived mast cells is spatiotemporally restricted to a subset expressing high levels of cell surface FcepsilonRI and MHC II. BMC Immunol. 2010, 11, 34. [Google Scholar] [CrossRef]
- Carroll-Portillo, A.; Cannon, J.L.; Holmes, A.; Kawakami, Y.; Kawakami, T.; Cambi, A.; Lidke, D.S. Mast cells and dendritic cells form synapses that facilitate antigen transfer for T cell activation. J. Cell. Biol. 2015, 210, 851–864. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Alysandratos, K.D.; Angelidou, A.; Delivanis, D.A.; Sismanopoulos, N.; Zhang, B.; Asadi, S.; Vasiadi, M.; Weng, Z.; Miniati, A.; et al. Mast cells and inflammation. Biochim. Biophys. Acta 2010, 1822, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Papadopoulou, N.; Kempuraj, D.; Boucher, W.S.; Sugimoto, K.; Cetrulo, C.L.; Theoharides, T.C. Human mast cells express corticotropin-releasing hormone (CRH) receptors and CRH leads to selective secretion of vascular endothelial growth factor. J. Immunol. 2005, 174, 7665–7675. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, L.H.; Perrin, M.H.; Brown, M.R.; Rivier, J.E. Verification of both the sequence and confromational specificity of neurotensin in binding to mast cells. Biochem. Biophys. Res. Commun. 1977, 76, 1079–1085. [Google Scholar] [CrossRef]
- Donelan, J.; Boucher, W.; Papadopoulou, N.; Lytinas, M.; Papaliodis, D.; Theoharides, T.C. Corticotropin-releasing hormone induces skin vascular permeability through a neurotensin-dependent process. Proc. Natl. Acad. Sci. USA 2006, 103, 7759–7764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoermann, G.; Gleixner, K.V.; Dinu, G.E.; Kundi, M.; Greiner, G.; Wimazal, F.; Hadzijusufovic, E.; Mitterbauer, G.; Mannhalter, C.; Valent, P.; et al. The KIT D816V allele burden predicts survival in patients with mastocytosis and correlates with the WHO type of the disease. Allergy 2014, 69, 810–813. [Google Scholar] [CrossRef]
- Patel, A.B.; Theoharides, T.C. Methoxyluteolin Inhibits Neuropeptide-stimulated Proinflammatory Mediator Release via mTOR Activation from Human Mast Cells. J. Pharmacol. Exp. Ther. 2017, 361, 462–471. [Google Scholar] [CrossRef] [Green Version]
- Mustain, W.C.; Rychahou, P.G.; Evers, B.M. The role of neurotensin in physiologic and pathologic processes. Curr. Opin. Endocrinol. Diabetes Obes. 2011, 18, 75–82. [Google Scholar] [CrossRef]
- Caceda, R.; Kinkead, B.; Nemeroff, C.B. Neurotensin: Role in psychiatric and neurological diseases. Peptides 2006, 27, 2385–2404. [Google Scholar] [CrossRef]
- Mashaghi, A.; Marmalidou, A.; Tehrani, M.; Grace, P.M.; Pothoulakis, C.; Dana, R. Neuropeptide substance P and the immune response. Cell. Mol. Life Sci. 2016, 73, 4249–4264. [Google Scholar] [CrossRef] [Green Version]
- O’Connor, T.M.; O’Connell, J.; O’Brien, D.I.; Goode, T.; Bredin, C.P.; Shanahan, F. The role of substance P in inflammatory disease. J. Cell. Physiol. 2004, 201, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Hokfelt, T.; Pernow, B.; Wahren, J.; Substance, P. A pioneer amongst neuropeptides. J. Intern. Med. 2001, 249, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Douglas, S.D.; Leeman, S.E. Neurokinin-1 receptor: Functional significance in the immune system in reference to selected infections and inflammation. Ann. N. Y. Acad. Sci. 2011, 1217, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, Y.; Dong, H.; Xu, Y.; Zhang, S. Induction of Microglial Activation by Mediators Released from Mast Cells. Cell. Physiol. Biochem. 2016, 38, 1520–1531. [Google Scholar] [CrossRef] [PubMed]
- Skaper, S.D.; Facci, L.; Giusti, P. Neuroinflammation, microglia and mast cells in the pathophysiology of neurocognitive disorders: A review. CNS Neurol. Disord. Drug Targets 2014, 13, 1654–1666. [Google Scholar] [CrossRef] [PubMed]
- Girolamo, F.; Coppola, C.; Ribatti, D. Immunoregulatory effect of mast cells influenced by microbes in neurodegenerative diseases. Brain Behav. Immun. 2017, 65, 68–89. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C.; Stewart, J.M.; Hatziagelaki, E.; Kolaitis, G. Brain “fog,” inflammation and obesity: Key aspects of 2 neuropsychiatric disorders improved by luteolin. Front. Neurosci. 2015, 9, 225. [Google Scholar] [CrossRef]
- Kalogeromitros, D.; Syrigou, E.I.; Makris, M.; Kempuraj, D.; Stavrianeas, N.G.; Vasiadi, M.; Theoharides, T.C. Nasal provocation of patients with allergic rhinitis and the hypothalamic-pituitary-adrenal axis. Ann. Allergy Asthma Immunol. 2007, 98, 269–273. [Google Scholar] [CrossRef]
- Louveau, A.; Smirnov, I.; Keyes, T.J.; Eccles, J.D.; Rouhani, S.J.; Peske, J.D.; Derecki, N.C.; Castle, D.; Mandell, J.W.; Lee, K.S.; et al. Structural and functional features of central nervous system lymphatic vessels. Nature 2015, 523, 337–341. [Google Scholar] [CrossRef]
- Abbott, N.J. Inflammatory mediators and modulation of blood-brain barrier permeability. Cell. Mol. Neurobiol. 2000, 20, 131–147. [Google Scholar] [CrossRef]
- Pan, W.; Stone, K.P.; Hsuchou, H.; Manda, V.K.; Zhang, Y.; Kastin, A.J. Cytokine signaling modulates blood-brain barrier function. Curr. Pharm. Des. 2011, 17, 3729–3740. [Google Scholar] [CrossRef] [PubMed]
- Srikantha, P.; Mohajeri, M.H. The Possible Role of the Microbiota-Gut-Brain-Axis in Autism Spectrum Disorder. Int. J. Mol. Sci. 2019, 20, 2115. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C.; Doyle, R. Autism, gut-blood-brain barrier and mast cells. J. Clin. Psychopharm. 2008, 28, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, M.; Sapone, A.; Senger, S.; Camhi, S.S.; Kadzielski, S.M.; Buie, T.M.; Kelly, D.L.; Cascella, N.; Fasano, A. Blood-brain barrier and intestinal epithelial barrier alterations in autism spectrum disorders. Mol. Autism. 2016, 7, 49. [Google Scholar] [CrossRef] [PubMed]
- Rozniecki, J.J.; Sahagian, G.; Jacobson, S.; Kempuraj, D.; Theoharides, T.C. Acute restraint stress induces brain metastases of luciferase-tagged breast cancer cells in mice. Brain Behav. Immun. 2009, 1366, 204–210. [Google Scholar]
- Cowan, C.S.M.; Hoban, A.E.; Ventura-Silva, A.P.; Dinan, T.G.; Clarke, G.; Cryan, J.F. Gutsy Moves: The Amygdala as a Critical Node in Microbiota to Brain Signaling. Bioessays 2018, 40, 1700172. [Google Scholar] [CrossRef]
- McCusker, R.H.; Kelley, K.W. Immune-neural connections: How the immune system’s response to infectious agents influences behavior. J. Exp. Biol. 2013, 216, 84–98. [Google Scholar] [CrossRef]
- Herbert, M.R. Contributions of the environment and environmentally vulnerable physiology to autism spectrum disorders. Curr. Opin. Neurol. 2010, 23, 103–110. [Google Scholar] [CrossRef]
- Deth, R.; Muratore, C.; Benzecry, J.; Power-Charnitsky, V.A.; Waly, M. How environmental and genetic factors combine to cause autism: A redox/methylation hypothesis. NeuroToxicology 2008, 29, 190–201. [Google Scholar] [CrossRef]
- Sealey, L.A.; Hughes, B.W.; Sriskanda, A.N.; Guest, J.R.; Gibson, A.D.; Johnson-Williams, L.; Pace, D.G.; Bagasra, O. Environmental factors in the development of autism spectrum disorders. Environ. Int. 2016, 88, 288–298. [Google Scholar] [CrossRef]
- Modabbernia, A.; Velthorst, E.; Reichenberg, A. Environmental risk factors for autism: An evidence-based review of systematic reviews and meta-analyses. Mol. Autism. 2017, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- McCall, R.B. Childhood IQ’s as Predictors of Adult Educational and Occupational Status. Science 1977, 197, 482–483. [Google Scholar] [CrossRef] [PubMed]
- Moffitt, T.E.; Gabrielli, W.F.; Mednick, S.A.; Schulsinger, F. Socioeconomic status, IQ, and delinquency. J. Abnorm. Psychol. 1981, 90, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.; Frankovich, J.; Cooperstock, M.; Cunningham, M.W.; Latimer, M.E.; Murphy, T.K.; Pasternack, M.; Thienemann, M.; Williams, K.; Walter, J.; et al. Clinical evaluation of youth with pediatric acute-onset neuropsychiatric syndrome (PANS): Recommendations from the 2013 PANS Consensus Conference. J. Child Adolesc. Psychopharmacol. 2015, 25, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C. Mold and Immunity. Clin. Ther. 2018, 40, 882–884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ratnaseelan, A.M.; Tsilioni, I.; Theoharides, T.C. Effects of Mycotoxins on Neuropsychiatric Symptoms and Immune Processes. Clin. Ther. 2018, 40, 903–917. [Google Scholar] [CrossRef] [PubMed]
- Spengler, J.; Neas, L.M.; Nakai, C.; Dockery, D.W.; Speizer, F.E.; Ware, J.; Raizenne, M. Respiratory symptoms and housing characteristics. Indoor Air 1994, 4, 72–82. [Google Scholar] [CrossRef]
- Ismaiel, A.A.; Papenbrock, J. Mycotoxins: Producing Fungi and Mechanisms of Phytotoxicity. Agriculture 2015, 5, 492–537. [Google Scholar] [CrossRef] [Green Version]
- Rocha, M.E.B.; Chagas, O.F.; Maia, F.E.F.; Guedes, M.I.F.; Rondina, D. Mycotoxins and their effects on human and animal health. Food Control 2014, 36, 159–165. [Google Scholar] [CrossRef]
- American Academy of Pediatrics, Committee on Environmental Health Toxic effects of indoor molds. Pediatrics 1998, 101, 712–714. [CrossRef]
- Andersson, M.A.; Nikulin, M.; Koljalg, U.; Andersson, M.C.; Rainey, F.; Reijula, K.; Hintikka, E.L.; Salkinoja-Salonen, M. Bacteria, molds, and toxins in water-damaged building materials. Appl. Environ. Microbiol. 1997, 63, 387–393. [Google Scholar] [PubMed]
- Peltola, J.; Andersson, M.A.; Haahtela, T.; Mussalo-Rauhamaa, H.; Rainey, F.A.; Kroppenstedt, R.M.; Samson, R.A.; Salkinoja-Salonen, M.S. Toxic-metabolite-producing bacteria and fungus in an indoor environment. Appl. Environ. Microbiol. 2001, 67, 3269–3274. [Google Scholar] [CrossRef] [PubMed]
- Etzel, R.A. Mycotoxins. JAMA 2002, 287, 425–427. [Google Scholar] [CrossRef] [PubMed]
- Fog, N.K. Mycotoxin production by indoor molds. Fungal Genet. Biol. 2003, 39, 103–117. [Google Scholar]
- Gorny, R.L.; Reponen, T.; Willeke, K.; Schmechel, D.; Robine, E.; Boissier, M.; Grinshpun, S.A. Fungal fragments as indoor air biocontaminants. Appl. Environ. Microbiol. 2002, 68, 3522–3531. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hope, J. A review of the mechanism of injury and treatment approaches for illness resulting from exposure to water-damaged buildings, mold, and mycotoxins. Sci. World J. 2013, 2013, 767482. [Google Scholar] [CrossRef] [PubMed]
- Brasel, T.L.; Douglas, D.R.; Wilson, S.C.; Straus, D.C. Detection of airborne Stachybotrys chartarum macrocyclic trichothecene mycotoxins on particulates smaller than conidia. Appl. Environ. Microbiol. 2005, 71, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Brasel, T.L.; Martin, J.M.; Carriker, C.G.; Wilson, S.C.; Straus, D.C. Detection of airborne Stachybotrys chartarum macrocyclic trichothecene mycotoxins in the indoor environment. Appl. Environ. Microbiol. 2005, 71, 7376–7388. [Google Scholar] [CrossRef] [PubMed]
- Charpin-Kadouch, C.; Maurel, G.; Felipo, R.; Queralt, J.; Ramadour, M.; Dumon, H.; Garans, M.; Botta, A.; Charpin, D. Mycotoxin identification in moldy dwellings. J. Appl. Toxicol. 2006, 26, 475–479. [Google Scholar] [CrossRef]
- Sava, V.; Reunova, O.; Velasquez, A.; Harbison, R.; Sanchez-Ramos, J. Acute neurotoxic effects of the fungal metabolite ochratoxin-A. NeuroToxicology 2006, 27, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Sava, V.; Reunova, O.; Velasquez, A.; Sanchez-Ramos, J. Can low level exposure to ochratoxin-A cause parkinsonism? J. Neurol. Sci. 2006, 249, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Fromme, H.; Gareis, M.; Volkel, W.; Gottschalk, C. Overall internal exposure to mycotoxins and their occurrence in occupational and residential settings–An overview. Int. J. Hyg. Environ. Health 2016, 219, 143–165. [Google Scholar] [CrossRef] [PubMed]
- Ziats, M.N.; Grosvenor, L.P.; Rennert, O.M. Functional genomics of human brain development and implications for autism spectrum disorders. Transl. Psychiatry 2015, 5, 665. [Google Scholar] [CrossRef]
- Hendry, K.M.; Cole, E.C. A review of mycotoxins in indoor air. J. Toxicol. Environ. Health 1993, 38, 183–198. [Google Scholar] [CrossRef] [PubMed]
- Brasel, T.L.; Campbell, A.W.; Demers, R.E.; Ferguson, B.S.; Fink, J.; Vojdani, A.; Wilson, S.C.; Straus, D.C. Detection of trichothecene mycotoxins in sera from individuals exposed to Stachybotrys chartarum in indoor environments. Arch. Environ. Health 2004, 59, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Campbell, A.W.; Thrasher, J.D.; Gray, M.R.; Vojdani, A. Mold and mycotoxins: Effects on the neurological and immune systems in humans. Adv. Appl. Microbiol. 2004, 55, 375–406. [Google Scholar] [PubMed]
- Crago, B.R.; Gray, M.R.; Nelson, L.A.; Davis, M.; Arnold, L.; Thrasher, J.D. Psychological, neuropsychological, and electrocortical effects of mixed mold exposure. Arch. Environ. Health 2003, 58, 452–463. [Google Scholar] [CrossRef] [PubMed]
- Baldo, J.V.; Ahmad, L.; Ruff, R. Neuropsychological performance of patients following mold exposure. Appl. Neuropsychol. 2002, 9, 193–202. [Google Scholar] [CrossRef]
- Brown, R.C.; Lockwood, A.H.; Sonawane, B.R. Neurodegenerative diseases: An overview of environmental risk factors. Environ. Health Perspect. 2005, 113, 1250–1256. [Google Scholar] [CrossRef]
- Gordon, W.A.; Cantor, J.B.; Johanning, E.; Charatz, H.J.; Ashman, T.A.; Breeze, J.L.; Haddad, L.; Abramowitz, S. Cognitive impairment associated with toxigenic fungal exposure: A replication and extension of previous findings. Appl. Neuropsychol. 2004, 11, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Casas, L.; Torrent, M.; Zock, J.P.; Doekes, G.; Forns, J.; Guxens, M.; Taubel, M.; Heinrich, J.; Sunyer, J. Early life exposures to home dampness, pet ownership and farm animal contact and neuropsychological development in 4 year old children: A prospective birth cohort study. Int. J. Hyg. Environ. Health 2013, 216, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Anyanwu, E.C.; Campbell, A.W.; Vojdani, A. Neurophysiological effects of chronic indoor environmental toxic mold exposure on children. Sci. World J. 2003, 3, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Jedrychowski, W.; Maugeri, U.; Perera, F.; Stigter, L.; Jankowski, J.; Butscher, M.; Mroz, E.; Flak, E.; Skarupa, A.; Sowa, A. Cognitive function of 6-year old children exposed to mold-contaminated homes in early postnatal period. Prospective birth cohort study in Poland. Physiol. Behav. 2011, 104, 989–995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doi, K.; Uetsuka, K. Mechanisms of mycotoxin-induced neurotoxicity through oxidative stress-associated pathways. Int. J. Mol. Sci. 2011, 12, 5213–5237. [Google Scholar] [CrossRef] [PubMed]
- Campbell, A.W.; Thrasher, J.D.; Madison, R.A.; Vojdani, A.; Gray, M.R.; Johnson, A. Neural autoantibodies and neurophysiologic abnormalities in patients exposed to molds in water-damaged buildings. Arch. Environ. Health 2003, 58, 464–474. [Google Scholar] [CrossRef]
- Rea, W.J.; Didriksen, N.; Simon, T.R.; Pan, Y.; Fenyves, E.J.; Griffiths, B. Effects of toxic exposure to molds and mycotoxins in building-related illnesses. Arch. Environ. Health 2003, 58, 399–405. [Google Scholar] [CrossRef]
- Kilburn, K.H. Indoor mold exposure associated with neurobehavioral and pulmonary impairment: A preliminary report. Arch. Environ. Health 2003, 58, 390–398. [Google Scholar] [CrossRef]
- Karunasena, E.; Larranaga, M.D.; Simoni, J.S.; Douglas, D.R.; Straus, D.C. Building-associated neurological damage modeled in human cells: A mechanism of neurotoxic effects by exposure to mycotoxins in the indoor environment. Mycopathologia 2010, 170, 377–390. [Google Scholar] [CrossRef]
- Thrasher, J.D.; Crawley, S. The biocontaminants and complexity of damp indoor spaces: More than what meets the eyes. Toxicol. Ind. Health 2009, 25, 583–615. [Google Scholar] [CrossRef]
- Alassane-Kpembi, I.; Kolf-Clauw, M.; Gauthier, T.; Abrami, R.; Abiola, F.A.; Oswald, I.P.; Puel, O. New insights into mycotoxin mixtures: The toxicity of low doses of Type B trichothecenes on intestinal epithelial cells is synergistic. Toxicol. Appl. Pharmacol. 2013, 272, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Rzhetsky, A.; Bagley, S.C.; Wang, K.; Lyttle, C.S.; Cook, E.H.; Altman, R.B.; Gibbons, R.D. Environmental and state-level regulatory factors affect the incidence of autism and intellectual disability. PLoS Comput. Biol. 2014, 10, e1003518. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.T.; Wais, J.; Crawford, D.A. Prenatal exposure to common environmental factors affects brain lipids and increases risk of developing autism spectrum disorders. Eur. J. Neurosci. 2015, 42, 2742–2760. [Google Scholar] [CrossRef] [PubMed]
- Kilburn, K.H.; Thrasher, J.D.; Immers, N.B. Do terbutaline- and mold-associated impairments of the brain and lung relate to autism? Toxicol. Ind. Health 2009, 25, 703–710. [Google Scholar] [CrossRef] [PubMed]
- De, S.B.; Brera, C.; Mezzelani, A.; Soricelli, S.; Ciceri, F.; Moretti, G.; Debegnach, F.; Bonaglia, M.C.; Villa, L.; Molteni, M.; et al. Role of mycotoxins in the pathobiology of autism: A first evidence. Nutr. Neurosci. 2019, 22, 132–144. [Google Scholar]
- Tobel, J.S.; Antinori, P.; Zurich, M.G.; Rosset, R.; Aschner, M.; Gluck, F.; Scherl, A.; Monnet-Tschudi, F. Repeated exposure to Ochratoxin A generates a neuroinflammatory response, characterized by neurodegenerative M1 microglial phenotype. NeuroToxicology 2014, 44, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Doebler, J.A.; Martin, L.J.; Morse, J.D.; Ballough, G.P.; Strauss, J.A.; Anthony, A. Mesenteric mast cell degranulation in acute T-2 toxin poisoning. Toxicol. Lett. 1992, 62, 33–38. [Google Scholar] [CrossRef]
- Yarom, R.; Bergmann, F.; Yagen, B. Cutaneous injury by topical T-2 toxin: Involvement of microvessels and mast cells. Toxicon 1987, 25, 167–174. [Google Scholar] [CrossRef]
- Saluja, R.; Metz, M.; Maurer, M. Role and relevance of mast cells in fungal infections. Front. Immunol. 2012, 3, 146. [Google Scholar] [CrossRef] [PubMed]
- Lindau, M.; Almkvist, O.; Mohammed, A.H. Effects of stress on learning and memory. Concept Cogn. Emot. Behav. 2016, 10, 270–274. [Google Scholar]
- Theoharides, T.C.; Kavalioti, M. Effect of stress on learning and motivation-relevance to autism spectrum disorder. Int. J. Immunopath. Pharmacol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Moscarello, J.M.; LeDoux, J.E. Active avoidance learning requires prefrontal suppression of amygdala-mediated defensive reactions. J. Neurosci. 2013, 33, 3815–3823. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Kim, J.J. Amygdala regulates risk of predation in rats foraging in a dynamic fear environment. Proc. Natl. Acad. Sci. USA 2010, 107, 21773–21777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volkmar, F.; Siegel, M.; Woodbury-Smith, M.; King, B.; McCracken, J.; State, M. Practice parameter for the assessment and treatment of children and adolescents with autism spectrum disorder. J. Am. Acad. Child. Adolesc. Psychiatry 2014, 53, 237–257. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.W.; Canavera, K.; Kleinpeter, F.L.; Maccubbin, E.; Taga, K. The fears, phobias and anxieties of children with autism spectrum disorders and Down syndrome: Comparisons with developmentally and chronologically age matched children. Child. Psychiatry Hum. Dev. 2005, 36, 3–26. [Google Scholar] [CrossRef] [PubMed]
- Philip, R.C.; Whalley, H.C.; Stanfield, A.C.; Sprengelmeyer, R.; Santos, I.M.; Young, A.W.; Atkinson, A.P.; Calder, A.J.; Johnstone, E.C.; Lawrie, S.M.; et al. Deficits in facial, body movement and vocal emotional processing in autism spectrum disorders. Psychol. Med. 2010, 40, 1919–1929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hollocks, M.J.; Howlin, P.; Papadopoulos, A.S.; Khondoker, M.; Simonoff, E. Differences in HPA-axis and heart rate responsiveness to psychosocial stress in children with autism spectrum disorders with and without co-morbid anxiety. Psychoneuroendocrinology 2014, 46, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, J.; Glod, M.; Connolly, B.; McConachie, H. The relationship between anxiety and repetitive behaviours in autism spectrum disorder. J. Autism. Dev. Disord. 2012, 42, 2404–2409. [Google Scholar] [CrossRef] [PubMed]
- Steensel, F.J.; Bogels, S.M.; Perrin, S. Anxiety disorders in children and adolescents with autistic spectrum disorders: A meta-analysis. Clin. Child. Fam. Psychol. Rev. 2011, 14, 302–317. [Google Scholar] [CrossRef]
- Spratt, E.G.; Nicholas, J.S.; Brady, K.T.; Carpenter, L.A.; Hatcher, C.R.; Meekins, K.A.; Furlanetto, R.W.; Charles, J.M. Enhanced cortisol response to stress in children in autism. J. Autism. Dev. Disord. 2012, 42, 75–81. [Google Scholar] [CrossRef]
- Kushki, A.; Drumm, E.; Pla, M.M.; Tanel, N.; Dupuis, A.; Chau, T.; Anagnostou, E. Investigating the autonomic nervous system response to anxiety in children with autism spectrum disorders. PLoS ONE 2013, 8, 59730. [Google Scholar] [CrossRef] [PubMed]
- MacKinnon, N.; Kingsbury, M.; Mahedy, L.; Evans, J.; Colman, I. The Association Between Prenatal Stress and Externalizing Symptoms in Childhood: Evidence From the Avon Longitudinal Study of Parents and Children. Biol. Psychiatry 2018, 83, 100–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beversdorf, D.Q.; Manning, S.E.; Hillier, A.; Anderson, S.L.; Nordgren, R.E.; Walters, S.E.; Nagaraja, H.N.; Cooley, W.C.; Gaelic, S.E.; Bauman, M.L. Timing of prenatal stressors and autism. J. Autism. Dev. Disord. 2005, 35, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Ronald, A.; Pennell, C.E.; Whitehouse, A.J. Prenatal maternal stress associated with ADHD and autistic traits in early childhood. Front. Psychol. 2010, 1, 223. [Google Scholar] [CrossRef] [PubMed]
- Crafa, D.; Warfa, N. Maternal migration and autism risk: Systematic analysis. Int. Rev. Psychiatry 2015, 27, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Simcock, G.; Kildea, S.; Elgbeili, G.; Laplante, D.P.; Cobham, V.; King, S. Prenatal maternal stress shapes children’s theory of mind: The QF2011 Queensland Flood Study. J. Dev. Orig. Health Dis. 2017, 8, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Babenko, O.; Kovalchuk, I.; Metz, G.A. Stress-induced perinatal and transgenerational epigenetic programming of brain development and mental health. Neurosci. Biobehav. Rev. 2015, 48, 70–91. [Google Scholar] [CrossRef] [PubMed]
- Tordjman, S.; Somogyi, E.; Coulon, N.; Kermarrec, S.; Cohen, D.; Bronsard, G.; Bonnot, O.; Weismann-Arcache, C.; Botbol, M.; Lauth, B.; et al. Environment interactions in autism spectrum disorders: Role of epigenetic mechanisms. Front. Psychiatry 2014, 5, 53. [Google Scholar] [CrossRef]
- Pedersen, J.M.; Mortensen, E.L.; Christensen, D.S.; Rozing, M.; Brunsgaard, H.; Meincke, R.H.; Petersen, G.L.; Lund, R. Prenatal and early postnatal stress and later life inflammation. Psychoneuroendocrinology 2018, 88, 158–166. [Google Scholar] [CrossRef]
- Bauman, M.L.; Kemper, T.L. The neuropathology of the autism spectrum disorders: What have we learned? Novartis. Found. Symp. 2003, 251, 112–122. [Google Scholar] [PubMed]
- Bliss-Moreau, E.; Moadab, G.; Santistevan, A.; Amaral, D.G. The effects of neonatal amygdala or hippocampus lesions on adult social behavior. Behav. Brain Res. 2017, 322, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Bondarenko, E.; Hodgson, D.M.; Nalivaiko, E. Amygdala mediates respiratory responses to sudden arousing stimuli and to restraint stress in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 306, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Amaral, D.G.; Bauman, M.D.; Schumann, C.M. The amygdala and autism: Implications from non-human primate studies. Genes. Brain Behav. 2003, 2, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Bauman, M.D.; Lavenex, P.; Mason, W.A.; Capitanio, J.P.; Amaral, D.G. The development of mother-infant interactions after neonatal amygdala lesions in rhesus monkeys. J. Neurosci. 2004, 24, 711–721. [Google Scholar] [CrossRef]
- Avino, T.A.; Barger, N.; Vargas, M.V.; Carlson, E.L.; Amaral, D.G.; Bauman, M.D.; Schumann, C.M. Neuron numbers increase in the human amygdala from birth to adulthood, but not in autism. Proc. Natl. Acad. Sci. USA 2018, 115, 3710–3715. [Google Scholar] [CrossRef] [Green Version]
- Ressler, K.J. Amygdala activity, fear, and anxiety: Modulation by stress. Biol. Psychiatry 2010, 67, 1117–1119. [Google Scholar] [CrossRef]
- Bliss-Moreau, E.; Bauman, M.D.; Amaral, D.G. Neonatal amygdala lesions result in globally blunted affect in adult rhesus macaques. Behav. Neurosci. 2011, 125, 848–858. [Google Scholar] [CrossRef]
- Bliss-Moreau, E.; Toscano, J.E.; Bauman, M.D.; Mason, W.A.; Amaral, D.G. Neonatal amygdala lesions alter responsiveness to objects in juvenile macaques. Neuroscience 2011, 178, 123–132. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.S.; Ku, Y.H.; Li, L.S.; Lu, Y.C.; Ding, X.; Wang, Y.G. Corticotropin releasing factor and substance P mediate the nucleus amygdaloideus centralis-nucleus ventromedialis-nucleus dorsomedialis pressor system. Brain Res. 1999, 842, 392–398. [Google Scholar] [CrossRef]
- Almehmadi, K.A.; Theoharides, T.C. Increased expression of miR-155p5 in amygdala of children with Autism Spectrum Disorder. Autism. Res. 2019, in press. [Google Scholar]
- Takahashi, L.K.; Hubbard, D.T.; Lee, I.; Dar, Y.; Sipes, S.M. Predator odor-induced conditioned fear involves the basolateral and medial amygdala. Behav. Neurosci. 2007, 121, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Holmes, N.M.; Parkes, S.L.; Killcross, A.S.; Westbrook, R.F. The basolateral amygdala is critical for learning about neutral stimuli in the presence of danger, and the perirhinal cortex is critical in the absence of danger. J. Neurosci. 2013, 33, 13112–13125. [Google Scholar] [CrossRef] [PubMed]
- Truitt, W.A.; Sajdyk, T.J.; Dietrich, A.D.; Oberlin, B.; McDougle, C.J.; Shekhar, A. From anxiety to autism: Spectrum of abnormal social behaviors modeled by progressive disruption of inhibitory neuronal function in the basolateral amygdala in Wistar rats. Psychopharmacology 2007, 191, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Singh, L.K.; Boucher, W.; Pang, X.; Letourneau, R.; Seretakis, D.; Green, M.; Theoharides, T.C. Potent mast cell degranulation and vascular permeability triggered by urocortin through activation of CRH receptors. J. Pharmacol. Exp. Ther. 1999, 288, 1349–1356. [Google Scholar] [PubMed]
- Alysandratos, K.D.; Asadi, S.; Angelidou, A.; Zhang, B.; Sismanopoulos, N.; Yang, H.; Critchfield, A.; Theoharides, T.C. Neurotensin and CRH interactions augment human mast cell activation. PLoS ONE 2012, 7, 48934. [Google Scholar] [CrossRef] [PubMed]
- Asadi, S.; Alysandratos, K.D.; Angelidou, A.; Miniati, A.; Sismanopoulos, N.; Vasiadi, M.; Zhang, B.; Kalogeromitros, D.; Theoharides, T.C. Substance P (SP) induces expression of functional corticotropin-releasing hormone receptor-1 (CRHR-1) in human mast cells. J. Invest. Dermatol. 2012, 132, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Kempuraj, D.; Papadopoulou, N.G.; Lytinas, M.; Huang, M.; Kandere-Grzybowska, K.; Madhappan, B.; Boucher, W.; Christodoulou, S.; Athanassiou, A.; Theoharides, T.C. Corticotropin-releasing hormone and its structurally related urocortin are synthesized and secreted by human mast cells. Endocrinology 2004, 145, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C.; Donelan, J.M.; Papadopoulou, N.; Cao, J.; Kempuraj, D.; Conti, P. Mast cells as targets of corticotropin-releasing factor and related peptides. Trends Pharmacol. Sci. 2004, 25, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Asadi, S.; Theoharides, T.C. Corticotropin-releasing hormone and extracellular mitochondria augment IgE-stimulated human mast-cell vascular endothelial growth factor release, which is inhibited by luteolin. J. Neuroinflam. 2012, 9, 85. [Google Scholar] [CrossRef] [PubMed]
- Chrousos, G.P. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N. Engl. J. Med. 1995, 332, 1351–1362. [Google Scholar] [CrossRef] [PubMed]
- Frank, M.G.; Weber, M.D.; Watkins, L.R.; Maier, S.F. Stress-induced neuroinflammatory priming: A liability factor in the etiology of psychiatric disorders. Neurobiol. Stress 2016, 4, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Amidfar, M.; Won, E. A review on inflammatory cytokine-induced alterations of the brain as potential neural biomarkers in post-traumatic stress disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 91, 103–122. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Missig, G.; Finger, B.C.; Landino, S.M.; Alexander, A.J.; Mokler, E.L.; Robbins, J.O.; Manasian, Y.; Kim, W.; Kim, K.S.; et al. Maternal and Early Postnatal Immune Activation Produce Dissociable Effects on Neurotransmission in mPFC-Amygdala Circuits. J. Neurosci. 2018, 38, 3358–3372. [Google Scholar] [CrossRef] [PubMed]
- Michopoulos, V.; Powers, A.; Gillespie, C.F.; Ressler, K.J.; Jovanovic, T. Inflammation in Fear- and Anxiety-Based Disorders: PTSD, GAD, and Beyond. Neuropsychopharmacology 2017, 42, 254–270. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, D.E.; Rainnie, D.G. Prenatal Stress Alters the Development of Socioemotional Behavior and Amygdala Neuron Excitability in Rats. Neuropsychopharmacology 2015, 40, 2135–2145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaidan, H.; Leshem, M.; Gaisler-Salomon, I. Prereproductive stress to female rats alters corticotropin releasing factor type 1 expression in ova and behavior and brain corticotropin releasing factor type 1 expression in offspring. Biol. Psychiatry 2013, 74, 680–687. [Google Scholar] [CrossRef] [PubMed]
- Bercum, F.M.; Rodgers, K.M.; Benison, A.M.; Smith, Z.Z.; Taylor, J.; Kornreich, E.; Grabenstatter, H.L.; Dudek, F.E.; Barth, D.S. Maternal Stress Combined with Terbutaline Leads to Comorbid Autistic-Like Behavior and Epilepsy in a Rat Model. J. Neurosci. 2015, 35, 15894–15902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tottenham, N.; Sheridan, M.A. A review of adversity, the amygdala and the hippocampus: A consideration of developmental timing. Front. Hum. Neurosci. 2009, 3, 68. [Google Scholar] [CrossRef] [PubMed]
- Bilbo, S.D.; Block, C.L.; Bolton, J.L.; Hanamsagar, R.; Tran, P.K. Beyond infection–Maternal immune activation by environmental factors, microglial development, and relevance for autism spectrum disorders. Exp. Neurol. 2018, 299, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Barreau, F.; Salvador-Cartier, C.; Houdeau, E.; Bueno, L.; Fioramonti, J. Long-term alterations of colonic nerve-mast cell interactions induced by neonatal maternal deprivation in rats. Gut 2008, 57, 582–590. [Google Scholar] [CrossRef]
- Huang, M.; Pang, X.; Karalis, K.; Theoharides, T.C. Stress-induced interleukin-6 release in mice is mast cell-dependent and more pronounced in Apolipoprotein E knockout mice. Cardiovasc. Res. 2003, 59, 241–249. [Google Scholar] [CrossRef] [Green Version]
- Dong, H.; Zhang, X.; Wang, Y.; Zhou, X.; Qian, Y.; Zhang, S. Suppression of Brain Mast Cells Degranulation Inhibits Microglial Activation and Central Nervous System Inflammation. Mol. Neurobiol. 2017, 54, 997–1007. [Google Scholar] [CrossRef]
- Garay, P.A.; Hsiao, E.Y.; Patterson, P.H.; McAllister, A.K. Maternal immune activation causes age- and region-specific changes in brain cytokines in offspring throughout development. Brain Behav. Immun. 2013, 31, 54–68. [Google Scholar] [CrossRef]
- Mueller, F.S.; Polesel, M.; Richetto, J.; Meyer, U.; Weber-Stadlbauer, U. Mouse models of maternal immune activation: Mind your caging system! Brain Behav. Immun. 2018, 73, 643–660. [Google Scholar] [CrossRef]
- Kentner, A.C.; Bilbo, S.D.; Brown, A.S.; Hsiao, E.Y.; McAllister, A.K.; Meyer, U.; Pearce, B.D.; Pletnikov, M.V.; Yolken, H.; Bauman, M.D. Maternal immune activation: Reporting guidelines to improve the rigor, reproducibility, and transparency of the model. Neuropsychopharmacology 2019, 44, 245–258. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Kavalioti, M. Stress, inflammation and natural treatments. J. Biol. Regul. Homeost. Agents 2018, 32, 1345–1347. [Google Scholar]
- Puri, N.; Roche, P.A. Mast cells possess distinct secretory granule subsets whose exocytosis is regulated by different SNARE isoforms. Proc. Natl. Acad. Sci. USA 2008, 105, 2580–2585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taracanova, A.; Tsilioni, I.; Conti, P.; Norwitz, E.R.; Leeman, S.E.; Theoharides, T.C. Substance P and IL-33 administered together stimulate a marked secretion of IL-1beta from human mast cells, inhibited by methoxyluteolin. Proc. Natl. Acad. Sci. USA 2018, 115, 9381–9390. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Kelley, K.W.; Johnson, R.W. Luteolin reduces IL-6 production in microglia by inhibiting JNK phosphorylation and activation of AP-1. Proc. Natl. Acad. Sci. USA 2008, 105, 7534–7539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Theoharides, T.C.; Kavalioti, M.; Tsilioni, I. Mast Cells, Stress, Fear and Autism Spectrum Disorder. Int. J. Mol. Sci. 2019, 20, 3611. https://doi.org/10.3390/ijms20153611
Theoharides TC, Kavalioti M, Tsilioni I. Mast Cells, Stress, Fear and Autism Spectrum Disorder. International Journal of Molecular Sciences. 2019; 20(15):3611. https://doi.org/10.3390/ijms20153611
Chicago/Turabian StyleTheoharides, Theoharis C., Maria Kavalioti, and Irene Tsilioni. 2019. "Mast Cells, Stress, Fear and Autism Spectrum Disorder" International Journal of Molecular Sciences 20, no. 15: 3611. https://doi.org/10.3390/ijms20153611



