Lack of Evidence for Microplastic Contamination from Water-Soluble Detergent Capsules
Abstract
:1. Introduction
2. Materials and Methods
2.1. Water-Soluble Detergents for Laundry and Dishwashing Machine
2.2. Washing Conditions
2.2.1. Laundry Washes
2.2.2. Dishwashing
2.3. Blank Samples and Microplastic Catching Devices
- Tap water, at the beginning (sample A—0001-00) and in the middle (sample B—0002-00) of the testing process, to check whether microplastic particles could come from the incoming water.
- Empty cycles, consisting of two wastewater samples, one from laundry (0003-00) and the other one from dishwashing (0004-00), without detergents nor textile garments or dishes, to check microplastics coming from machine devices.
- Two wastewater samples, one from laundry (0005-00) and the other one from dishwashing (0006-00), only with liquid detergent, to check whether the laundry/dishwashing detergents contributes to the release of microplastics.
- Two wastewater samples, one from laundry (0007-00) with only textile and the other one from dishwashing (0008-00) with only tableware, to check their contribution to microplastic release.
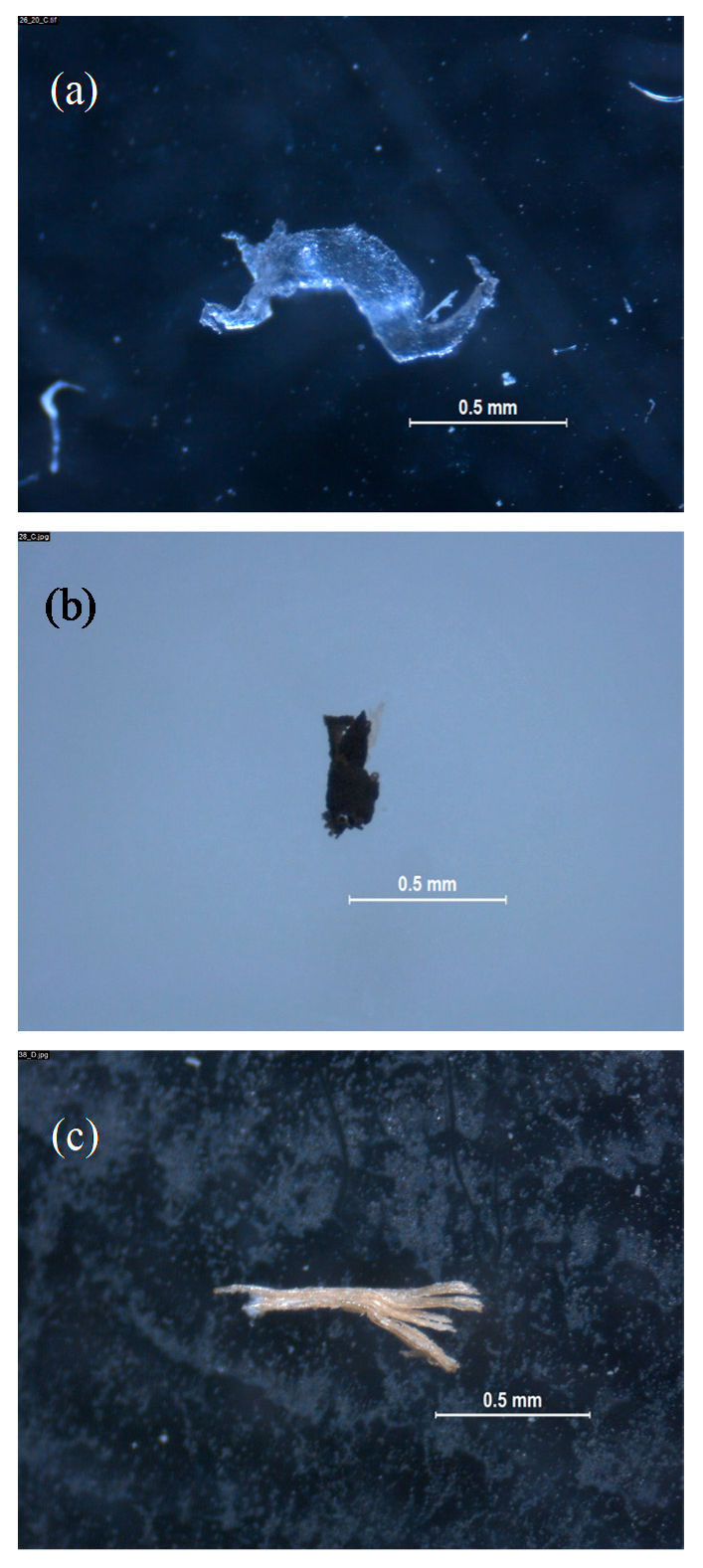
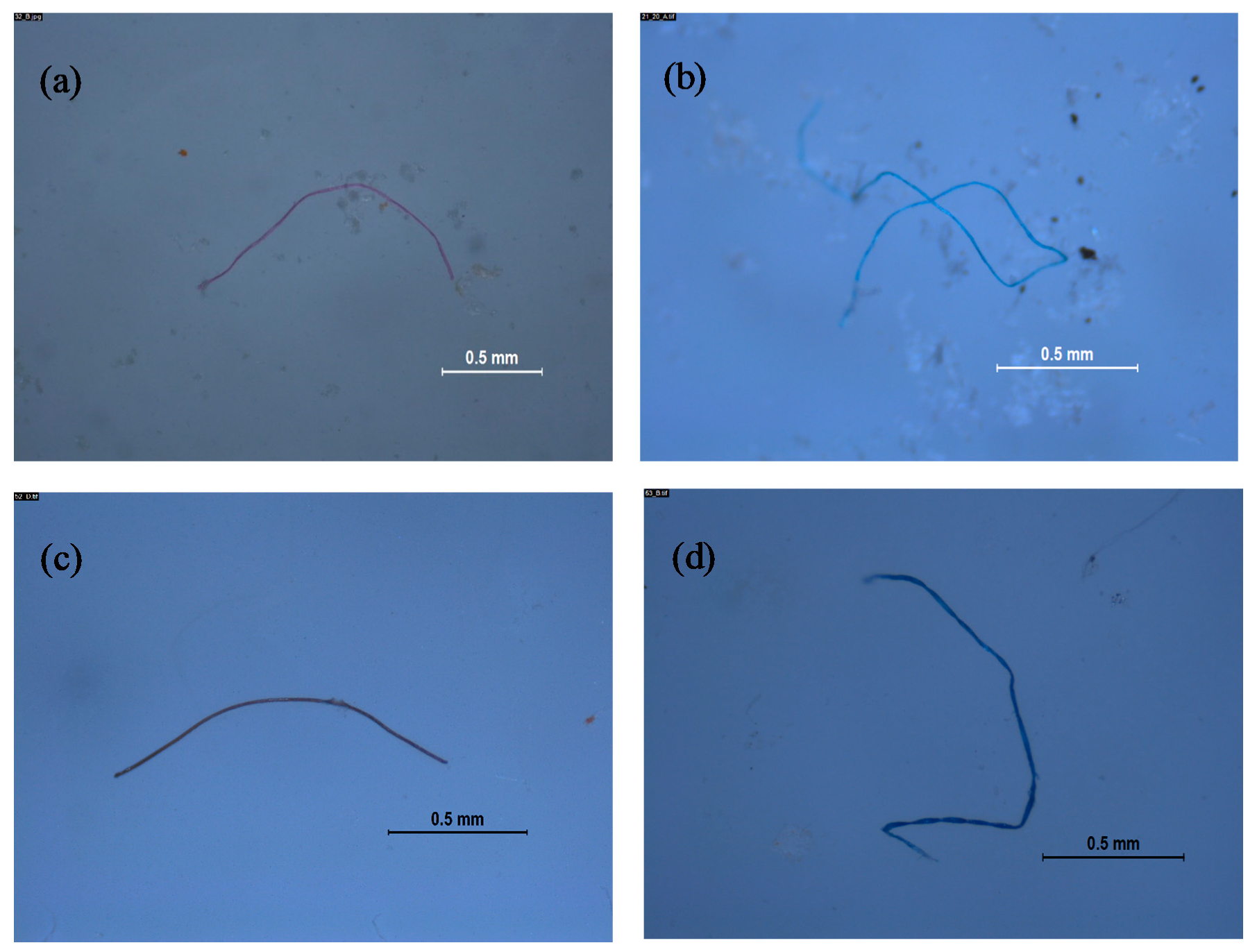
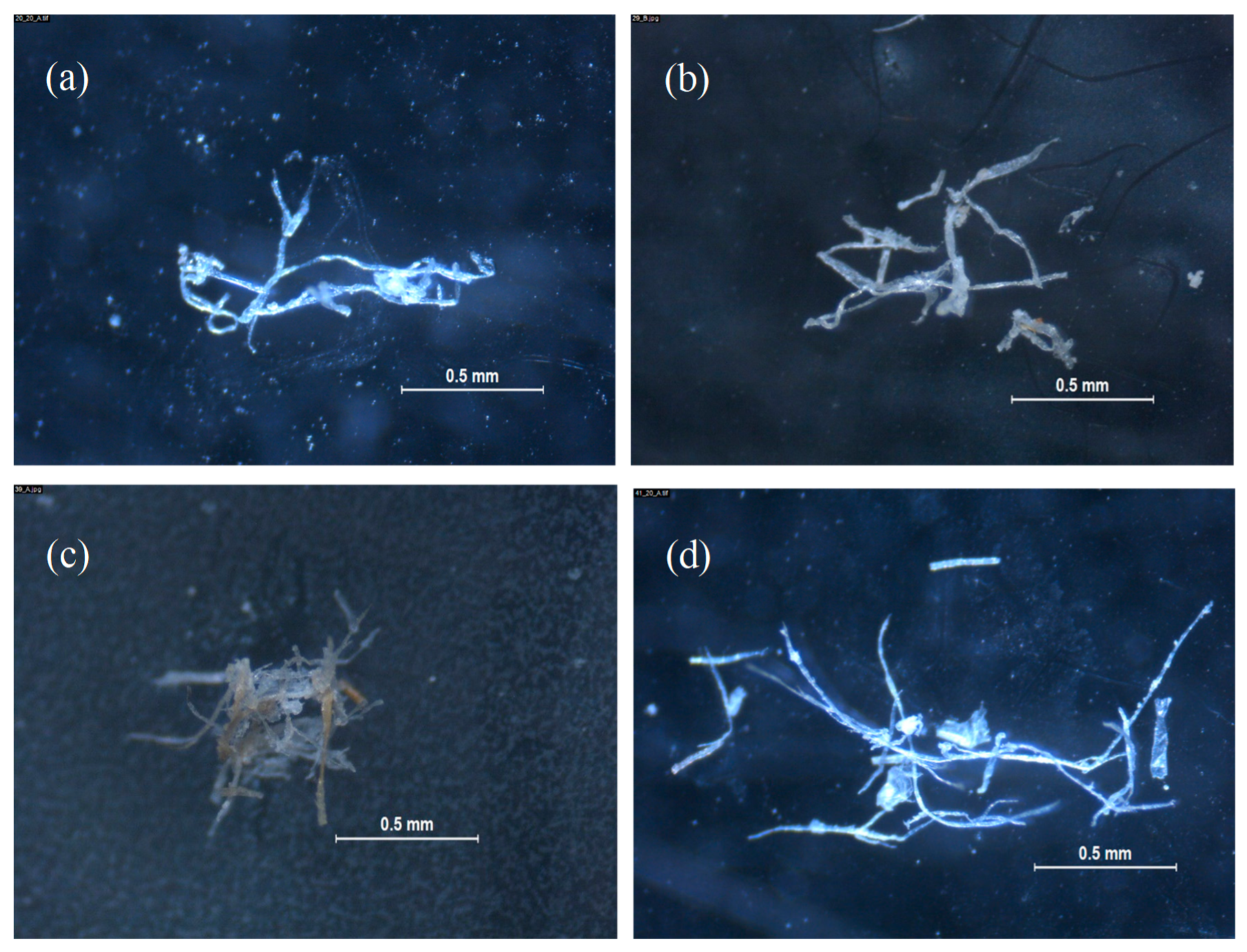
2.4. Microplastic Identification
3. Results and Discussion
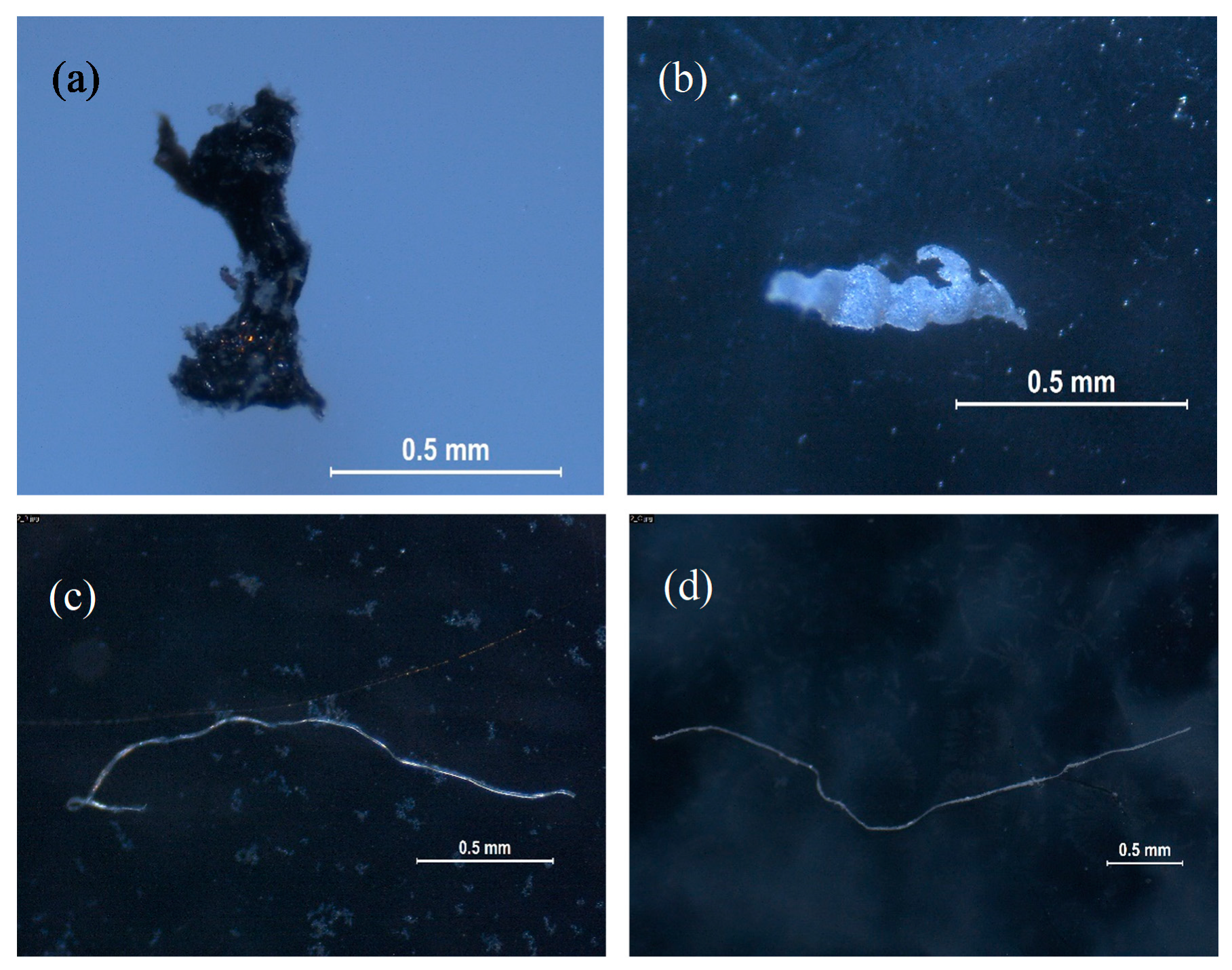
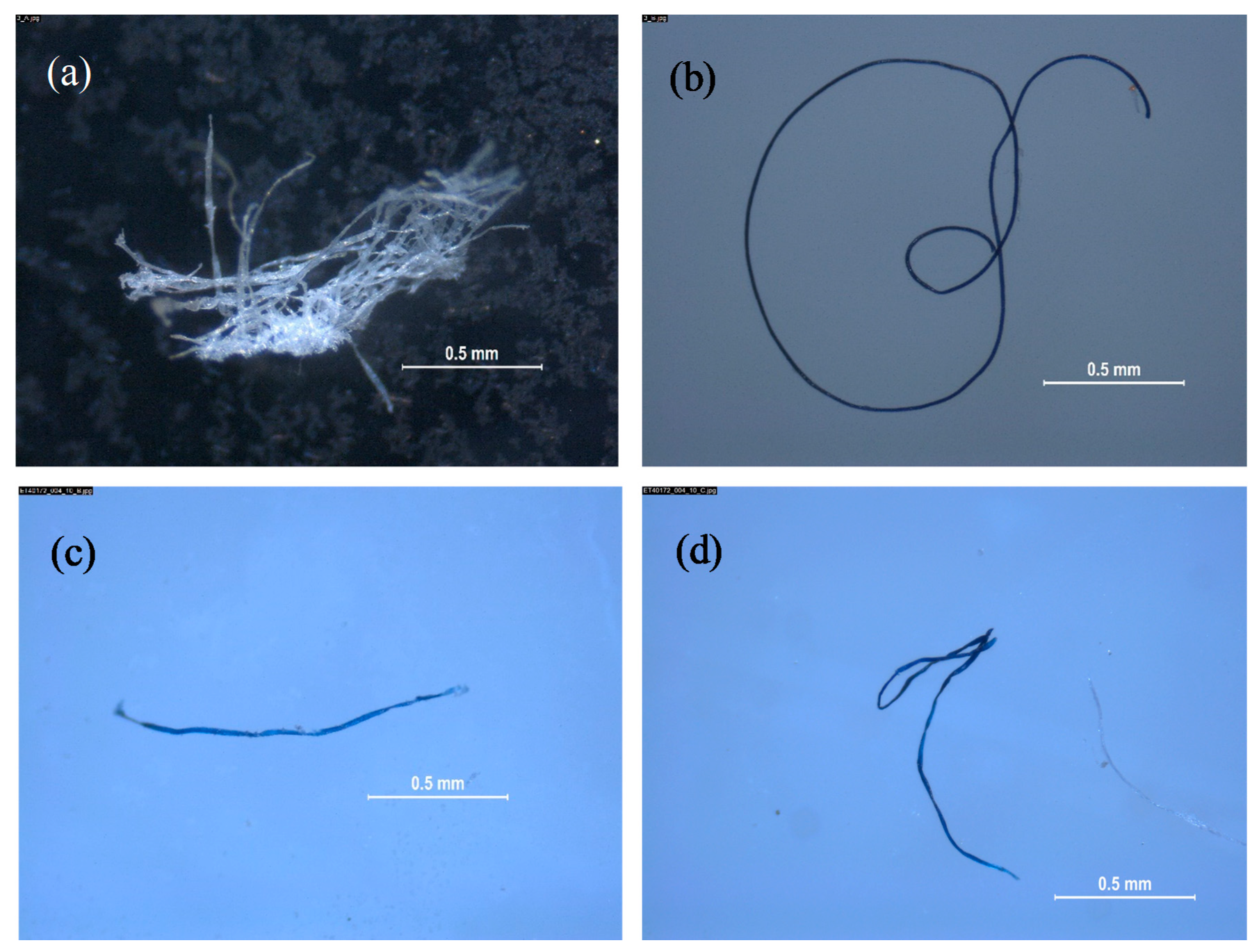
3.1. Enveloping Material, Blank Samples, Empty Cycles, and Catching Devices
3.2. Microplastics in Water-Soluble Detergent Capsules
3.2.1. Laundry Capsules
3.2.2. Dishwashing Capsules
4. Conclusions
- A total of 103 samples were analyzed throughout the project, including 5 enveloping materials, 10 samples including blank samples, empty cycles and catching devices samples in duplicate, and 39 water-soluble capsules also analyzed in duplicate.
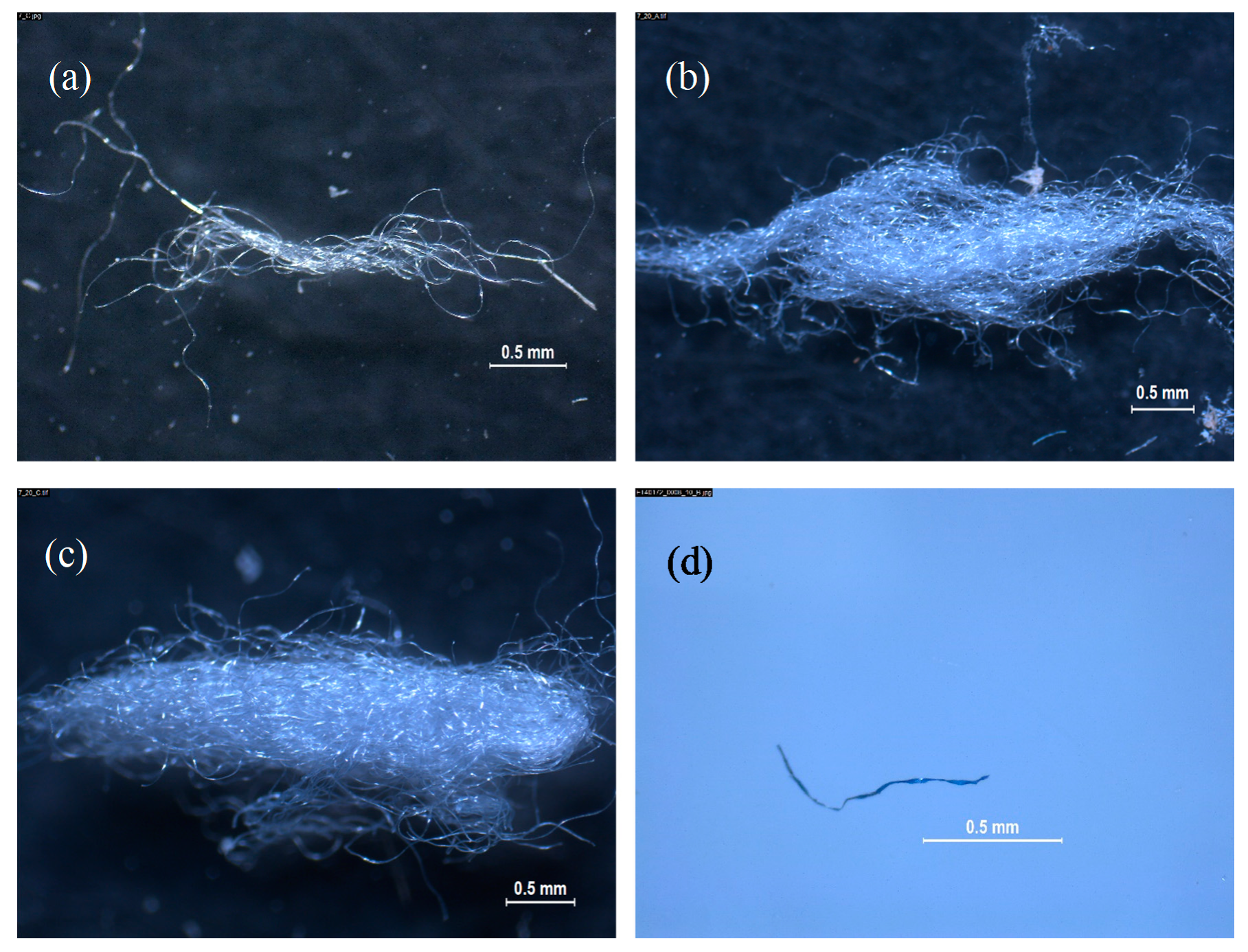
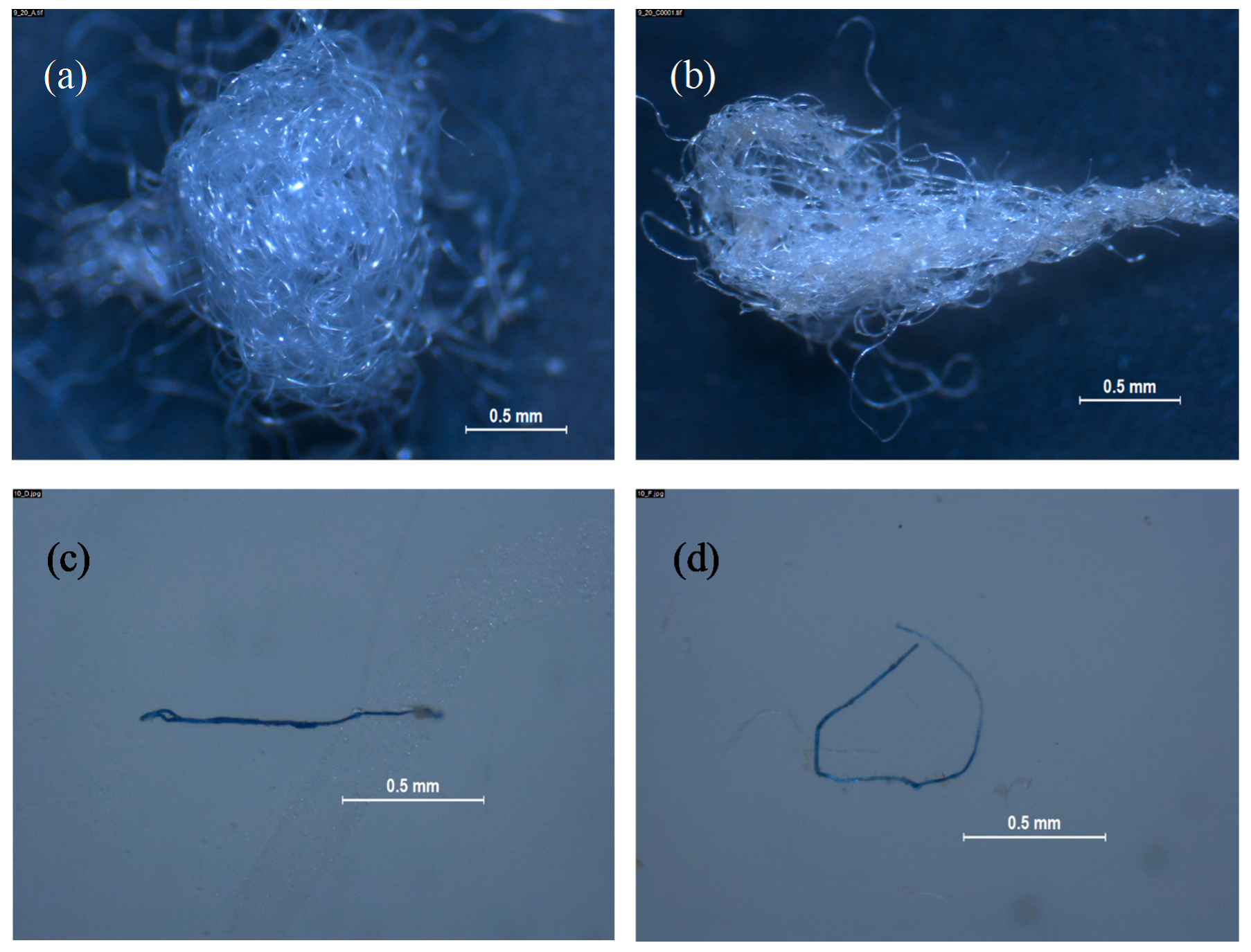
- Both devices tested for catching microplastics reported fibers in their composition, although wastewater from 0010-00 displayed less fibers than wastewater from 0009-00.
- The vast majority of fibers isolated from laundry wastewater samples were present as a knotty mass, difficult to efficiently be counted, meanwhile separate fibers were mostly present in dishwashing wastewater. In this sense, an artificial calculated rate (CR) was designed to standardize results from images picked up by stereomicroscope and their classification and quantification.
- The variability in duplicate experiments for the same commercial brand was not predictable, with some implicit variance from an unidentified source.
- A total of 70% of wastewater from water-soluble washing detergents reported a low CR parameter, in the first tertile, with only two brand models with a high fiber shedding from polyester blankets, i.e., 0015-00 and 0017-00.
- Wastewater samples from dishwashing water-soluble detergent capsules were much less contaminated with microplastics than those from laundry detergents.
- Detected microplastics in dishwashing wastewater included an extrapolated count of 0.29 fibers per liter, three fragmented microplastics, i.e., PUR, PVP, and PAAM, not representing more than 0.08 items per liter, and some non-polymeric microparticles.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Chemicals Agency (ECHA). Annex XV Restriction Report Proposal for a Restriction; Report version number 1 (20 March 2019); European Chemicals Agency: Helsinki, Finland, 2019.
- Hartmann, N.B.; Huffer, T.; Thompson, R.C.; Hassellöv, M.; Verschoor, A.; Daugaard, A.E.; Rist, S.; Karlsson, T.; Brennholt, N.; Cole, M.; et al. Are we speaking the same language? Recommendations for a definition and categorization framework for plastic debris. Environ. Sci. Technol. 2019, 53, 1039–1047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gigault, J.; Ter Halle, A.; Baudrimont, M.; Pascal, P.Y.; Gauffre, F.; Phi, T.L.; Hadr, H.E.; Grassl, B.; Reynaud, S. Current opinion: What is a nanoplastic? Environ. Pollut. 2018, 235, 1030–1034. [Google Scholar] [CrossRef] [PubMed]
- Crawford, C.B.; Quinn, B. Microplastic Pollutants; Elsevier: Oxford, UK, 2017; 315p. [Google Scholar]
- Bayo, J.; Martínez, A.; Guillén, M.; Olmos, S.; Roca, M.J.; Alcolea, A. Microbeads in Commercial Facial Cleansers: Threatening the Environment. Clean-Soil Air Water 2017, 45, 1600683. [Google Scholar] [CrossRef]
- Hale, R.C.; Seeley, M.E.; La Guardia, M.J.; Mai, L.; Zeng, E.Y. A global perspective on microplastics. J. Geophys. Res. Oceans 2020, 125, e14719. [Google Scholar] [CrossRef]
- Gregory, M.R. Environmental implications of plastic debris in marine settings entanglement, ingestion, smothering, hangers-on, hitch-hiking and alien invasions. Philos. Trans. R. Soc. B 2009, 364, 2013–2025. [Google Scholar] [CrossRef]
- Bayo, J.; Rojo, D.; Olmos, S. Abundance, morphology and chemical composition of microplastics in sand and sediments from a protected coastal area: The Mar Menor lagoon (SE Spain). Environ. Pollut. 2019, 252, 1357–1366. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef]
- Rochman, C.M.; Hoh, E.; Kurobe, T.; Teh, S.J. Ingested plastic transfers hazardous chemicals to fish and induces hepatic stress. Sci. Rep. 2013, 3, 3263. [Google Scholar] [CrossRef]
- Cox, K.D.; Covernton, G.A.; Davies, H.L.; Dower, J.F.; Juanes, F.; Dudas, S.E. Human consumption of microplastics. Environ. Sci. Technol. 2019, 53, 7068–7074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakir, A.; Rowland, S.J.; Thompson, R.C. Competitive sorption of persistent organic pollutants onto microplastics in the marine environment. Mar. Pollut. Bull. 2012, 64, 2782–2789. [Google Scholar] [CrossRef] [PubMed]
- Carr, S.A.; Liu, J.; Tesoro, A.G. Transport and fate of microplastic particles in wastewater treatment plants. Water Res. 2016, 91, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Talvitie, J.; Mikola, A.; Setälä, O.; Heinonen, M.; Koistinen, A. How well is microlitter purified from wastewater?—A detailed study on the stepwise removal of microlitter in a tertiary level wastewater treatment plant. Water Res. 2017, 109, 164–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enfrin, M.; Dumée, L.F.; Lee, J. Nano/microplastics in water and wastewater treatment processes–origin, impact and potential solutions. Water Res. 2019, 161, 621–638. [Google Scholar] [CrossRef] [PubMed]
- Prata, J.C. Microplastics in wastewater: State of the knowledge on sources, fate and solutions. Mar. Pollut Bull. 2018, 129, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Boucher, J.; Friot, D. Primary Microplastics in the Oceans: A Global Evaluation of Sources; International Union for Conservation of Nature: Gland, Switzerland, 2017; 43p. [Google Scholar]
- Bayo, J.; Olmos, S.; López-Castellanos, J. Microplastics in an urban wastewater treatment plant: The influence of physicochemical parameters and environmental factors. Chemosphere 2020, 238, 124593. [Google Scholar] [CrossRef]
- Browne, M.A.; Crump, P.; Niven, S.J.; Teuten, E.; Tonkin, A.; Galloway, T.; Thompson, R. Accumulation of microplastic on shorelines worldwide: Sources and sinks. Environ. Sci. Technol. 2011, 45, 9175–9179. [Google Scholar] [CrossRef]
- Bilgin, M.; Yurtsever, M.; Karadagli, F. Microplastic removal by aerated grit chambers versus settling tanks of a municipal wastewater treatment plant. J. Water Process. Eng. 2020, 38, 101604. [Google Scholar] [CrossRef]
- Almroth, B.M.C.; Åstrom, L.; Roslund, S.; Petersson, H.; Johansson, M.; Persson, N. Quantifying shedding of synthetic fibers from textiles; a source of microplastics released into the environment. Environ. Sci. Pollut. Res. 2018, 25, 1191–1199. [Google Scholar] [CrossRef] [Green Version]
- Bayo, J.; López-Castellanos, J.; Olmos, S. Membrane bioreactor and rapid sand filtration for the removal of microplastics in an urban wastewater treatment plant. Mar. Pollut. Bull. 2020, 156, 111211. [Google Scholar] [CrossRef]
- Sun, J.; Dai, X.; Wang, Q.; van Loosdrecht, M.C.; Ni, B.J. Microplastics in wastewater treatment plants: Detection, occurrence and removal. Water Res. 2019, 152, 21–37. [Google Scholar] [CrossRef]
- Napper, I.E.; Thompson, R.C. Release of synthetic microplastic plastic fibres from domestic washing machines: Effects of fabric type and washing conditions. Mar. Pollut. Bull. 2016, 112, 39–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Falco, F.; Gullo, M.P.; Gentile, G.; Di Pace, E.; Cocca, M.; Gelabert, L.; Brouta-Agnésa, M.; Rovira, A.; Escudero, R.; Villalba, R.; et al. Evaluation of microplastic release caused by textile washing processes of synthetic fabrics. Environ. Pollut. 2018, 236, 916–925. [Google Scholar] [CrossRef] [PubMed]
- Fontana, G.D.; Mossotti, R.; Montarsolo, A. Assessment of microplastics release from polyester fabrics: The impact of different washing conditions. Environ. Pollut. 2020, 264, 113960. [Google Scholar] [CrossRef] [PubMed]
- Hummel, D.O. Atlas of Plastics Additives: Analysis by Spectrometric Methods; Springer: Berlin/Heidelberg, Germany, 2002; 537p. [Google Scholar]
- Frias, J.P.G.L.; Gago, J.; Otero, V.; Sobral, P. Microplastics in coastal sediments from Southern Portuguese shelf waters. Mar. Environ. Res. 2016, 114, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Jiang, Q.; Hu, X.; Zhong, X. Occurrence and identification of microplastics in tap water from China. Chemosphere 2020, 252, 126493. [Google Scholar] [CrossRef] [PubMed]
- Plastics Europe. Plastics—The Facts. An Analysis of European Plastics Production, Demand and Waste Data; Plastics Europe: Brussels, Belgium, 2020. [Google Scholar]
- Kniggendorf, A.K.; Wetzel, C.; Roth, B. Microplastics detection in streaming tap water with Raman spectroscopy. Sensors 2019, 19, 1839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estahbanati, S.; Fahrenfeld, N.L. Influence of wastewater treatment plant discharges on microplastic concentrations in surface water. Chemosphere 2016, 162, 277–284. [Google Scholar] [CrossRef]
- Lv, X.; Dong, Q.; Zuo, Z.; Liu, Y.; Huang, X.; Wu, W.M. Microplastics in a municipal wastewater treatment plant: Fate, dynamic distribution, removal efficiencies, and control strategies. J. Clean. Prod. 2019, 225, 579–586. [Google Scholar] [CrossRef]
- Okoffo, E.D.; O’Brien, S.; O’Brien, J.W.; Tscharke, B.J.; Thomas, K.V. Wastewater treatment plants as a source of plastics in the environment: A review of occurrence, methods for identification, quantification and fate. Environ Sci Water Res. 2019, 5, 1908–1931. [Google Scholar] [CrossRef]
- McIlwraith, H.K.; Lin, J.; Erdle, L.M.; Mallos, N.; Diamond, M.L.; Rochman, C.M. Capturing microfibers–marketed technologies reduce microfiber emissions from washing machines. Mar. Pollut. Bull. 2019, 139, 40–45. [Google Scholar] [CrossRef]
- Hernandez, E.; Nowack, B.; Mitrano, D.M. Polyester textiles as a source of microplastics from households: A mechanistic study to understand microfiber release during washing. Environ. Sci. Technol. 2017, 51, 7036–7046. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Hay, J.N.; Jenkins, M.J. FTIR spectroscopic analysis of poly (ethylene terephthalate) on crystallization. Eur. Polym. J. 2012, 48, 1586–1610. [Google Scholar] [CrossRef]
- Cincinelli, A.; Scopetani, C.; Chelazzi, D.; Lombardini, E.; Martellini, T.; Katsoyiannis, A.; Fossi, M.C.; Corsolini, S. Microplastic in the surface waters of the Ross Sea (Antarctica): Occurrence, distribution and characterization by FTIR. Chemosphere 2017, 175, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wang, Q.; Ruan, Y.; Wu, R.; Chen, L.; Zhang, K.; Lam, P.K. Intra-day microplastic variations in wastewater: A case study of a sewage treatment plant in Hong Kong. Mar. Pollut. Bull. 2020, 160, 111535. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.; Tang, F.H.; Maggi, F. Sinking of microbial-associated microplastics in natural waters. PLoS ONE 2020, 15, e0228209. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). Scientific Opinion on the safety of polyvinylpyrrolidone-vinyl acetate copolymer for the proposed uses as a food additive. EFSA J. 2010, 8, 1948. [Google Scholar]
- Schafer, J.; Reindel, W.; Steffen, R.; Mosehauer, G.; Chinn, J. Use of a novel extended blink test to evaluate the performance of two polyvinylpyrrolidone-containing, silicone hydrogel contact lenses. Clin. Ophthalmol. 2018, 12, 819–825. [Google Scholar] [CrossRef] [Green Version]
- Kurakula, M.; Rao, G.K. Pharmaceutical assessment of polyvinylpyrrolidone PVP) as excipient from conventional to controlled delivery systems with a spotlight on COVID-19 inhibition. J. Drug Deliv. Sci. Technol. 2020, 60, 102046. [Google Scholar] [CrossRef]
- Xiong, B.; Loss, R.D.; Shields, D.; Pawlik, T.; Hochreiter, R.; Zydney, A.L.; Kumar, M. Polyacrylamide degradation and its implications in environmental systems. NPJ Clean Water 2018, 1, 17. [Google Scholar] [CrossRef]
- Liu, H.; Bu, Y.; Sanjayan, J.G.; Nazari, A.; Shen, Z. Suitability of polyacrylamide superabsorbent polymers as the internal curing agent of well cement. Constr. Build. Mater. 2016, 112, 253–260. [Google Scholar] [CrossRef]
- Kwaśniewska, D.; Wieczorek, D.; Staszak, K. Effect of amphoteric surfactants on surface and wetting properties of hand dishwashing liquids. Pol. J. Cosmet. 2019, 22, 301–304. [Google Scholar]
- Nigamatzyanova, L.; Fakhrullin, R. Dark-field hyperspectral microscopy for label-free microplastics and nanoplastics detection and identification in vivo: A Caenorhabditis elegans study. Environ. Pollut. 2021, 271, 116337. [Google Scholar] [CrossRef] [PubMed]
- Boyle, K.; Örmeci, B. Microplastics and nanoplastics in the freshwater and terrestrial environment: A review. Water 2020, 12, 2633. [Google Scholar] [CrossRef]
- Ricciardi, M.; Pironti, C.; Motta, O.; Miele, Y.; Proto, A.; Montano, L. Microplastics in the Aquatic Environment: Occurrence, Persistence, Analysis, and Human Exposure. Water 2021, 13, 973. [Google Scholar] [CrossRef]




| Country/Use | Code | Brand Model |
|---|---|---|
| Spain/Laundry | 0014-00 | Carrefour |
| Spain/Laundry | 0015-00 | Skip Powercaps |
| Spain/Laundry | 0016-00 | Colon |
| Spain/Laundry | 0017-00 | Wipp Express |
| Spain/Laundry | 0018-00 | Ariel |
| Spain/Dishwashing | 0019-00 | Fairy Platinum Plus All in One |
| Spain/Dishwashing | 0020-00 | W5 Limón |
| Spain/Dishwashing | 0021-00 | Finish Max Power |
| Spain/Dishwashing | 0022-00 | Finish Quantum |
| Spain/Dishwashing | 0023-00 | Bosque Verde |
| Portugal/Laundry | 0024-00 | Persil Dup Caps |
| Portugal/Laundry | 0025-00 | Skip Ultimate |
| Portugal/Laundry | 0026-00 | Auchan |
| Portugal/Laundry | 0027-00 | Continente |
| Portugal/Laundry | 0028-00 | Epsil |
| Portugal/Dishwashing | 0029-00 | Fairy Platinum All in One |
| Portugal/Dishwashing | 0031-00 | Super Pop |
| Portugal/Dishwashing | 0032-00 | Unamat |
| Portugal/Dishwashing | 0033-00 | W5 All in One |
| Belgium/Laundry | 0034-00 | Formil Duo Power |
| Belgium/Laundry | 0035-00 | Delhaize |
| Belgium/Laundry | 0036-00 | Dash |
| Belgium/Laundry | 0037-00 | Persil Discs |
| Belgium/Laundry | 0038-00 | Le Chat |
| Belgium/Dishwashing | 0039-00 | Sun |
| Belgium/Dishwashing | 0040-00 | W5 Tablettes |
| Belgium/Dishwashing | 0041-00 | Dreft |
| Belgium/Dishwashing | 0042-00 | Boni Selection |
| Belgium/Dishwashing | 0043-00 | Frosch |
| Italy/Laundry | 0044-00 | Dash |
| Italy/Laundry | 0045-00 | Dexal |
| Italy/Laundry | 0046-00 | Dixan |
| Italy/Laundry | 0047-00 | Sole |
| Italy/Laundry | 0048-00 | Formil Monodosi |
| Italy/Dishwashing | 0049-00 | Conad Verso Natura |
| Italy/Dishwashing | 0050-00 | Svelto |
| Italy/Dishwashing | 0051-00 | Pril |
| Italy/Dishwashing | 0052-00 | Fairy Platinum Plus |
| Italy/Dishwashing | 0053-00 | Coop Vivi Verde |
| Sample | Code | Specification |
|---|---|---|
| Blank/Tap water | 0001-00 | Sample A |
| Blank/Tap water | 0002-00 | Sample B |
| Blank/Wastewater/Laundry-machine | 0003-00 | Sample C |
| Blank/Wastewater/Dishwashing-machine | 0004-00 | Sample D |
| Blank/Liquid Detergent (Formil)/Laundry-machine | 0005-00 | Sample E |
| Blank/Liquid Detergent (Somat)/Dishwashing-machine | 0006-00 | Sample F |
| Blank/Textile without detergent/Laundry-machine | 0007-00 | Sample G |
| Blank/Dishes without detergent/Dishwashing-machine | 0008-00 | Sample H |
| Catching device/Cora Ball/Laundry-machine | 0009-00 | Cora Ball |
| Catching device/Guppyfriend/Laundry-machine | 0010-00 | Guppyfriend |
| Sample | Code | CR Value |
|---|---|---|
| Blank/Tap water | 0001-00 | 1 |
| Blank/Tap water | 0002-00 | 4 |
| Blank/Wastewater/Laundry-machine | 0003-00 | 2 |
| Blank/Wastewater/Dishwashing-machine | 0004-00 | 2 |
| Blank/Liquid Detergent (Formil)/Laundry-machine | 0005-00 | 39 |
| Blank/Liquid Detergent (Somat)/Dishwashing-machine | 0006-00 | 1 |
| Blank/Textile without detergent/Laundry-machine | 0007-00 | 43 |
| Blank/Dishes without detergent/Dishwashing-machine | 0008-00 | 1 |
| Catching device/Cora Ball/Laundry-machine | 0009-00 | 44 |
| Catching device/Guppyfriend/Laundry-machine | 0010-00 | 13 |
| Country | Code | CR Value |
|---|---|---|
| Spain | 0014-00 | 11 |
| Spain | 0015-00 | 61 |
| Spain | 0016-00 | 1 |
| Spain | 0017-00 | 66 |
| Spain | 0018-00 | 0 |
| Portugal | 0024-00 | 1 |
| Portugal | 0025-00 | 41 |
| Portugal | 0026-00 | 26 |
| Portugal | 0027-00 | 6 |
| Portugal | 0028-00 | 14 |
| Belgium | 0034-00 | 4 |
| Belgium | 0035-00 | 2 |
| Belgium | 0036-00 | 22 |
| Belgium | 0037-00 | 9 |
| Belgium | 0038-00 | 13 |
| Italy | 0044-00 | 38 |
| Italy | 0045-00 | 7 |
| Italy | 0046-00 | 23 |
| Italy | 0047-00 | 15 |
| Italy | 0048-00 | 17 |
| Country | Code | CR Value |
|---|---|---|
| Spain | 0019-00 | 0 |
| Spain | 0020-00 | 1 |
| Spain | 0021-00 | 1 |
| Spain | 0022-00 | 2 |
| Spain | 0023-00 | 2 |
| Portugal | 0029-00 | 1 |
| Portugal | 0031-00 | 0 |
| Portugal | 0032-00 | 2 |
| Portugal | 0033-00 | 2 |
| Belgium | 0039-00 | 2 |
| Belgium | 0040-00 | 0 |
| Belgium | 0041-00 | 1 |
| Belgium | 0042-00 | 0 |
| Belgium | 0043-00 | 0 |
| Italy | 0049-00 | 1 |
| Italy | 0050-00 | 0 |
| Italy | 0051-00 | 1 |
| Italy | 0052-00 | 4 |
| Italy | 0053-00 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bayo, J.; Ramos, B.; López-Castellanos, J.; Rojo, D.; Olmos, S. Lack of Evidence for Microplastic Contamination from Water-Soluble Detergent Capsules. Microplastics 2022, 1, 121-140. https://doi.org/10.3390/microplastics1010008
Bayo J, Ramos B, López-Castellanos J, Rojo D, Olmos S. Lack of Evidence for Microplastic Contamination from Water-Soluble Detergent Capsules. Microplastics. 2022; 1(1):121-140. https://doi.org/10.3390/microplastics1010008
Chicago/Turabian StyleBayo, Javier, Belén Ramos, Joaquín López-Castellanos, Dolores Rojo, and Sonia Olmos. 2022. "Lack of Evidence for Microplastic Contamination from Water-Soluble Detergent Capsules" Microplastics 1, no. 1: 121-140. https://doi.org/10.3390/microplastics1010008





