Relationship of Microplastics to Body Size for Two Estuarine Fishes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Sampling
2.2. Lab Methods
2.3. Data Analysis
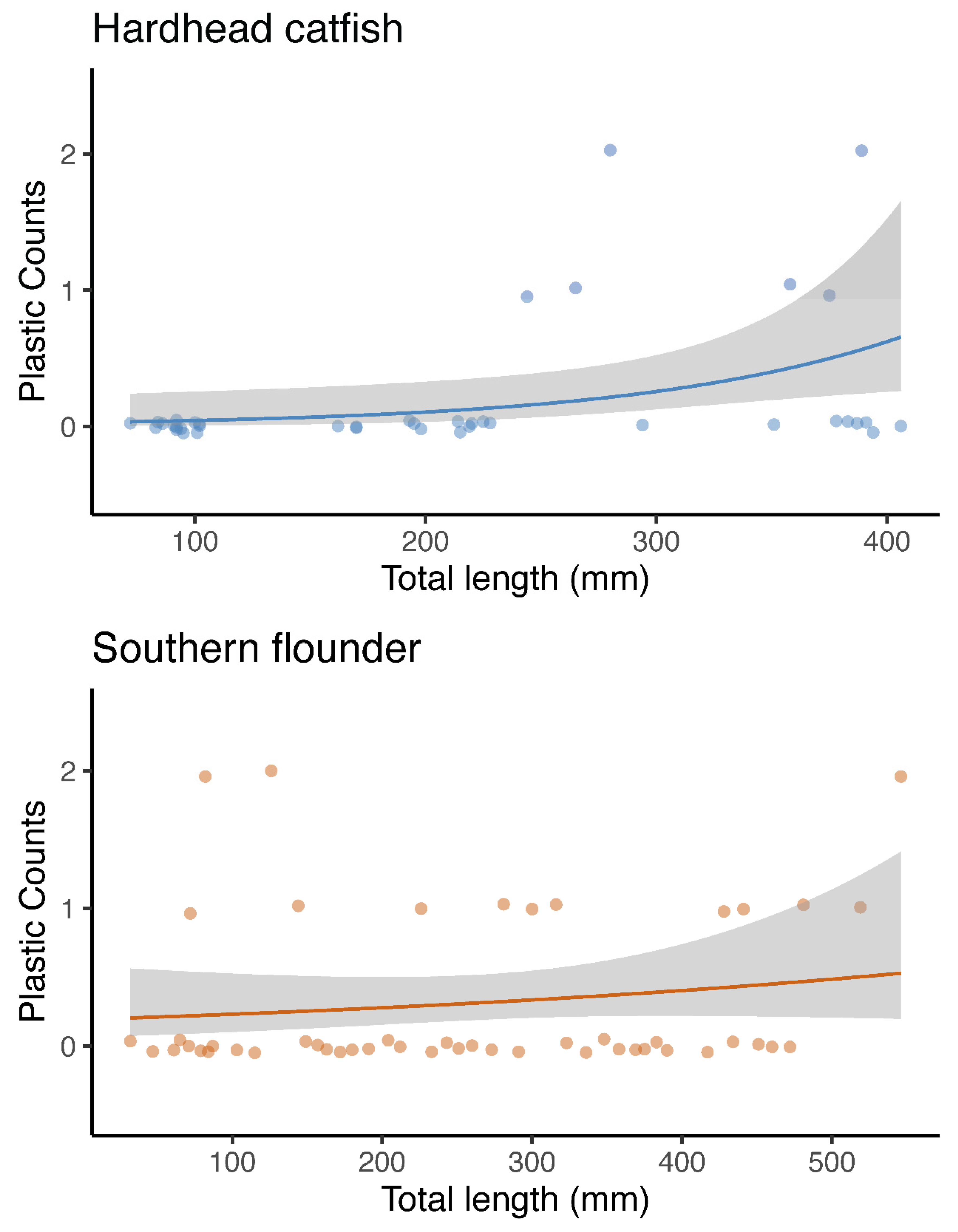
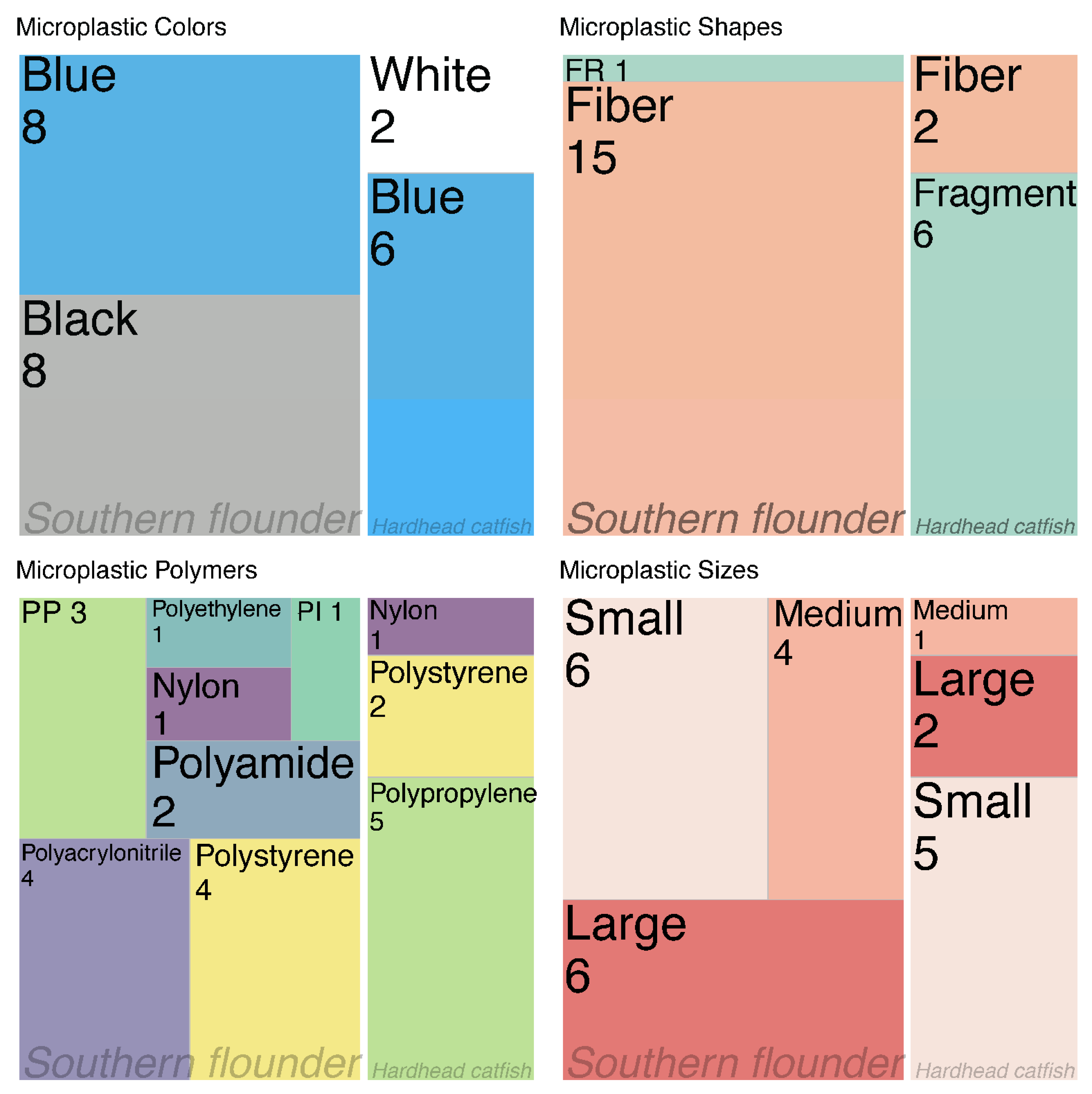
3. Results
3.1. Microplastics and Total Length
3.2. Microplastics Descriptions
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duis, K.; Coors, A. Microplastics in the aquatic and terrestrial environment: Sources (with a specific focus on personal care products), fate and effects. Environ. Sci. Eur. 2016, 28, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Wezel, A.; Caris, I.; Kools, S.A.E. Release of primary microplastics from consumer products to wastewater in the Netherlands. Environ. Toxicol. Chem. 2015, 35, 1627–1631. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef]
- Neto, J.A.B.; Gaylarde, C.; Beech, I.; Bastos, A.C.; da Silva Quaresma, V.; de Carvalho, D.G. Microplastics and attached microorganisms in sediments of the Vitória bay estuarine system in SE Brazil. Ocean Coast. Manag. 2019, 169, 247–253. [Google Scholar] [CrossRef]
- McEachern, K.; Alegria, H.; Kalagher, A.L.; Hansen, C.; Morrison, S.; Hastings, D. Microplastics in Tampa Bay, Florida: Abundance and variability in estuarine waters and sediments. Mar. Pollut. Bull. 2019, 148, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Bessa, F.; Barría, P.; Neto, J.; Frias, J.; Otero, V.; Sobral, P.; Marques, J.C. Occurrence of microplastics in commercial fish from a natural estuarine environment. Mar. Pollut. Bull. 2018, 128, 575–584. [Google Scholar] [CrossRef]
- Kazour, M.; Jemaa, S.; El Rakwe, M.; Duflos, G.; Hermabassiere, L.; Dehaut, A.; Le Bihanic, F.; Cachot, J.; Cornille, V.; Rabhi, K.; et al. Juvenile fish caging as a tool for assessing microplastics contamination in estuarine fish nursery grounds. Environ. Sci. Pollut. Res. 2018, 27, 3548–3559. [Google Scholar] [CrossRef]
- Peters, C.A.; Hendrickson, E.; Minor, E.C.; Schreiner, K.; Halbur, J.; Bratton, S.P. Pyr-GC/MS analysis of microplastics extracted from the stomach content of benthivore fish from the Texas Gulf Coast. Mar. Pollut. Bull. 2018, 137, 91–95. [Google Scholar] [CrossRef]
- Anderson, J.C.; Park, B.J.; Palace, V.P. Microplastics in aquatic environments: Implications for Canadian ecosystems. Environ. Pollut. 2016, 218, 269–280. [Google Scholar] [CrossRef] [Green Version]
- Qiang, L.; Cheng, J. Exposure to microplastics decreases swimming competence in larval zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2019, 176, 226–233. [Google Scholar] [CrossRef]
- Batel, A.; Linti, F.; Scherer, M.; Erdinger, L.; Braunbeck, T. Transfer of benzo[a]pyrene from microplastics toArtemianauplii and further to zebrafish via a trophic food web experiment: CYP1A induction and visual tracking of persistent organic pollutants. Environ. Toxicol. Chem. 2016, 35, 1656–1666. [Google Scholar] [CrossRef] [PubMed]
- Di Mauro, R.; Kupchik, M.J.; Benfield, M.C. Abundant plankton-sized microplastic particles in shelf waters of the northern Gulf of Mexico. Environ. Pollut. 2017, 230, 798–809. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y. Fish Resources of the Gulf of Mexico. In Habitats and Biota of the Gulf of Mexico: Before the Deepwater Horizon Oil Spill; Ward, C.H., Ed.; Springer: New York, NY, USA, 2017; pp. 869–1038. [Google Scholar]
- Muncy, R.J.; Wingo, W.M. Species Profiles: Life Histories and Environmental Requirements of Coastal Fishes and Invertebrates (Gulf of Mexico): Sea Catfish and Gafftopsail Catfish; U.S. Fish and Wildlife Service: Washington, DC, USA, 1983; p. 17.
- Conover, D.O.; Munch, S.B. Sustaining Fisheries Yields over Evolutionary Time Scales. Science 2002, 297, 94–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pensinger, L.G.; Polito, M.J.; Midway, S.R. Ontogenetic stability in the trophic niche of a common Gulf of Mexico fish, Ariopsis felis. Environ. Biol. Fishes 2021, 104, 569–579. [Google Scholar] [CrossRef]
- Midway, S.; Scharf, F.S. Histological Analysis Reveals Larger Size at Maturity for Southern Flounder with Implications for Biological Reference Points. Mar. Coast. Fish. 2012, 4, 628–638. [Google Scholar] [CrossRef]
- Midway, S.R.; White, J.W.; Scharf, F.S. The Potential for Cryptic Population Structure to Sustain a Heavily Exploited Marine Flatfish Stock. Mar. Coast. Fish. 2018, 10, 411–423. [Google Scholar] [CrossRef] [Green Version]
- Burke, J.S. Role of feeding and prey distribution of summer and southern flounder in selection of estuarine nursery habitats. J. Fish Biol. 1995, 47, 355–366. [Google Scholar] [CrossRef]
- Phillips, M.B.; Bonner, T. Occurrence and amount of microplastic ingested by fishes in watersheds of the Gulf of Mexico. Mar. Pollut. Bull. 2015, 100, 264–269. [Google Scholar] [CrossRef]
- Louisiana Department of Wildlife and Fisheries. Independent Sampling Activities: Field Manual; Marine Fisheries Section: Baton Rouge, LA, USA, 2015; 42p. [Google Scholar]
- Foekema, E.M.; De Gruijter, C.; Mergia, M.T.; van Franeker, J.A.; Murk, A.J.; Koelmans, A. Plastic in North Sea Fish. Environ. Sci. Technol. 2013, 47, 8818–8824. [Google Scholar] [CrossRef]
- Toner, K.; Midway, S.R. Historic fish samples from the Southeast USA lack microplastics. Sci. Total Environ. 2021, 776, 145923. [Google Scholar] [CrossRef]
- Kery, M. Introduction to WinBUGS for Ecologists: Bayesian Approach to Regression, ANOVA, Mixed Models and Related Analyses; Academic Press: Cambridge, MA, USA, 2010; p. 320. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Zhang, F.; Wang, X.; Xu, J.; Zhu, L.; Peng, G.; Xu, P.; Li, D. Food-web transfer of microplastics between wild caught fish and crustaceans in East China Sea. Mar. Pollut. Bull. 2019, 146, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Wessel, C.C.; Lockridge, G.R.; Battiste, D.; Cebrian, J. Abundance and characteristics of microplastics in beach sediments: Insights into microplastic accumulation in northern Gulf of Mexico estuaries. Mar. Pollut. Bull. 2016, 109, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Beckwith, V.K.; Fuentes, M.M. Microplastic at nesting grounds used by the northern Gulf of Mexico loggerhead recovery unit. Mar. Pollut. Bull. 2018, 131, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, G.V.; Barletta, M.; Lima, A.R.A. Use of estuarine resources by top predator fishes. How do ecological patterns affect rates of contamination by microplastics? Sci. Total Environ. 2018, 655, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Nerland, I.L.; Halsband, C.; Allan, I.; Thomas, K.V. Microplastics in Marine Environments: Occurrence, Distribution and Effects; Norsk Institutt for Vannforskning: Oslo, Norway, 2014. [Google Scholar]
- Lusher, A.; McHugh, M.; Thompson, R. Occurrence of microplastics in the gastrointestinal tract of pelagic and demersal fish from the English Channel. Mar. Pollut. Bull. 2012, 67, 94–99. [Google Scholar] [CrossRef]
- Boerger, C.M.; Lattin, G.L.; Moore, S.L.; Moore, C.J. Plastic ingestion by planktivorous fishes in the North Pacific Central Gyre. Mar. Pollut. Bull. 2010, 60, 2275–2278. [Google Scholar] [CrossRef]
- Alvarez-Zeferino, J.C.; Ojeda-Benítez, S.; Cruz-Salas, A.A.; Martínez-Salvador, C.; Vazquez Morillas, A. Dataset of quantification and classification of microplastics in Mexican sandy beaches. Data Brief 2020, 33, 106473. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gad, A.K.; Midway, S.R. Relationship of Microplastics to Body Size for Two Estuarine Fishes. Microplastics 2022, 1, 211-220. https://doi.org/10.3390/microplastics1010014
Gad AK, Midway SR. Relationship of Microplastics to Body Size for Two Estuarine Fishes. Microplastics. 2022; 1(1):211-220. https://doi.org/10.3390/microplastics1010014
Chicago/Turabian StyleGad, Ahmed K., and Stephen R. Midway. 2022. "Relationship of Microplastics to Body Size for Two Estuarine Fishes" Microplastics 1, no. 1: 211-220. https://doi.org/10.3390/microplastics1010014





