Kawasaki Disease: Management Challenges during COVID-19 Pandemic with an Upsurge in Multisystem Inflammatory Syndrome in Children
Abstract
:1. Introduction
2. Etiology and Pathogenesis
3. Definition of Kawasaki Disease
3.1. Classic Kawasaki Disease
3.2. Incomplete (Atypical) Kawasaki Disease
4. Kawasaki Disease and Bacillus Calmette-Guerin (BCG) Scar Reactivation
5. Recurrent Kawasaki Disease
6. Kawasaki Disease and COVID-19
7. Multisystem Inflammatory Syndrome in Children and Kawasaki Disease
8. Complications of Kawasaki Disease
9. Treatment of Kawasaki Disease
9.1. Standard Treatment
9.1.1. Intravenous Immunoglobulin
Prediction of IVIG Resistance in Kawasaki Disease—Kobayashi Score
9.1.2. Aspirin
9.2. Second-Line Treatments
10. Prognosis
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kawasaki, T. Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children. Arerugi 1967, 16, 178–222. [Google Scholar] [PubMed]
- Holman, R.C.; Curns, A.T.; Belay, E.D.; Steiner, C.A.; Schonberger, L.B. Kawasaki syndrome hospitalizations in the United States, 1997 and 2000. Pediatrics 2003, 112, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Harnden, A.; Tulloh, R.; Burgner, D. Kawasaki disease. BMJ 2014, 349, g5336. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.T.; Wu, M.H. The global epidemiology of Kawasaki disease: Review and future perspectives. Glob. Cardiol. Sci. Pract. 2017, 31, e201720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rowley, A.H.; Shulman, S.T. Kawasaki disease. In Nelson Textbook of Pediatrics, 19th ed.; Behrman, R.E., Kliegman, R.M., Jenson, H.B., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 823–826. [Google Scholar]
- McCrindle, B.W.; Rowley, A.H.; Newburger, J.W.; Burns, J.C.; Bolger, A.F.; Gewitz, M.; Baker, A.L.; Jackson, M.A.; Takahashi, M.; Shah, P.B.; et al. Diagnosis, Treatment, and Long-Term Management of Kawasaki Disease: A Scientific Statement for Health Professionals from the American Heart Association. Circulation 2017, 135, e927–e999. [Google Scholar] [CrossRef]
- Newburger, J.W.; Takahashi, M.; Gerber, M.A.; Gewitz, M.H.; Tani, L.Y.; Burns, J.C.; Shulman, S.T.; Bolger, A.F.; Ferrieri, P.; Baltimore, R.S.; et al. Diagnosis, treatment, and long-term management of Kawasaki disease: A statement for health professionals from the committee on rheumatic fever, endocarditis, and Kawasaki disease, council on cardiovascular disease in the young, American Heart Association. Pediatrics 2004, 114, 1708–1733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noval Rivas, M.; Arditi, M. Kawasaki disease: Pathophysiology and insights from mouse models. Nat. Rev. Rheu. Matol. 2020, 16, 391–405. [Google Scholar] [CrossRef]
- Rowley, A.H. Is Kawasaki disease an infectious disorder? Int. J. Rheum. Dis. 2018, 21, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.Y.; Lu, C.Y.; Shao, P.L.; Lee, P.I.; Lin, M.T.; Fan, T.Y.; Cheng, A.L.; Lee, W.L.; Hu, J.J.; Yeh, S.J.; et al. Viral infections associated with Kawasaki disease. J. Formos. Med. Assoc. 2014, 113, 148–154. [Google Scholar] [CrossRef] [Green Version]
- Hall, M.; Hoyt, L.; Ferrieri, P.; Schlievert, P.M.; Jenson, H.B. Kawasaki syndrome-like illness associated with infection caused by entero toxin B-secreting Staphylococcus aureus. Clin. Infect. Dis. 1999, 29, 586–589. [Google Scholar] [CrossRef] [Green Version]
- Rowley, A.H.; Shulman, S.T.; Mask, C.A.; Finn, L.S.; Terai, M.; Baker, S.C.; Galliani, C.A.; Takahashi, K.; Naoe, S.; Kalelkar, M.B.; et al. IgA plasma cell infiltration of proximal respiratory tract, pancreas, kidney, and coronary artery in acute Kawasaki disease. J. Infect Dis. 2000, 182, 1183–1191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rife, E.; Gedalia, A. Kawasaki Disease: An Update. Curr. Rheumatol. Rep. 2020, 22, 75. [Google Scholar] [CrossRef] [PubMed]
- Ueno, Y.; Takano, N.; Kanegane, H.; Yokoi, T.; Yachie, A.; Miyawaki, T.; Taniguchi, N. The acute phase nature of inter leukin 6: Studies in Kawasaki disease and other febrile illnesses. Clin. Exp. Immunol. 1989, 76, 337–342. [Google Scholar]
- Rowley, A.H. Kawasaki disease: AHA statement and recommendations. Contemp. Pediatr. 2018, 35, 10. [Google Scholar]
- Sundel, R.P.; Petty, R.E. Kawasaki disease. In Textbook of Pediatric Rheumatology, 6th ed.; Cassidy, J.T., Petty, R.E., Laxer, R.M., Lindsley, C.B., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 2011; pp. 505–520. [Google Scholar]
- Mastrangelo, G.; Cimaz, R.; Calabri, G.B.; Simonini, G.; Lasagni, D.; Resti, M.; Trapani, S. Kawasaki disease in infants less than one year of age: An Italian cohort from a single center. BMC Pediatr. 2019, 19, 321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, K.Y.Y.; Chua, M.C.; Tan, N.W.H.; Chandran, S. Reactivation of BCG inoculation site in a child with febrile exanthema of 3 days duration: An early indicator of incomplete Kawasaki disease. BMJ Case Rep. 2020, 13, e239648. [Google Scholar] [CrossRef] [PubMed]
- Trollfors, B.; Sigurdsson, V.; Dahlgren-Aronsson, A. Prevalence of Latent TB and Effectiveness of BCG Vaccination against Latent Tuberculosis: An Observational Study. Int. J. Infect. Dis. 2021, 109, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Kadowaki, J.; Yamaguchi, M.; Sato, S. Three cases suspected as acute febrile mucocutaneous lymph node syndrome: Emphasis on cutaneous changes at the BCG and tuberculin inoculated site in one case. Jpn. J. Pediatr. 1972, 25, 901–905. [Google Scholar]
- Chalmers, D.; Corban, J.G.; Moore, P.P. BCG site inflammation: A useful diagnostic sign in incomplete Kawasaki disease. J. Paediatr. Child Health 2008, 44, 525–526. [Google Scholar] [CrossRef]
- Takayama, J.; Yanase, Y.; Kawasaki, T. A study on erythematous change at the site of the BCG inoculation. Acta Paediatr. Jpn. 1982, 86, 567–572. [Google Scholar]
- Sireci, G.; Dieli, F.; Salerno, A. T cells recognize an immunodominant epitope of heat shock protein 65 in Kawasaki disease. Mol. Med. 2000, 6, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.C.; Lee, P.C.; Wang, C.C.; Hwang, B.T.; Meng, C.C.; Tsai, M.C. Reaction at the bacillus Calmette–Guérin inoculation site in patients with Kawasaki disease. Pediatr. Neonatol. 2013, 54, 43–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tseng, H.C.; Ho, J.C.; Guo, M.M.; Lo, M.H.; Hsieh, K.S.; Tsai, W.C.; Kuo, H.C.; Lee, C.H. Bull’s eye dermatoscopy pattern at bacillus Calmette-Guérin inoculation site correlates with systemic involvements in patients with Kawasaki disease. J. Dermatol. 2016, 43, 1044–1050. [Google Scholar] [CrossRef] [PubMed]
- Kollmann, T.R.; Klein, E.J.; Stefanelli, C.B.; Marcuse, E.K. Purified protein derivative anergy in Kawasaki disease. Pediatr. Infect. Dis. J. 2001, 20, 81–82. [Google Scholar] [CrossRef] [PubMed]
- Medaglia, A.A.; Siracusa, L.; Gioè, C.; Giordano, S.; Cascio, A.; Colomba, C. Kawasaki disease recurrence in the COVID-19 era: A systematic review of the literature. Ital. J. Pediatr. 2021, 47, 95. [Google Scholar] [CrossRef]
- Yang, H.M.; Du, Z.D.; Fu, P.P. Clinical features of recurrent Kawasaki disease and its risk factors. Eur. J. Pediatr. 2013, 172, 1641–1647. [Google Scholar] [CrossRef]
- Maddox, R.A.; Belay, E.D.; Holman, R.C. Recurrent Kawasaki syndrome in the United States. In Proceedings of the Abstracts of the Ninth International Kawasaki Symposium, Taipei, Taiwan, 10–12 April 2008. [Google Scholar]
- Nakamura, Y.; Hirose, K.; Yanagawa, H.; Kato, H.; Kawasaki, T. Incidence rate of recurrent Kawasaki disease in Japan. Acta Paediatr. 1994, 83, 1061–1064. [Google Scholar] [CrossRef]
- Sudo, D.; Nakamura, Y. Nationwide surveys show that the incidence of recurrent Kawasaki disease in Japan has hardly changed over the last 30 years. Acta Paediatr. 2017, 106, 796–800. [Google Scholar] [CrossRef]
- Hirata, S.; Nakamura, Y.; Yanagawa, H. Incidence rate of recurrent Kawasaki disease and related risk factors: From the results of nationwide surveys of Kawasaki disease in Japan. Acta Paediatr. 2001, 90, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Oki, I.; Tanihara, S.; Ojima, T.; Yanagawa, H. Cardiac sequelae in recurrent cases of Kawasaki disease: A comparison between the initial episode of the disease and a recurrence in the same patients. Pediatrics 1998, 102, E66. [Google Scholar] [CrossRef] [Green Version]
- Verdoni, L.; Mazza, A.; Gervasoni, A.; Martelli, L.; Ruggeri, M.; Ciuffreda, M.; Bonanomi, E.; D’Antiga, L. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: An observational cohort study. Lancet 2020, 395, 1771–1778. [Google Scholar] [CrossRef]
- Toubiana, J.; Poirault, C.; Corsia, A.; Bajolle, F.; Fourgeaud, J.; Angoulvant, F.; Debray, A.; Basmaci, R.; Salvador, E.; Biscardi, S.; et al. Kawasaki-like multisystem inflammatory syndrome in children during the COVID-19 pandemic in Paris, France: Prospective observational study. BMJ 2020, 369, m2094. [Google Scholar] [CrossRef] [PubMed]
- Jones, V.G.; Mills, M.; Suarez, D.; Hogan, C.A.; Yeh, D.; Segal, J.B.; Nguyen, E.L.; Barsh, G.R.; Maskatia, S.; Mathew, R. COVID-19 and Kawasaki disease: Novel virus and novel case. Hosp. Pediatr. 2020, 10, 537–540. [Google Scholar] [CrossRef]
- Riphagen, S.; Gomez, X.; Gonzalez-Martinez, C.; Wilkinson, N.; Theocharis, P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet 2020, 395, 1607–1608. [Google Scholar] [CrossRef]
- Belhadjer, Z.; Méot, M.; Bajolle, F.; Khraiche, D.; Legendre, A.; Abakka, S.; Auriau, J.; Grimaud, M.; Oualha, M.; Beghetti, M.; et al. Acute heart failure in multisystem inflammatory syndrome in children in the context of global SARS-CoV-2 pandemic. Circulation 2020, 142, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Esmaeilzadeh, H.; Mortazavi, N.; Salehi, A.; Fatemian, H.; Dehghani, S.M.; Vali, M.; Vardanjani, H.M. Effect of COVID-19 on Kawasaki Disease: Decrease Age of Onset and Increase Skin Manifestation. BMC Pediatr. 2021, 21, 571. [Google Scholar] [CrossRef] [PubMed]
- Yung, C.F.; Nadua, K.D.; Oh, B.K.; Thoon, K.C. Epidemiologic trends in Kawasaki disease during coronavirus disease-19 in Singapore. J. Pediatr. 2020, 226, 314–315. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, E.; Bamford, A.; Kenny, J.; Kaforou, M.; Jones, C.E.; Shah, P.; Ramnarayan, P.; Fraisse, A.; Miller, O.; Davies, P.; et al. Clinical Characteristics of 58 Children with a Pediatric Inflammatory Multisystem Syndrome Temporally Associated with SARS-CoV-2. JAMA 2020, 324, 259–269. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Rapid Risk Assessment: Paediatric Inflammatory Multisystem Syndrome and SARS-CoV-2 Infection in Children. Published 15 May 2020. Available online: https://www.ecdc.europa.eu/en/publications-data/paediatric-inflammatory-multisystem-syndrome-and-sars-cov-2-rapid-risk-assessment (accessed on 25 January 2022).
- World Health Organization. Multisystem Inflammatory Syndrome in Children and Adolescents with COVID-19. Published 15 May 2020. Available online: https://www.who.int/publications-detail/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19 (accessed on 25 January 2022).
- Matucci-Cerinic, C.; Caorsi, R.; Consolaro, A.; Rosina, S.; Civino, A.; Ravelli, A. Multisystem Inflammatory Syndrome in Children: Unique Disease or Part of the Kawasaki Disease Spectrum? Front. Pediatr. 2021, 9, 680813. [Google Scholar] [CrossRef] [PubMed]
- Bukulmez, H. Current Understanding of Multisystem Inflammatory Syndrome (MIS-C) Following COVID-19 and Its Distinction from Kawasaki Disease. Curr. Rheumatol. Rep. 2021, 23, 58. [Google Scholar] [CrossRef]
- Colomba, C.; La Placa, S.; Saporito, L.; Corsello, G.; Ciccia, F.; Medaglia, A.; Romanin, B.; Serra, N.; Di Carlo, P.; Cascio, A. Intestinal Involvement in Kawasaki Disease. J. Pediatr. 2018, 202, 186–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouldali, N.; Toubiana, J.; Antona, D.; Javouhey, E.; Madhi, F.; Lorrot, M.; Léger, P.L.; Galeotti, C.; Claude, C.; Wiedemann, A.; et al. Association of intravenous immunoglobulins plus methylprednisolone vs immunoglobulins alone with course of fever in multisystem inflammatory syndrome in children. JAMA 2021, 325, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.C. Kawasaki disease. Adv. Pediatr. Infect Dis. 2001, 48, 157–177. [Google Scholar]
- Marchesi, A.; Rigante, D.; Cimaz, R.; Ravelli, A.; de Jacobis, I.T.; Rimini, A.; Cardinale, F.; Cattalini, M.; De Zorzi, A.; Dellepiane, R.M.; et al. Revised recommendations of the Italian Society of Pediatrics about the general management of Kawasaki disease. Ital. J. Pediatr. 2021, 47, 16. [Google Scholar] [CrossRef]
- Kanegaye, J.T.; Wilder, M.S.; Molkara, D.; Frazer, J.R.; Pancheri, J.; Tremoulet, A.H.; Watson, V.E.; Best, B.M.; Burns, J.C. Recognition of a Kawasaki disease shock syndrome. Pediatrics 2009, 123, e783–e789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burns, J.C.; Capparelli, E.V.; Brown, J.A.; Newburger, J.W.; Glode, M.P.; US/Canadian Kawasaki Syndrome Study Group. Intravenous gamma-globulin treatment and retreatment in Kawasaki disease. Pediatr. Infect. Dis. J. 1998, 17, 1144–1148. [Google Scholar] [CrossRef]
- Oates-Whitehead, R.M.; Baumer, J.H.; Haines, L.; Love, S.; Maconochie, I.K.; Gupta, A.; Roman, K.; Dua, J.S.; Flynn, I. Intravenous immunoglobulin for the treatment of Kawasaki disease in children. Cochrane Database Syst. Rev. 2003, 4, CD004000. [Google Scholar]
- Furusho, K.; Kamiya, T.; Nakano, H.; Kiyosawa, N.; Shinomiya, K.; Hayashidera, T.; Tamura, T.; Hirose, O.; Manabe, Y.; Yokoyama, T. High-dose intravenous gammaglobulin for Kawasaki disease. Lancet 1984, 2, 1055–1058. [Google Scholar] [CrossRef]
- Newburger, J.W.; Takahashi, M.; Burns, J.C.; Beiser, A.; Chung, K.J.; Duffy, C.E.; Glode, M.P.; Mason, W.H.; Reddy, V.; Sanders, S.; et al. The treatment of Kawasaki syndrome with intravenous gamma globulin. N. Engl. J. Med. 1986, 315, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Gerding, R. Kawasaki disease: A review. J. Pediatr. Health Care 2011, 25, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Inoue, Y.; Takeuchi, K.; Okada, Y.; Tamura, K.; Tomomasa, T.; Kobayashi, T.; Morikawa, A. Prediction of intravenous immunoglobulin unresponsiveness in patients with Kawasaki disease. Circulation 2006, 113, 2606–2612. [Google Scholar] [CrossRef]
- Kibata, T.; Suzuki, Y.; Hasegawa, S.; Matsushige, T.; Kusuda, T.; Hoshide, M.; Takahashi, K.; Okada, S.; Wakiguchi, H.; Moriwake, T. Coronary artery lesions and the increasing incidence of Kawasaki disease resistant to initial immune globulin. Int. J. Cardiol. 2016, 214, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Sleeper, L.A.; Minich, L.L.; McCrindle, B.M.; Li, J.S.; Mason, W.; Colan, S.D.; Atz, A.M.; Printz, B.F.; Baker, A.; Vetter, V.L.; et al. Pediatric Heart Network Investigators. Evaluation of Kawasaki disease risk-scoring systems for intravenous immunoglobulin resistance. J. Pediatr. 2011, 158, 831–835.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, S.; Sutton, N.; Blackstock, S.; Gormley, S.; Hoggart, C.J.; Levin, M.; Herberg, J.A. Predicting IVIG resistance in UK Kawasaki disease. Arch. Dis. Child. 2015, 100, 366–368. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Lee, H.; Eun, L. Verification of Current Risk Scores for Kawasaki Disease in Korean Children. J. Korean Med. Sci. 2017, 32, 1991–1996. [Google Scholar] [CrossRef]
- Arane, K.; Mendelsohn, K.; Mimouni, M.; Mimouni, F.; Koren, Y.; Simon, D.B.; Bahat, H.; Helou, M.H.; Mendelson, A.; Hezkelo, N. Japanese scoring systems to predict resistance to intravenous immunoglobulin in Kawasaki disease were unreliable for Caucasian Israeli children. Acta Paediatr. 2018, 107, 2179–2184. [Google Scholar] [CrossRef]
- Platt, B.; Belarski, E.; Manaloor, J.; Ofner, S.; Carroll, A.E.; John, C.C.; Wood, J.B. Comparison of Risk of Recrudescent Fever in Children with Kawasaki Disease Treated with Intravenous Immunoglobulin and Low-Dose vs High-Dose Aspirin. JAMA Netw. Open 2020, 3, e1918565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freeman, A.F.; Shulman, S.T. Kawasaki disease: Summary of the American Heart Association guidelines. Am. Fam. Physician 2006, 74, 1141–1148. [Google Scholar]
- Kato, H.; Koike, S.; Yokoyama, T. Kawasaki disease: Effect of treatment on coronary artery involvement. Pediatrics 1979, 63, 175–179. [Google Scholar] [CrossRef]
- Kijima, Y.; Kamiya, T.; Suzuki, A.; Hirose, O.; Manabe, H. A trial procedure to prevent aneurysm formation of the coronary arteries by steroid pulse therapy in Kawasaki disease. Jpn. Circ. J. 1982, 46, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Sundel, R.P.; Baker, A.L.; Fulton, D.R.; Newburger, J.W. Corticosteroids in the initial treatment of Kawasaki disease: Report of a randomized trial. J. Pediatr. 2003, 142, 611–616. [Google Scholar] [CrossRef]
- Newburger, J.W.; Sleeper, L.A.; McCrindle, B.W.; Minich, L.L.; Gersony, W.; Vetter, V.L.; Atz, A.M.; Li, J.S.; Takahashi, M.; Baker, A.L.; et al. Randomized trial of pulsed corticosteroid therapy for primary treatment of Kawasaki disease. N. Engl. J. Med. 2007, 356, 663–675. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Okada, Y.; Shinohara, M.; Kobayashi, T.; Kobayashi, T.; Tomomasa, T.; Takeuchi, K.; Morikawa, A. A multicenter prospective randomized trial of corticosteroids in primary therapy for Kawasaki disease: Clinical course and coronary artery outcome. J. Pediatr. 2006, 149, 336–341. [Google Scholar] [CrossRef] [PubMed]
- De Graeff, N.; Groot, N.; Ozen, S.; Eleftheriou, D.; Avcin, T.; Bader-Meunier, B.; Dolezalova, P.; Feldman, B.M.; Kone-Paut, I.; Lahdenne, P.; et al. European consensus-based recommendations for the diagnosis and treatment of Kawasaki disease-the SHARE initiative. Rheumatology 2019, 58, 672–682. [Google Scholar] [CrossRef]
- Buda, P.; Friedman-Gruszczyńska, J.; Książyk, J. Anti-inflammatory Treatment of Kawasaki Disease: Comparison of Current Guidelines and Perspectives. Front. Med. 2021, 8, 738850. [Google Scholar] [CrossRef] [PubMed]
- Orenstein, J.M.; Shulman, S.T.; Fox, L.M.; Baker, S.C.; Takahashi, M.; Bhatti, T.R.; Russo, P.A.; Mierau, G.W.; de Chadarévian, J.P.; Perlman, E.J.; et al. Three linked vasculopathic processes characterize Kawasaki disease: A light and transmission electron microscopic study. PLoS ONE 2012, 7, e38998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holve, T.J.; Patel, A.; Chau, Q.; Marks, A.R.; Meadows, A.; Zaroff, J.G. Long-Term cardiovascular outcomes in survivors of Kawasaki disease. Pediatrics 2014, 133, e305–e311. [Google Scholar] [CrossRef] [Green Version]
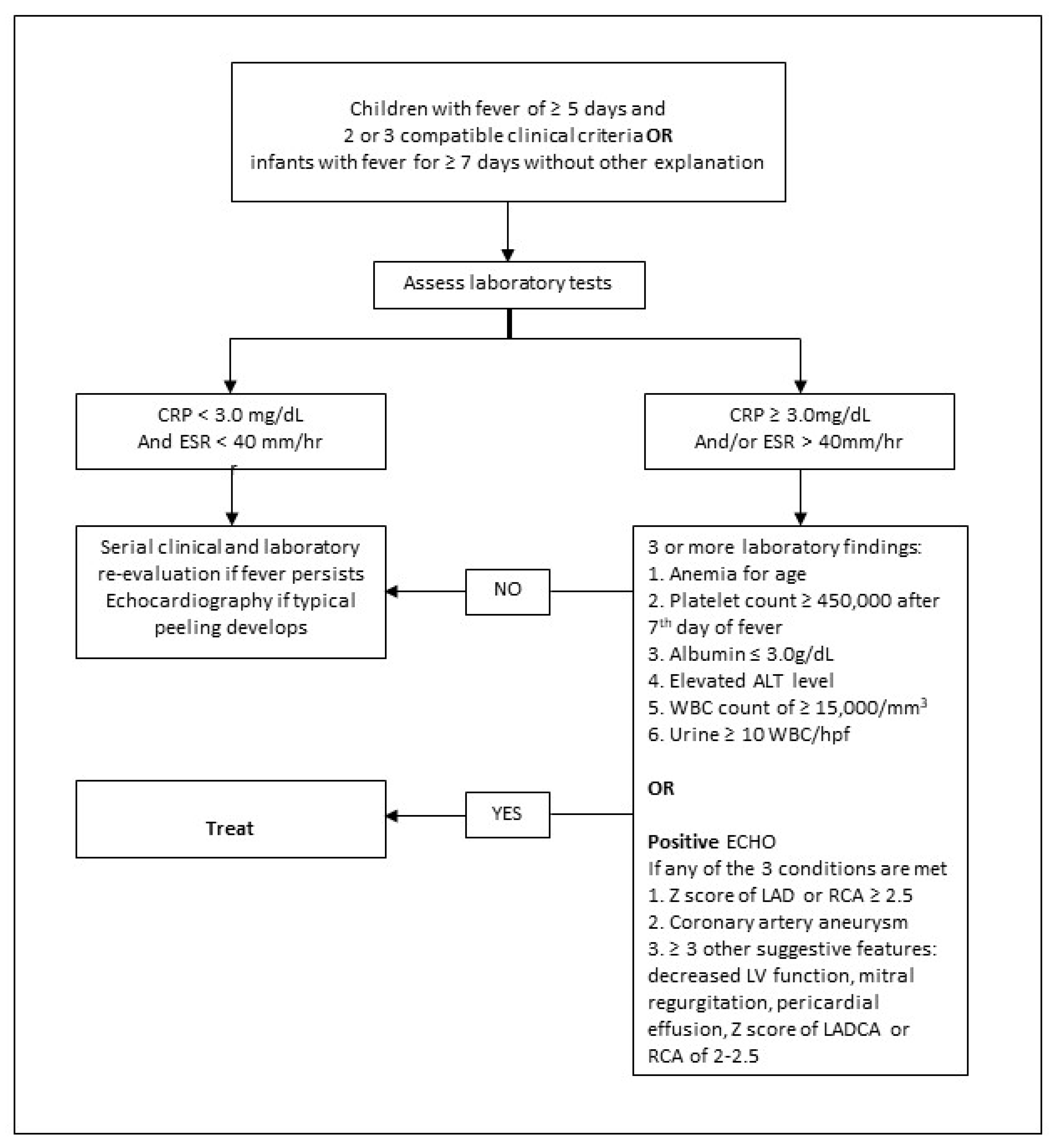
| The diagnosis of Kawasaki disease is considered confirmed by the presence of fever for at least 5 days and four of the five principal clinical features with no other explainable causes: |
|
Supplementary laboratory criteria
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hendriks, G.; Chandran, S. Kawasaki Disease: Management Challenges during COVID-19 Pandemic with an Upsurge in Multisystem Inflammatory Syndrome in Children. Rheumato 2022, 2, 34-45. https://doi.org/10.3390/rheumato2020005
Hendriks G, Chandran S. Kawasaki Disease: Management Challenges during COVID-19 Pandemic with an Upsurge in Multisystem Inflammatory Syndrome in Children. Rheumato. 2022; 2(2):34-45. https://doi.org/10.3390/rheumato2020005
Chicago/Turabian StyleHendriks, Gillian, and Suresh Chandran. 2022. "Kawasaki Disease: Management Challenges during COVID-19 Pandemic with an Upsurge in Multisystem Inflammatory Syndrome in Children" Rheumato 2, no. 2: 34-45. https://doi.org/10.3390/rheumato2020005






