The Utility of the Shock Index for Predicting Survival, Function and Health Status Outcomes in Major Trauma Patients: A Registry-Based Cohort Study
Abstract
:1. Introduction
2. Methods
2.1. Study Design
2.2. Study Setting and Participants
2.3. Patient Selection
2.4. Procedures
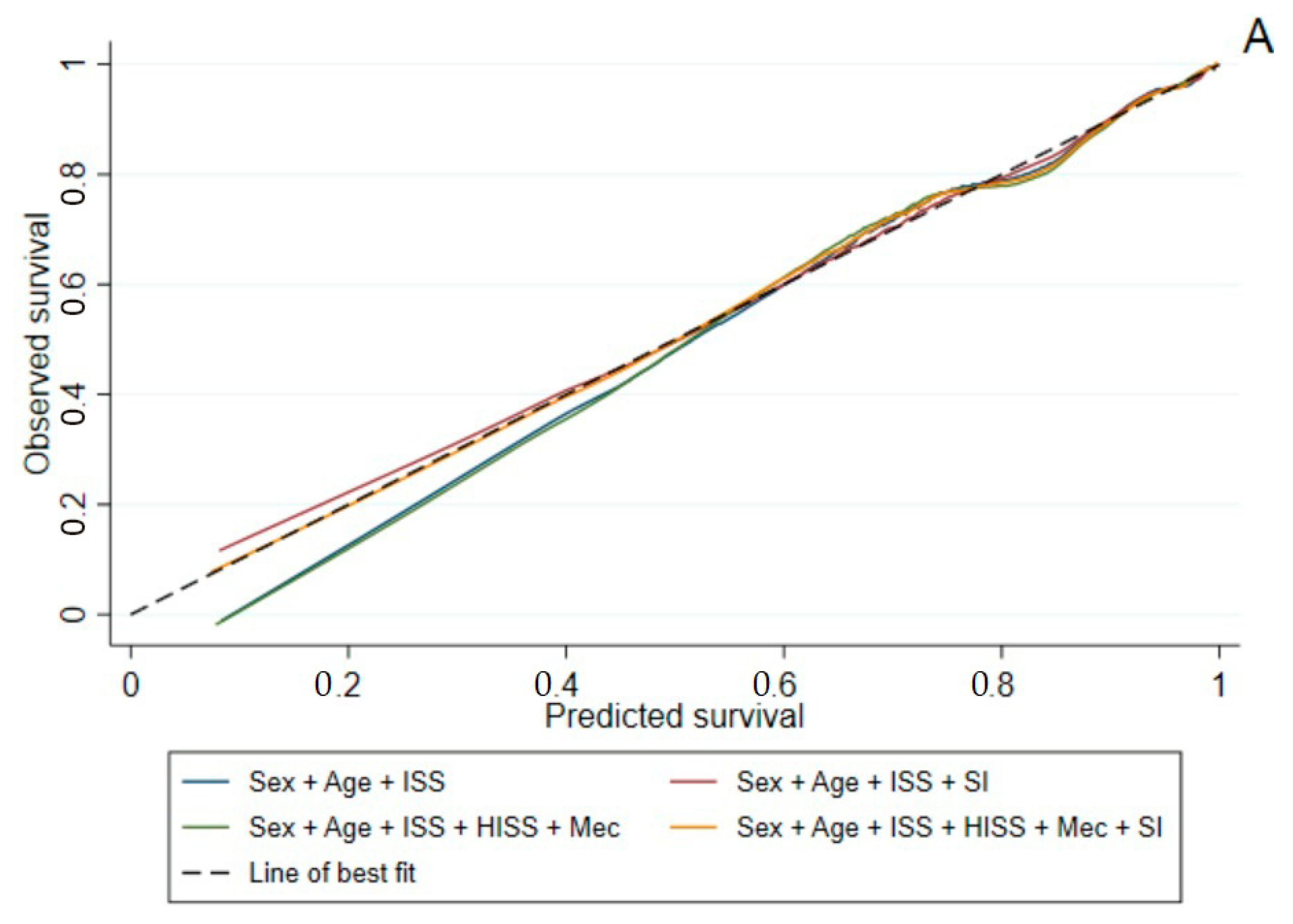
2.5. Statistical Analysis
3. Results
3.1. Overview of the Dataset
3.2. Contribution of Shock Index to Prediction of Outcomes
3.2.1. Survival to Hospital Discharge
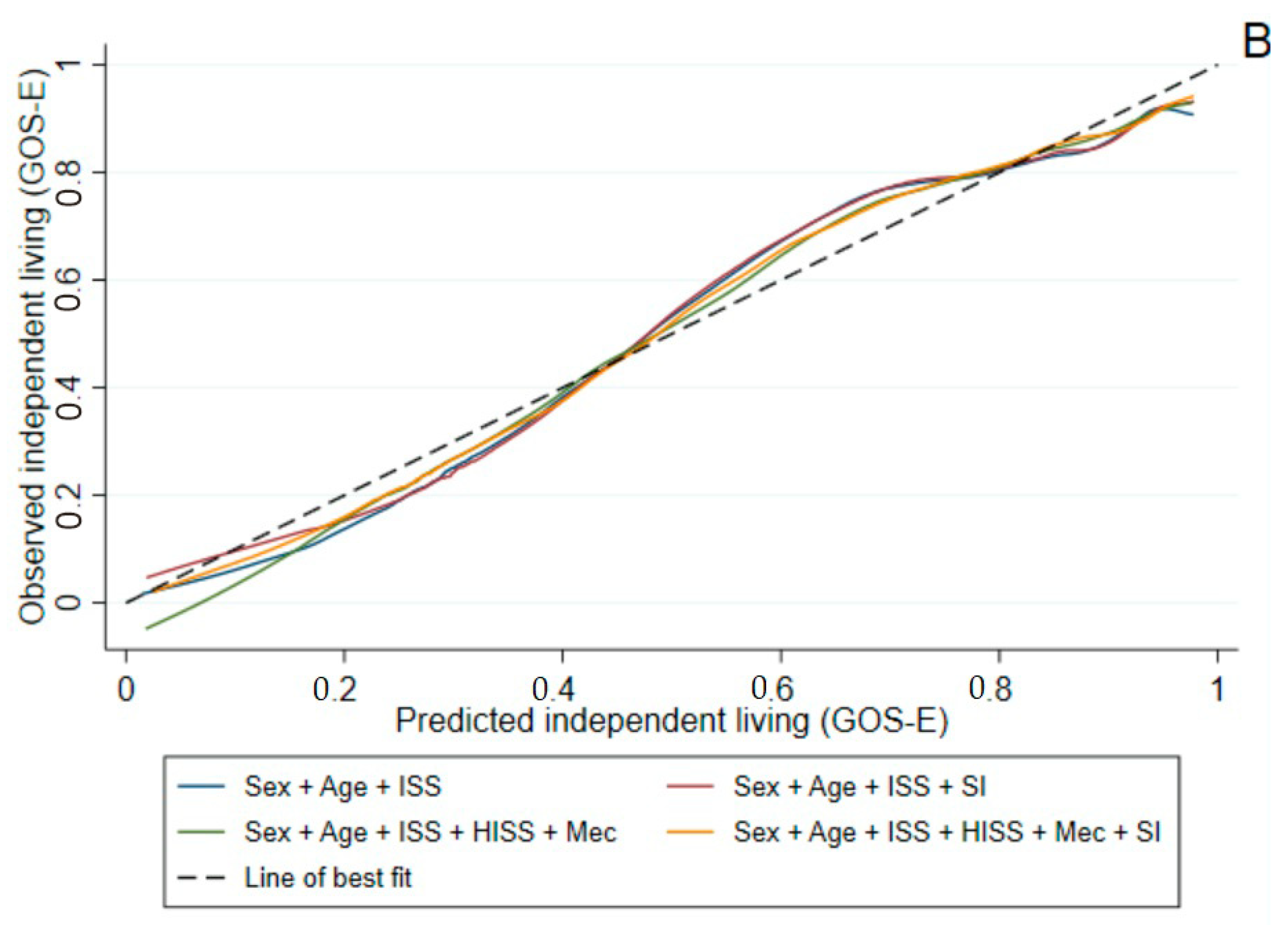
3.2.2. GOS-E at Six Months Post-Injury
3.2.3. EQ-5D-3L Summary Score at Six Months Post-Injury
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Kauvar, D.S.; Wade, C.E. The epidemiology and modern management of traumatic hemorrhage: US and international perspectives. Crit. Care 2005, 9, S1–S9. [Google Scholar] [CrossRef] [Green Version]
- Goldman, S.; Siman-Tov, M.; Bahouth, H.; Kessel, B.; Klein, Y.; Michaelson, M.; Miklosh, B.; Rivkind, A.; Shaked, G.; Simon, D.; et al. The Contribution of the Israeli Trauma System to the Survival of Road Traffic Casualties. Traffic Inj. Prev. 2014, 16, 368–373. [Google Scholar] [CrossRef]
- Cameron, P.A.; Gabbe, B.J.; Cooper, D.J.; Walker, T.; Judson, R.; McNeil, J. A statewide system of trauma care in Victoria: Effect on patient survival. Med. J. Aust. 2008, 189, 546–550. [Google Scholar] [CrossRef]
- Alharbi, R.J.; Shrestha, S.; Lewis, V.; Miller, C. The effectiveness of trauma care systems at different stages of development in reducing mortality: A systematic review and meta-analysis. World J. Emerg. Surg. 2021, 16, 38. [Google Scholar] [CrossRef]
- Champion, H.R.; Sacco, W.J.; Copes, W.S.; Gann, D.S.; Gennarelli, T.A.; Flanagan, M.E. A revision of the Trauma Score. J. Trauma-Inj. Infect. Crit. Care 1989, 29, 623–629. [Google Scholar] [CrossRef]
- Boyd, C.R.; Tolson, M.A.; Copes, W.S. Evaluating trauma care: The TRISS method. Trauma Score and the Injury Severity Score. J. Trauma-Inj. Infect. Crit. Care 1987, 27, 370–378. [Google Scholar] [CrossRef]
- Gabbe, B.J.; Cameron, P.A.; Wolfe, R. TRISS: Does it get better than this? Acad. Emerg. Med. 2004, 11, 181–186. [Google Scholar] [CrossRef]
- Gabbe, B.J.; Cameron, P.A.; Finch, C.F. Is the revised trauma score still useful? ANZ J. Surg. 2003, 73, 944–948. [Google Scholar] [CrossRef]
- Demetriades, D.; Chan, L.S.; Velmahos, G.; Berne, T.V.; Cornwell, E.E., III; Belzberg, H.; Asensio, J.A.; Murray, J.; Berne, J.; Shoemaker, W. TRISS methodology in trauma: The need for alternatives. Br. J. Surg. 1998, 85, 379–384. [Google Scholar] [CrossRef]
- Tisherman, S.A.; Schmicker, R.H.; Brasel, K.J.; Bulger, E.M.; Kerby, J.D.; Minei, J.P.; Powell, J.L.; Reiff, D.A.; Rizoli, S.B.; Schreiber, M.A. Detailed Description of All Deaths in Both the Shock and Traumatic Brain Injury Hypertonic Saline Trials of the Resuscitation Outcomes Consortium. Ann. Surg. 2015, 261, 586–590. [Google Scholar] [CrossRef] [Green Version]
- Olaussen, A.; Blackburn, T.; Mitra, B.; Fitzgerald, M. Shock index for prediction of critical bleeding post-trauma: A systematic review. Emerg. Med. Australas. 2014, 26, 223–228. [Google Scholar] [CrossRef]
- Allgower, M.; Burri, C. Shockindex. Dtsch. Med. Wochenschr. 1967, 92, 1947–1950. [Google Scholar]
- El-Menyar, A.; Goyal, P.; Tilley, E.; Latifi, R. The clinical utility of shock index to predict the need for blood transfusion and outcomes in trauma. J. Surg. Res. 2018, 227, 52–59. [Google Scholar] [CrossRef]
- Wang, I.-J.; Bae, B.-K.; Park, S.-W.; Cho, Y.-M.; Lee, D.-S.; Min, M.-K.; Ryu, J.-H.; Kim, G.-H.; Jang, J.-H. Pre-hospital modified shock index for prediction of massive transfusion and mortality in trauma patients. Am. J. Emerg. Med. 2019, 38, 187–190. [Google Scholar] [CrossRef]
- Sahu, N.; Yee, S.; Das, M.; Trinh, S.; Amoruso, R.; Connolly, M.; Rama, A.; Zuberi, J. Shock Index as a Marker for Mortality Rates in Those Admitted to the Medical Intensive Care Unit from the Emergency Department. Cureus 2020, 12, e7903. [Google Scholar] [CrossRef]
- Hashmi, A.; Rhee, P.; Pandit, V.; Kulvatunyou, N.; Tang, A.; O’Keeffe, T.; Zangbar, B.; Wynne, J.; Gries, L.; Vercruysse, G.; et al. Shock Index Predicts Mortality in Geriatric Trauma Patients: An Analysis of The National Trauma Data Bank. J. Surg. Res. 2014, 186, 687. [Google Scholar] [CrossRef]
- Jouini, S.; Jebali, A.; Hedhli, H.; Ben Kaddour, R.; Mrabet, A.; Hebaieb, F. Predictive value of shock index ≥ 1 in severe trauma patients in emergency department. La Tunis. Med. 2019, 97, 802–807. [Google Scholar]
- National, State and Territory Population [Internet]: Australien Bureau of Statistics 2020. Available online: https://www.abs.gov.au/statistics/people/population/national-state-and-territory-population/latest-release (accessed on 12 November 2020).
- Annual Report 2018–2019; Victorian State Trauma Outcomes Registry and Monitoring Group (VSTORM): Melbourne, VIC, Australia, 2020; pp. 10, 59.
- Haider, A.A.; Azim, A.; Rhee, P.; Kulvatunyou, N.; Ibraheem, K.; Tang, A.; O’Keeffe, T.; Iftikhar, H.; Vercruysse, G.; Joseph, B. Substituting systolic blood pressure with shock index in the National Trauma Triage Protocol. J. Trauma Acute Care Surg. 2016, 81, 1136–1141. [Google Scholar] [CrossRef]
- Kehoe, A.; Smith, J.E.; Edwards, A.; Yates, D.; Lecky, F. The changing face of major trauma in the UK. Emerg. Med. J. 2015, 32, 911–915. [Google Scholar] [CrossRef] [Green Version]
- Stevenson, M.; Segui-Gomez, M.; Lescohier, I.; Di Scala, C.; McDonald-Smith, G. An overview of the injury severity score and the new injury severity score. Inj. Prev. 2001, 7, 10–13. [Google Scholar] [CrossRef]
- Wilson, J.L.; Pettigrew, L.E.; Teasdale, G.M. Structured Interviews for the Glasgow Outcome Scale and the Extended Glasgow Outcome Scale: Guidelines for Their Use. J. Neurotrauma 1998, 15, 573–585. [Google Scholar] [CrossRef]
- Gabbe, B.J.; Williamson, O.D.; Cameron, P.A.; Dowrick, A.S. Choosing outcome assessment instruments for trauma registries. Acad. Emerg. Med. 2005, 12, 751–758. [Google Scholar] [CrossRef]
- Singh, R.; Venkateshwara, G.; Nair, K.; Khan, M.; Saad, R. Agitation after traumatic brain injury and predictors of outcome. Brain Inj. 2013, 28, 336–340. [Google Scholar] [CrossRef]
- Grobet, C.; Marks, M.; Tecklenburg, L.; Audigé, L. Application and measurement properties of EQ-5D to measure quality of life in patients with upper extremity orthopaedic disorders: A systematic literature review. Arch. Orthop. Trauma. Surg. 2018, 138, 953–961. [Google Scholar] [CrossRef]
- Wilson, L.; Boase, K.; Nelson, L.D.; Temkin, N.R.; Giacino, J.T.; Markowitz, A.J.; Maas, A.; Menon, D.K.; Teasdale, G.; Manley, G.T. A Manual for the Glasgow Outcome Scale-Extended Interview. J. Neurotrauma 2021, 38, 2435–2446. [Google Scholar] [CrossRef]
- Altman, D.G.; Vergouwe, Y.; Royston, P.; Moons, K.G.M. Prognosis and prognostic research: Validating a prognostic model. BMJ 2009, 338, b605. [Google Scholar] [CrossRef]
- Royston, P.; Moons, K.G.M.; Altman, D.G.; Vergouwe, Y. Prognosis and prognostic research: Developing a prognostic model. BMJ 2009, 338, b604. [Google Scholar] [CrossRef]
- Hosmer, D.W.; Lemeshow, S.; Sturdivant, R.X.; Hosmer, D.W., Jr. Applied Logistic Regression, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 12–15, 157–169, 173–177. [Google Scholar]
- Bewick, V.; Cheek, L.; Ball, J. Statistics review 7: Correlation and regression. Crit. Care 2003, 7, 451–459. [Google Scholar] [CrossRef] [Green Version]
- Kenney, J.F.; Keeping, E.S. Mathematics of Statistics, 3rd ed.; Van Nostrand: Princeton, NJ, USA, 1962. [Google Scholar]
- Bruijns, S.R.; Guly, H.R.; Bouamra, O.; Lecky, F.; Lee, W.A. The value of traditional vital signs, shock index, and age-based markers in predicting trauma mortality. J. Trauma Acute Care Surg. 2013, 74, 1432–1437. [Google Scholar] [CrossRef]
- Zarzaur, B.L.; Croce, M.A.; Magnotti, L.J.; Fabian, T.C. Identifying Life-Threatening Shock in the Older Injured Patient: An Analysis of the National Trauma Data Bank. J. Trauma Inj. Infect. Crit. Care 2010, 68, 1134–1138. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.-C.; Rau, C.-S.; Kuo, S.C.H.; Chien, P.-C.; Hsieh, H.-Y.; Hsieh, C.-H. The Reverse Shock Index Multiplied by Glasgow Coma Scale Score (rSIG) and Prediction of Mortality Outcome in Adult Trauma Patients: A Cross-Sectional Analysis Based on Registered Trauma Data. Int. J. Environ. Res. Public Health 2018, 15, 2346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shibahashi, K.; Sugiyama, K.; Okura, Y.; Hoda, H.; Hamabe, Y. Can the shock index be a reliable predictor of early mortality after trauma in older patients? A retrospective cohort study. Acute Med. Surg. 2019, 6, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Filipescu, R.; Powers, C.; Yu, H.; Yu, J.; Rothstein, D.H.; Harmon, C.M.; Clemency, B.; Guo, W.A.; Bass, K.D. Improving the performance of the Revised Trauma Score using Shock Index, Peripheral Oxygen Saturation, and Temperature–a National Trauma Database study 2011 to 2015. Surgery 2020, 167, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Kruithof, N.; de Jongh, M.; de Munter, L.; Lansink, K.; Polinder, S. The effect of socio-economic status on non-fatal outcome after injury: A systematic review. Injury 2017, 48, 578–590. [Google Scholar] [CrossRef]
- Willemse-van Son, A.H.; Ribbers, G.M.; Verhagen, A.P.; Stam, H.J. Prognostic factors of long-term functioning and productivity after traumatic brain injury: A systematic review of prospective cohort studies. Clin. Rehabil. 2007, 21, 1024–1037. [Google Scholar] [CrossRef]
- Gabbe, B.J.; Simpson, P.M.; Cameron, P.; Ponsford, J.; Lyons, R.A.; Collie, A.; Fitzgerald, M.; Judson, R.; Teague, W.J.; Braaf, S.; et al. Long-term health status and trajectories of seriously injured patients: A population-based longitudinal study. PLoS Med. 2017, 14, e1002322. [Google Scholar] [CrossRef]
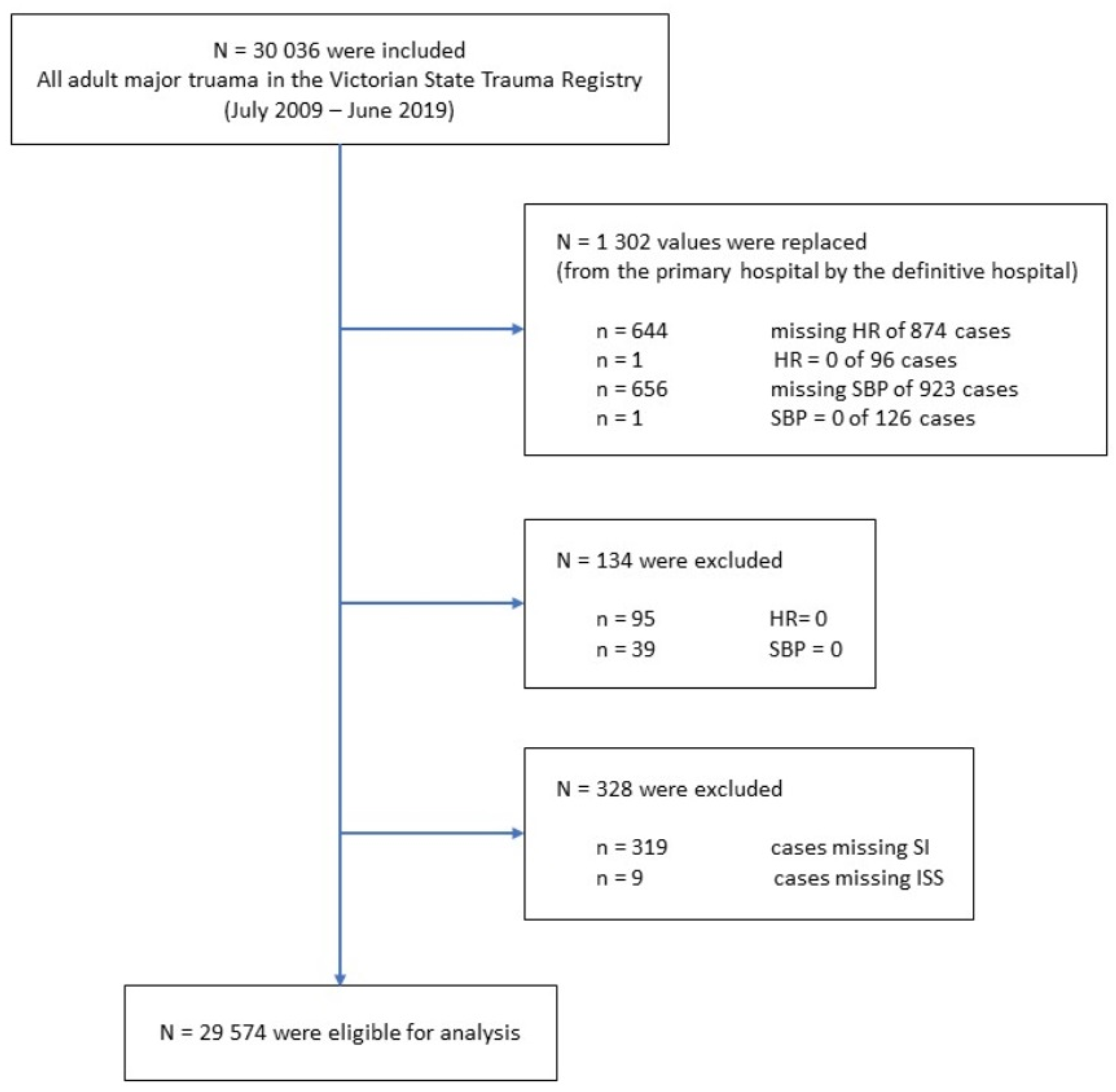
| Characteristics | Total Dataset n = 29,574 (100%) | Training Dataset n = 14,815 (50.1%) | Test Dataset n = 14,759 (49.9%) | |
|---|---|---|---|---|
| Age a | Mean (SD), years | 54.3 (±23.1) | 54.2 (±0.19) | 54.4 (±0.19) |
| Gender a | N (%) | |||
| Male | 20,707 (70.0) | 10,411 (70.3) | 10,296 (69.8) | |
| Female | 8867 (23.0) | 4404 (29.7) | 4463 (30.2) | |
| Injury Severity Score a | Median (IQR) | 17.0 (13.0–25.0) | 17 (13–25) | 17 (13–24) |
| Head Injury Severity Scale a | N (%) | |||
| No | 17,847 (60.3) | 8960 (60.5) | 8887 (60.2) | |
| Yes | 11,727 (39.7) | 5855 (39.5) | 5872 (39.8) | |
| Mechanism of Injury a | N (%) | |||
| Low fall (<1 m) | 8825 (29.8) | 4373 (29.52) | 4452 (30.2) | |
| Motor vehicle | 5747 (19.4) | 2842 (19.2) | 2905 (19.7) | |
| High fall | 3292 (11.1) | 1650 (11.1) | 1642 (11.1) | |
| Motorcycle | 2967 (10.0) | 1524 (10.3) | 1443 (9.8) | |
| Collision with person or object | 2293 (7.8) | 1175 (7.9) | 1118 (7.6) | |
| Pedestrian | 1606 (5.4) | 839 (5.7) | 767 (5.2) | |
| Pedal cyclist | 1574 (5.3) | 779 (5.3) | 795 (5.4) | |
| Other | 3270 (11.1) | 1633 (11.0) | 1637 (11.1) | |
| Shock Index a | N (%) | |||
| High SI ≥ 1 | 2230 (7.5) | 1134 (7.7) | 1096 (7.4) | |
| Low SI < 1 | 27,344 (92.5) | 13,681 (92.4) | 13,663 (92.6) | |
| Type of Injury b | N (%) | |||
| Blunt | 27,445 (92.8) | 13,722 (92.7) | 13,723 (93.0) | |
| Penetrating | 1193 (4.0) | 618 (4.2) | 575 (3.9) | |
| Burn | 638 (2.2) | 334 (2.3) | 304 (2.1) | |
| Other | 290 (1.0) | 135 (0.9) | 155 (1.1) | |
| Glasgow Coma Scale Score Group c | N (%) | |||
| 3–8 | 2568 (8.9) | 1288 (8.9) | 1280 (8.9) | |
| 9–12 | 1678 (5.8) | 834 (5.8) | 844 (5.9) | |
| 13–15 | 24,590 (85.3) | 12,323 (85.3) | 12,267 (85.2) | |
| Hospital Legth of Stay d | Median (IQR), days | 6.9 (3.8–12.8) | 6.9 (3.8–12.9) | 6.9 (3.9–12.8) |
| In-Hospital Mortality a | N (%) | |||
| No | 26,189 (88.6) | 13,087 (88.3) | 13,102 (88.8) | |
| Yes | 3385 (11.5) | 1728 (11.7) | 1657 (11.2) |
| Outcome | Training Dataset n = 14,815 (50.1%) | Test Dataset n = 14,759 (49.9%) | ||
|---|---|---|---|---|
| Survival to Discharge | N (%) | 29,574 (100) | ||
| No | 1728 (11.7) | 1657 (11.2) | ||
| Yes | 13,087 (88.3) | 13,102 (88.8) | ||
| GOS-E Score at 6 Months | N (% of the survivors) | 24,953 (95.3) | ||
| Dependent living | 4494 (36.0) | 4441 (35.6) | ||
| Independent living | 8000 (64.0) | 8018 (64.4) | ||
| EQ-5D-3L at 6 Months | N (% of the survivors) | 23,648 (90.3) | ||
| Mean (SD) | 0.53 (0.40) | 0.53 (0.40) |
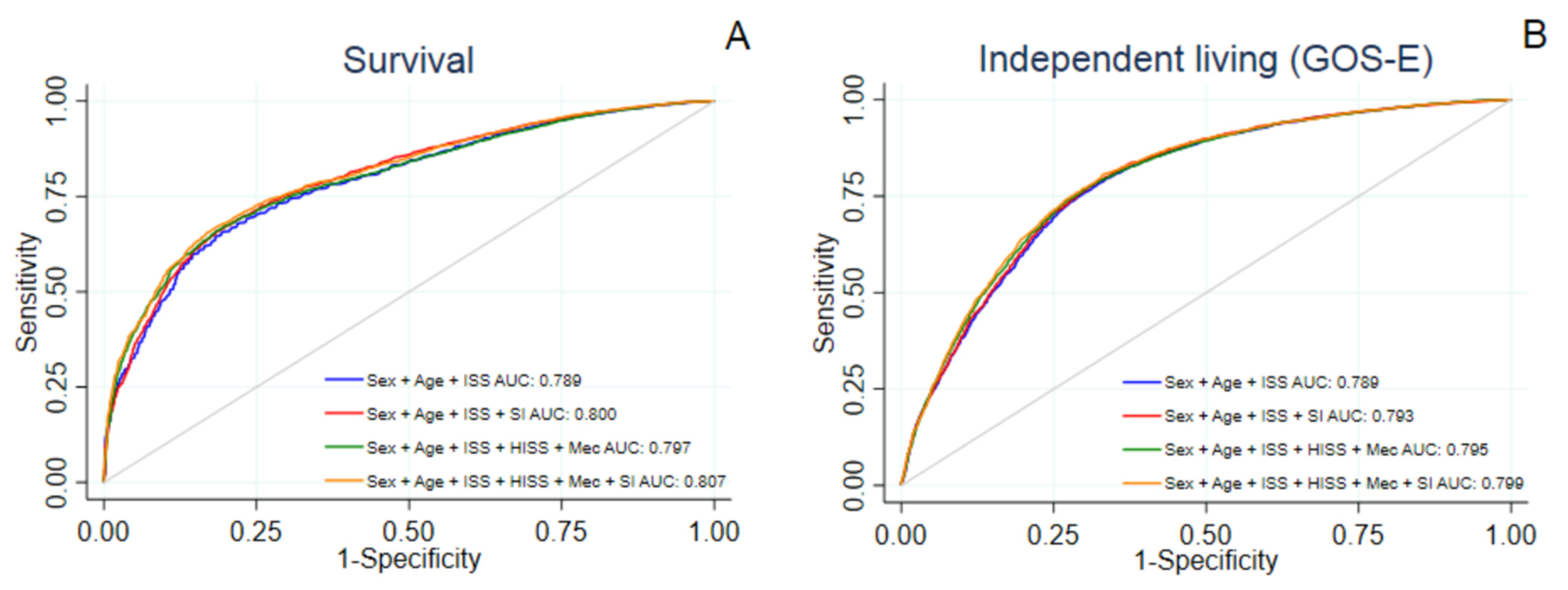
| Outcome | Prediction Model | AUC (95% CI) | H-L Statistic (p-Value) | LR-Test (p-Value) |
|---|---|---|---|---|
| Survival to Discharge | ||||
| Sex + Age + ISS | 0.789 (0.779–0.800) | 37.3 (<0.001) | - | |
| Sex + Age + ISS + SI | 0.800 (0.790–0.810) | 15.4 (0.05) | 141.0 (<0.001) * | |
| Sex + Age + ISS + HISS + Mechanism | 0.797 (0.787–0.807) | 32 (<0.001) | - | |
| Sex + Age + ISS + HISS + Mechanism + SI | 0.807 (0.797–0.816) | 38.1 (<0.001) | 146.6 (<0.001) ** | |
| TRISS | 0.764 (0.751–0.777) | 783.7 (<0.001) | - | |
| RTS | 0.696 (0.683–0.709) | 188.7 (<0.001) | - | |
| GOS-E at 6 Months | ||||
| Sex + Age + ISS | 0.789 (0.781–0.798) | 146.0 (<0.001) | - | |
| Sex + Age + ISS + SI | 0.793 (0.784–0.801) | 156.2 (<0.001) | 71.1 (<0.001) * | |
| Sex + Age + ISS + HISS + Mechanism | 0.795 (0.786–0.803) | 84.1 (<0.001) | - | |
| Sex + Age + ISS + HISS + Mechanism + SI | 0.799 (0.791–0.807) | 98.1 (<0.001) | 93.8 (<0.001) ** | |
| R2 | RMSE | |||
| EQ-5D-3L at 6 Months | ||||
| Sex + Age + ISS | 0.109 | 0.379 | ||
| Sex + Age + ISS + SI | 0.115 | 0.377 | ||
| Sex + Age + ISS + HISS + Mechanism | 0.110 | 0.379 | ||
| Sex + Age + ISS + HISS + Mechanism + SI | 0.116 | 0.377 |
| Outcome | Prediction Model | AUC (95% CI) | H-L Statistic (p-Value) |
|---|---|---|---|
| Survival to Discharge | |||
| Sex + Age + ISS | 0.774 (0.763–0.785) | 43.1 (<0.001) | |
| Sex + Age + ISS + SI | 0.789 (0.778–0.799) | 43.7 (<0.001) | |
| Sex + Age + ISS + HISS + Mechanism | 0.783 (0.773–0.794) | 41.3 (<0.001) | |
| Sex + Age + ISS + HISS + Mechanism + SI | 0.799 (0.788–0.809) | 27.1 (<0.001) | |
| TRISS | 0.768 (0.755–0.781) | 656.6 (<0.001) | |
| RTS | 0.703 (0.690–0.716) | 203.1 (<0.001) | |
| GOS-E at 6 Months | |||
| Sex + Age + ISS | 0.791 (0.782–0.799) | 178.0 (<0.001) | |
| Sex + Age + ISS + SI | 0.796 (0.787–0.804) | 178.6 (<0.001) | |
| Sex + Age + ISS + HISS + Mechanism | 0.798 (0.789–0.806) | 115.2 (<0.001) | |
| Sex + Age + ISS + HISS + Mechanism + SI | 0.803 (0.795–0.812) | 122.0 (<0.001) | |
| R2 | RMSE | ||
| EQ-5D-3L at 6 Months | |||
| Sex + Age + ISS | 0.112 | 0.370 | |
| Sex + Age + ISS + SI | 0.123 | 0.371 | |
| Sex + Age + ISS + HISS + Mechanism | 0.113 | 0.372 | |
| Sex + Age + ISS + HISS + Mechanism + SI | 0.123 | 0.373 |
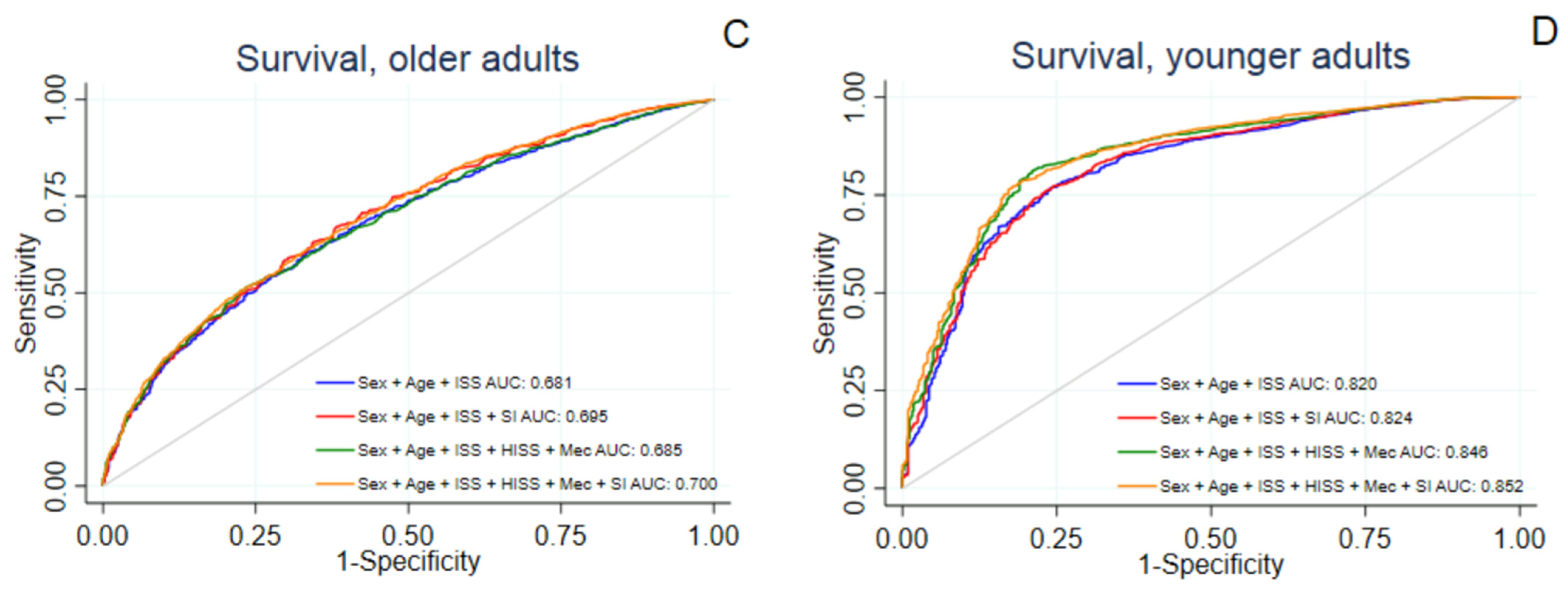
| Outcome | Prediction Model | AUC (95% CI) | H-L Statistic (p-Value) | LR-Test (p-Value) |
|---|---|---|---|---|
| Survival to Discharge | ||||
| Sex + Age + ISS | 0.681 (0.665–0.697) | 10.3 (0.24) | - | |
| Sex + Age + ISS + SI | 0.695 (0.679–0.711) | 7.8 (0.46) | 82.0 (<0.001) * | |
| Sex + Age + ISS + HISS + Mechanism | 0.685 (0.669–0.700) | 14.8 (0.064) | - | |
| Sex + Age + ISS + HISS + Mechanism + SI | 0.699 (0.683–0.715) | 12.2 (0.14) | 81.1 (<0.001) ** | |
| GOS-E at 6 Months | ||||
| Sex + Age + ISS | 0.751 (0.737–0.764) | 10.5 (0.23) | ||
| Sex + Age + ISS + SI | 0.753 (0.740–0.767) | 7.7 (0.47) | ||
| Sex + Age + ISS + HISS + Mechanism | 0.758 (0.744–0.771) | 9.0 (0.34) | ||
| Sex + Age + ISS + HISS + Mechanism + SI | 0.761 (0.748–0.775) | 8.9 (0.35) | ||
| R2 | RMSE | |||
| EQ-5D-3L at 6 Months | ||||
| Sex + Age + ISS | 0.095 | 0.392 | ||
| Sex + Age + ISS + SI | 0.102 | 0.390 | ||
| Sex + Age + ISS + HISS + Mechanism | 0.096 | 0.392 | ||
| Sex + Age + ISS + HISS + Mechanism + SI | 0.102 | 0.390 |
| Outcome | Prediction Model | AUC (95% CI) | H-L Statistic (p-Value) | LR-Test (p-Value) |
|---|---|---|---|---|
| Survival to Discharge | ||||
| Sex + Age + ISS | 0.820 (0.800–0.841) | 59.2 (<0.001) | - | |
| Sex + Age + ISS + SI | 0.824 (0.804–0.845) | 50.5 (<0.001) | 19.1 (<0.001) * | |
| Sex + Age + ISS + HISS + Mechanism | 0.846 (0.826–0.865) | 42.2 (<0.001) | - | |
| Sex + Age + ISS + HISS + Mechanism + SI | 0.852 (0.833–0.870) | 19.6 (0.012) | 28.7 (<0.001) ** | |
| GOS-E at 6 Months | ||||
| Sex + Age + ISS | 0.694 (0.678–0.710) | 74.6 (<0.001) | - | |
| Sex + Age + ISS + SI | 0.698 (0.683–0.714) | 49.3 (<0.001) | 22.6 (<0.001) * | |
| Sex + Age + ISS + HISS + Mechanism | 0.707 (0.691–0.722) | 47.3 (<0.001) | - | |
| Sex + Age + ISS + HISS + Mechanism + SI | 0.711 (0.695–0.727) | 28.9 (<0.001) | 31.8 (<0.001) ** | |
| R2 | RMSE | |||
| EQ-5D-3L at 6 Months | ||||
| Sex + Age + ISS | 0.066 | 0.361 | ||
| Sex + Age + ISS + SI | 0.071 | 0.361 | ||
| Sex + Age + ISS + HISS + Mechanism | 0.070 | 0.361 | ||
| Sex + Age + ISS + HISS + Mechanism + SI | 0.074 | 0.360 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wikström, L.; Kander, T.; Gabbe, B.J. The Utility of the Shock Index for Predicting Survival, Function and Health Status Outcomes in Major Trauma Patients: A Registry-Based Cohort Study. Trauma Care 2022, 2, 268-281. https://doi.org/10.3390/traumacare2020023
Wikström L, Kander T, Gabbe BJ. The Utility of the Shock Index for Predicting Survival, Function and Health Status Outcomes in Major Trauma Patients: A Registry-Based Cohort Study. Trauma Care. 2022; 2(2):268-281. https://doi.org/10.3390/traumacare2020023
Chicago/Turabian StyleWikström, Lena, Thomas Kander, and Belinda J. Gabbe. 2022. "The Utility of the Shock Index for Predicting Survival, Function and Health Status Outcomes in Major Trauma Patients: A Registry-Based Cohort Study" Trauma Care 2, no. 2: 268-281. https://doi.org/10.3390/traumacare2020023





