Forest Degradation: When Is a Forest Degraded?
Abstract
:1. Introduction
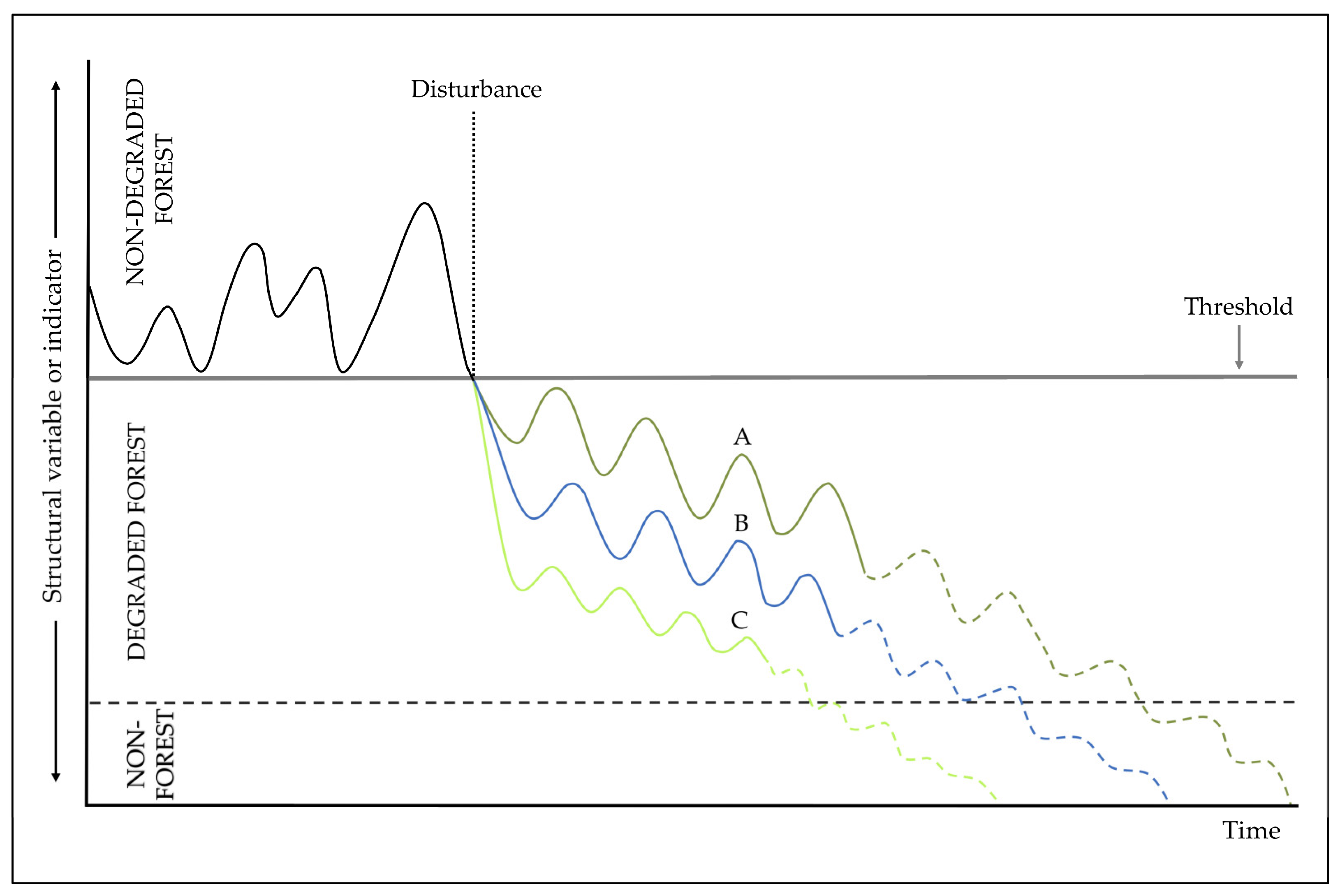
2. Loss of Resilience and the Degradation of Forests
3. What is a Degraded Forest?
4. Key Considerations in the Evaluation of Forest Degradation
4.1. Reference Forests
4.2. Degradation Indicators
4.3. Degradation Threshold
5. Principal Approaches to Identification of a Degraded Forest
6. Considerations for the Future
Author Contributions
Funding
Acknowledgements
Conflicts of Interest
References
- Hacia una Definición de Degradación de los Bosques: Análisis Comparativo de las Definiciones Existentes; Departamento Forestal, Organización de las Naciones Unidas para la Alimentación y la Agricultura (FAO): Roma, Italy, 2009.
- Stanturf, J.A.; Palik, B.J.; Dumroese, R.K. Contemporary forest restoration: A review emphasizing function. For. Ecol. Manag. 2014, 331, 292–323. [Google Scholar] [CrossRef]
- Sasaki, N.; Putz, F.E. Critical need for new definitions of “forest” and “forest degradation”. In global climate change agreements. Conserv. Lett. 2009, 2, 226–232. [Google Scholar] [CrossRef]
- Chazdon, R.L. Beyond deforestation: Restoring forests and ecosystem services on degraded lands. Science 2008, 320, 1458–1460. [Google Scholar] [CrossRef] [PubMed]
- Ahrends, A.; Burgess, N.D.; Milledge, S.A.H.; Bulling, M.T.; Fisher, B.; Smart, J.C.R.; Clarke, G.P.; Mhoro, B.E.; Lewis, S.L. Predictable waves of sequential forest degradation and biodiversity loss spreading from an African city. Proc. Natl. Acad. Sci. 2010, 107, 14556–14561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, I.D.; Guariguata, M.R.; Okabe, K.; Bahamondez, C.; Nasi, R.; Heymell, V.; Sabogal, C. An operational framework for defining and monitoring forest degradation. Ecol. Soc. 2013, 18, 20. [Google Scholar] [CrossRef]
- Lamb, D.; Stanturf, J.; Madsen, P. What is forest landscape restoration? In Forest Landscape Restoration: Integrating Natural and Social Sciences; Stanturf, J., Lamb, D., Madsen, P., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 3–23. [Google Scholar]
- Stanturf, J.A.; Palik, B.J.; Williams, M.I.; Dumroese, R.K.; Madsen, P. Forest restoration paradigms. J. Sustain. For. 2014, 33, S161–S194. [Google Scholar] [CrossRef]
- Modica, G.; Merlino, A.; Solano, F.; Mercurio, R. An index for the assessment of degraded Mediterranean forest ecosystems. For. Syst. 2015, 24, e037. [Google Scholar] [CrossRef]
- Assessing Forest Degradation: Towards the Development of Globally Applicable Guidelines; Forest Resources Assessment; FAO: Rome, Italy, 2011.
- Asner, G.P.; Broadbent, E.N.; Oliveira, P.J.C.; Keller, M.; Knapp, D.E.; Silva, J.N.M. Condition and fate of logged forests in the Brazilian Amazon. Proc. Natl. Acad. Sci. 2006, 103, 12947–12950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asner, G.P.; Hughes, R.F.; Vitousek, P.M.; Knapp, D.E.; Kennedy-Bowdoin, T.; Boardman, J.; Martin, R.E.; Eastwood, M.; Green, R.O. Invasive plants transform the three-dimensional structure of rain forests. Proc. Natl. Acad. Sci. 2008, 105, 4519–4523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murdiyarso, D.; Skutsch, M.; Guariguata, M.; Kanninen, M. Measuring and monitoring forest degradation for REDD: Implications of country circumstances. Cifor Infobriefs 2008. [Google Scholar] [CrossRef] [Green Version]
- Hosonuma, N.; Herold, M.; De Sy, V.; De Fries, R.S.; Brockhaus, M.; Verchot, L.; Angelsen, A.; Romijn, E. An assessment of deforestation and forest degradation drivers in developing countries. Environ. Res. Lett. 2012, 7, 44009. [Google Scholar] [CrossRef] [Green Version]
- Kissinger, G.; Herold, M.; De Sy, V. Drivers of Deforestation and Forest Degradation: A Synthesis Report for REDD+ Policymakers; The Government of the UK and Norway: Vancouver, BC, Canada, 2012. [Google Scholar]
- Bustamante, M.M.; Roitman, I.; Aide, T.M.; Alencar, A.; Anderson, L.O.; Aragão, L.; Asner, G.P.; Barlow, J.; Berenguer, E.; Chambers, J.; et al. Toward an integrated monitoring framework to assess the effects of tropical forest degradation and recovery on carbon stocks and biodiversity. Glob. Chang. Biol. 2016, 22, 92–109. [Google Scholar] [CrossRef] [PubMed]
- Simula, M.; Mansur, E. Un desafío mundial que reclama una respuesta local. Unasylva 2011, 62, 3–7. [Google Scholar]
- Thompson, I.D. Biodiversidad, umbrales ecosistémicos, resiliencia y degradación forestal. Unasylva 2011, 62, 25–30. [Google Scholar]
- Lund, H.G. What is A Degraded Forest? Forest Information Services: Gainesville, VA, USA, 2009. [Google Scholar]
- Hudson, P.F.; Alcántara-Ayala, I. Ancient and modern perspectives on land degradation. Catena 2006, 65, 102–106. [Google Scholar] [CrossRef]
- Putz, F.E.; Nasi, R. Carbon benefits from avoiding and repairing forest degradation. In Realising REDD+: National Strategy and Policy Options; Angelsen A, Ed.; CIFOR: Bogor, Indonesia, 2009; pp. 249–362. [Google Scholar]
- Morales-Barquero, L.; Skutsch, M.; Jardel-Peláez, J.E.; Ghilardi, A.; Kleinn, C.; Healey, R.J. Operationalizing the definition of forest degradation for REDD+, with application to Mexico. Forests 2014, 5, 1653–1681. [Google Scholar] [CrossRef]
- Putz, F.E.; Redford, K.H. The importance of defining ‘forest’: Tropical forest degradation, deforestation, long-term phase shifts, and further transitions. Biotropica 2010, 42, 10–20. [Google Scholar] [CrossRef]
- Armenteras, D.; González, T.M. Degradación de bosques: contexto y definiciones. In Degradación de bosques en Latinoamérica Síntesis conceptual, metodologías de evaluación y casos de estudio nacionales; Armenteras, D., González, T.M., Retana, J., Espelta, J.M., Eds.; IBERO-REDD: Madrid, España, 2016; pp. 10–12. [Google Scholar]
- Chazdon, R.L.; Brancalion, P.H.S.; Laestadius, L.; Bennett-Curry, A.; Buckingham, K.; Kumar, C.; Moll-Rocek, J.; Vieira, I.C.G.; Wilson, S.J. When is a forest a forest? Forest concepts and definitions in the era of forest and landscape restoration. Ambio 2016, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Proceedings: Second Expert Meeting on Harmonizing Forest-Related Definitions for Use by Various Stakeholders; FAO: Rome, Italy, 2002.
- Ghazoul, J.; Burivalova, Z.; Garcia-Ulloa, J.; King, L.A. Conceptualizing forest degradation. Trends Ecol. Evol. 2015, 30, 622–632. [Google Scholar] [CrossRef] [PubMed]
- White, P.S.; Pickett, S.T.A. Natural disturbance and patch dynamics: An introduction. In The Ecology of Natural Disturbance and Patch Dynamics; Pickett, S.T.A., White, P.S., Eds.; Academic Press: New York, NY, USA, 1985; pp. 3–13. [Google Scholar]
- Oliver, C.D.; Larson, B.C. Forest Stand Dynamics; McGraw-Hill: New York, NY, USA, 1990. [Google Scholar]
- Drever, C.R.; Peterson, G.; Messier, C.; Bergeron, Y.; Flannigan, M. Can forest management based on natural disturbances maintain ecological resilience? Can. J. For. Res. 2006, 36, 2285–2299. [Google Scholar] [CrossRef] [Green Version]
- Tivy, J. Biogeography: A Study of Plants in the Ecosphere; Longman Scientific and Technical: New York, NY, USA, 1993. [Google Scholar]
- Holling, C.S. Resilience and stability of ecological systems. Annu. Rev. Ecol. Syst. 1973, 4, 1–23. [Google Scholar] [CrossRef]
- Gunderson, L.H. Ecological resilience—In theory and application. Annu. Rev. Ecol. Syst. 2000, 31, 425–439. [Google Scholar] [CrossRef]
- Beisner, B.E.; Haydon, D.T.; Cuddington, K. Alternative stable states in ecology. Front. Ecol. Environ. 2003, 1, 376–382. [Google Scholar] [CrossRef] [Green Version]
- Thompson, I.D.; Ferreira, J.; Gardner, T.; Guariguata, M.R.; Koh, L.P.; Okabe, K.; Pan, Y.; Schmitt, C.B.; Tylianakis, J.M.; Barlow, J.; et al. Forest biodiversity, carbon and other ecosystem services: relationships and impacts of deforestation and forest degradation. In Understanding Relationships between Biodiversity, Carbon, Forests and People: The Key to Achieving REDD+ Objectives. A Global Assessment Report Prepared by the Global Forest Expert Panel on Biodiversity, Forest Management, and REDD+; Parrotta, J.A., Wildburger, C., Mansourian, S., Eds.; IUFRO World Series: Vienna, Austria, 2012; pp. 21–51. [Google Scholar]
- Bahamondez, C.; Thompson, I.D. Determining forest degradation, ecosystem state and resilience using a standard stand stocking measurement diagram: Theory into practice. Forestry 2016, 89, 290–300. [Google Scholar] [CrossRef]
- Perspectiva Mundial sobre la Diversidad Biológica 3; Secretaría del Convenio sobre la Diversidad Biológica (SCDB): Montreal, QC, Canada, 2010.
- Peterson, G.; Allen, C.; CS, H. Ecological resilience, biodiversity, and scale. Ecosystems 1998, 1, 6–18. [Google Scholar] [CrossRef]
- Walker, B.; Holling, C.S.; Carpenter, S.R.; Kinzig, A. Resilience, adaptability and transformability in Social-ecological systems. Ecol. Soc. 2004, 9, 5. [Google Scholar] [CrossRef]
- Thompson, I.D.; Mackey, B.; Mcnulty, S.; Mosseler, A. Forest Resilience, Biodiversity, and Climate Change A Synthesis of the Biodiversity/Resilience/Stability Relationship in Forest Ecosystems; Secretariat of the Convention on Biological Diversity: Montreal, QC, Canada, 2009. [Google Scholar]
- Hobbs, R.J.; Norton, D.A. Towards a conceptual framework for restoration ecology. Restor. Ecol. 1996, 4, 93–110. [Google Scholar] [CrossRef]
- Groffman, P.M.; Baron, J.S.; Blett, T.; Gold, A.J.; Goodman, I.; Gunderson, L.H.; Levinson, B.M.; Palmer, M.A.; Paerl, H.W.; Peterson, G.D.; et al. Ecological thresholds: The key to successful environmental management or an important concept with no practical application? Ecosystems 2006, 9, 1–13. [Google Scholar] [CrossRef]
- Global Forest Resources Assessment 2010; FAO: Rome, Italy, 2010.
- Thompson, I.D.; Okabe, K.; Tylianakis, J.M.; Kumar, P.; Brockerhoff, E.G.; Schellhorn, N.A.; Parrotta, J.A.; Nasi, R. Forest biodiversity and the delivery of ecosystem goods and services: Translating science into policy. Bioscience 2011, 61, 972–981. [Google Scholar] [CrossRef]
- Smallidge, P.; Greason, M. Forestry practices to avoid: Just say no to high-grading. In Enhancing the Stewardship of Your Forest; Smallidge, P.J., Sargent, S.E., Sullivan, K.L., Goff, G.R., Bryant, D.L., Eds.; Cornell University, Department of Natural Resources: New York, NY, USA, 2004; pp. 65–68. [Google Scholar]
- Zamorano-Elgueta, C.; Cayuela, L.; Rey-Benayas, J.M.; Donoso, P.J.; Geneletti, D.; Hobbs, R. The differential influences of human-induced disturbances on tree regeneration community: a landscape approach. Ecosphere 2014, 5, 1–17. [Google Scholar] [CrossRef]
- Chapin, F.S.; Sala, O.E.; Burke, I.C.; Grime, J.P.; Hooper, D.U.; Lauenroth, W.K.; Lombard, A.; Mooney, H.A.; Mosier, A.R.; Naeem, S.; et al. Ecosystem consequences of changing biodiversity experimental evidence and a research agenda for the future. Bioscience 1998, 48, 45–52. [Google Scholar] [CrossRef]
- Cadotte, M.W.; Carscadden, K.; Mirotchnick, N. Beyond species: functional diversity and the maintenance of ecological processes and services. J. Appl. Ecol. 2011, 48, 1079–1087. [Google Scholar] [CrossRef] [Green Version]
- Baraloto, C.; Hardy, O.J.; Paine, C.E.T.; Dexter, K.G.; Cruaud, C.; Dunning, L.T.; Gonzalez, M.; Molino, J.; Sabatier, D.; Savolainen, V.; et al. Using functional traits and phylogenetic trees to examine the assembly of tropical tree communities. J. Ecol. 2012, 100, 690–701. [Google Scholar] [CrossRef] [Green Version]
- Díaz, S.; Symstad, A.J.; Stuart Chapin, F.; Wardle, D.A.; Huenneke, L.F. Functional diversity revealed by removal experiments. Trends Ecol. Evol. 2003, 18, 140–146. [Google Scholar] [CrossRef]
- Standish, R.J.; Hobbs, R.J.; Mayfield, M.M.; Bestelmeyer, B.T.; Suding, K.N.; Battaglia, L.L.; Eviner, V.; Hawkes, C.V.; Temperton, V.M.; Cramer, V.A.; et al. Resilience in ecology: Abstraction, distraction, or where the action is? Biol. Conserv. 2014, 177, 43–51. [Google Scholar] [CrossRef] [Green Version]
- Guidelines for the Restoration, Management and Rehabilitation of Degraded and Secondary Tropical Forests; ITTO, CIFOR, FAO, IUCN, WWF International: Yokohama, Japan, 2002.
- Linder, P.; Östlund, L. Changes in the boreal forests of Sweden 1870–1991. Sven. Bot. Tidskr. 1992, 86, 199–215. [Google Scholar]
- Devi, U.; Behera, N. Assessment of plant diversity in response to forest degradation in a tropical dry deciduous forest of Eastern Ghats in Orissa. J. Trop. For. Sci. 2003, 15, 147–163. [Google Scholar]
- Holl, K.D.; Loik, M.E.; Lin, E.H.V.; Samuels, I.A. Tropical montane forest restoration in Costa Rica: Overcoming barriers to dispersal and establishment. Restor. Ecol. 2001, 8, 339–349. [Google Scholar] [CrossRef]
- Vargas, O. Guía Metodológica para la Restauración Ecológica del bosque altoandino; Universidad Nacional de Colombia: Bogota, Colombia, 2007. [Google Scholar]
- Tucker, C.M.; Randolph, J.C.; Evans, T.; Andersson, K.P.; Persha, L.M.; Green, G.M. An approach to assess relative degradation in dissimilar forests: Toward a comparative assessment of institutional outcomes. Ecol. Soc. 2008, 13, 4. [Google Scholar] [CrossRef]
- Keane, R.E.; Hessburg, P.F.; Landres, P.B.; Swanson, F.J. The use of historical range and variability (HRV) in landscape management. For. Ecol. Manag. 2009, 258, 1025–1037. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Steffen, W.; Burbidge, A.A.; Hughes, L.; Kitching, R.L.; Musgrave, W.; Stafford Smith, M.; Werner, P.A. Conservation strategies in response to rapid climate change: Australia as a case study. Biol. Conserv. 2010, 143, 1587–1593. [Google Scholar] [CrossRef]
- Chazdon, R.L. Tropical forest recovery: legacies of human impact and natural disturbances. Perspect. Plant Ecol. Evol. Syst. 2003, 6, 51–71. [Google Scholar] [CrossRef]
- Rehabilitation of Degraded Forests to Improve Livelihoods of Poor Farmers in South China; Liu, D. (Ed.) CIFOR: Bogor, Indonesia, 2003. [Google Scholar]
- van Nes, E.H.; Hirota, M.; Holmgren, M.; Scheffer, M. Tipping points in tropical tree cover: linking theory to data. Glob. Chang. Biol. 2014, 20, 1016–1021. [Google Scholar] [CrossRef] [PubMed]
- Baland, J.-M.; Bardhan, P.; Das, S.; Mookherjee, D. Forests to the people: Decentralization and forest degradation in the Indian Himalayas. World Dev. 2010, 38, 1642–1656. [Google Scholar] [CrossRef]
- Sasaki, N.; Asner, G.P.; Knorr, W.; Durst, P.B.; Priyadi, H.R.; Putz, F.E. Approaches to classifying and restoring degraded tropical forests for the anticipated REDD+ climate change mitigation mechanism. iForest-Biogeosciences For. 2011, 1–6. [Google Scholar] [CrossRef]
- Spies, T.A. Forest stand structure, composition, and function. In Creating a Forestry for the 21st Century: The Science of Ecosystem Management; Kohm, K.A., Franklin, J.F., Eds.; Island Press: Washington, DC, USA, 1997; pp. 11–30. [Google Scholar]
- Grushecky, S.T.; Ann Fajvan, M. Comparison of hardwood stand structure after partial harvesting using intensive canopy maps and geostatistical techniques. For. Ecol. Manag. 1999, 114, 421–432. [Google Scholar] [CrossRef]
- Deluca, T.; Fajvan, M.A.; Miller, G. Diameter-limit harvesting: Effects of residual trees on regeneration dynamics in Appalachian hardwoods. North. J. Appl. For. 2009, 26, 52–60. [Google Scholar] [CrossRef]
- Vásquez-Grandón, A.; Donoso, P.J.; Gerding, V. Degradación de los bosques: Concepto, proceso y estado —Un ejemplo de aplicación en bosques adultos nativos de Chile. In Silvicultura en bosques nativos. Experiencias en silvicultura y restauración en Chile, Argentina y el oeste de Estados Unidos; Donoso, P., Promis, A., Soto, D., Eds.; OSU College of Forestry: Valdivia, Chile, 2018; pp. 175–196. [Google Scholar]
- Ghazoul, J.; Chazdon, R. Degradation and recovery in changing forest landscapes: A multiscale conceptual framework. Annu. Rev. Environ. Resour. 2017, 42, 161–188. [Google Scholar] [CrossRef]
- Defining Secondary and Degraded Forests in Central America; CATIE: Cartago, Turrialba, Costa Rica, 2016.
- Nyland, R.D. Exploitative cutting and stand rehabilitation. In Silviculture. Concepts and Applications; Waveland Press, Inc.: Long Grove, IL, USA, 2016; pp. 539–560. [Google Scholar]
- Nyland, R.D. Rehabilitating cutover stands: Some ideas to ponder. In Proceedings of the Conference on Diameter-Limit Cutting in Northeastern Forests (GTR-NE-342); Kenefic, L.S., Nyland, R.D., Eds.; Department of Agriculture, Forest Service, Northeastern Research Station: Newtown Square, PA, USA, 2006; pp. 47–51. [Google Scholar]
- Cazzolla Gatti, R.; Castaldi, S.; Lindsell, J.A.; Coomes, D.A.; Marchetti, M.; Maesano, M.; Di Paola, A.; Paparella, F.; Valentini, R. The impact of selective logging and clearcutting on forest structure, tree diversity and above-ground biomass of African tropical forests. Ecol. Res. 2015, 30, 119–132. [Google Scholar] [CrossRef]
- Bijalwan, A. Structure, composition and diversity of degraded dry tropical forest in Balamdi Watershed of Chhattisgarh plain, India. J. Biodivers. 2010, 1, 119–124. [Google Scholar] [CrossRef]
- Khai, T.C.; Mizoue, N.; Kajisa, T.; Ota, T.; Yoshida, S. Stand structure, composition and illegal logging in selectively logged production forests of Myanmar: Comparison of two compartments subject to different cutting frequency. Glob. Ecol. Conserv. 2016, 7, 132–140. [Google Scholar] [CrossRef]
- Pillay, R.; Hua, F.; Loiselle, B.A.; Bernard, H.; Fletcher, R.J. Multiple stages of tree seedling recruitment are altered in tropical forests degraded by selective logging. Ecol. Evol. 2018, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Knight, A.T.; Cowling, R.M.; Rouget, M.; Balmford, A.; Lombard, A.T.; Campbell, B.M. Knowing but not doing: Selecting priority conservation areas and the research–Implementation gap. Conserv. Biol. 2008, 22, 610–617. [Google Scholar] [CrossRef] [PubMed]

| Criteria | Characteristics |
|---|---|
| Structure | Loss of canopy cover |
| Change in diametric structure (low frequency of intermediate diameter classes, absence of some diameter classes, low density of larger-diameter commercial species) | |
| Reduction of growing and biomass stock (basal area and volume) | |
| Composition | Loss of species (composition and biodiversity) |
| High density and dominance of non-commercial or secondary species | |
| High density and coverage of competitive species | |
| Regeneration | Very low or lack of tree species regeneration |
| Abundant regeneration of non-commercial and arborescent species |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vásquez-Grandón, A.; Donoso, P.J.; Gerding, V. Forest Degradation: When Is a Forest Degraded? Forests 2018, 9, 726. https://doi.org/10.3390/f9110726
Vásquez-Grandón A, Donoso PJ, Gerding V. Forest Degradation: When Is a Forest Degraded? Forests. 2018; 9(11):726. https://doi.org/10.3390/f9110726
Chicago/Turabian StyleVásquez-Grandón, Angélica, Pablo J. Donoso, and Víctor Gerding. 2018. "Forest Degradation: When Is a Forest Degraded?" Forests 9, no. 11: 726. https://doi.org/10.3390/f9110726




