Clinical Applications of Isothermal Diagnosis for Human Schistosomiasis
Abstract
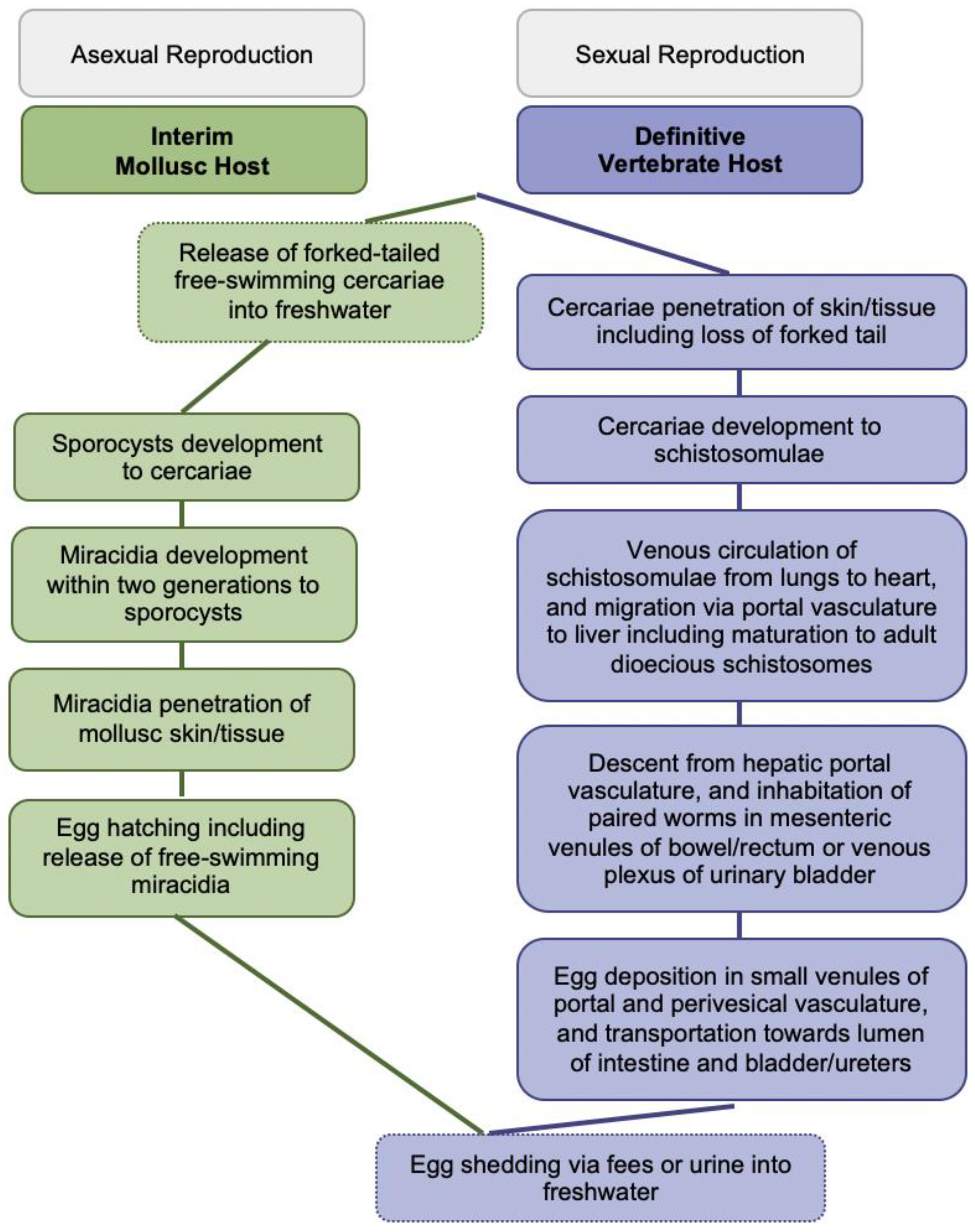
:1. Background
2. Schistosoma japonicum Assays
3. Schistosoma haematobium Assays
4. Schistosoma mansoni Assays
5. Assays on Multiple Schistosoma Species including Hybrids
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Avendaño, C.; Patarroyo, M. Loop-Mediated Isothermal Amplification as Point-of-Care Diagnosis for Neglected Parasitic Infections. Int. J. Mol. Sci. 2020, 21, 7981. [Google Scholar] [CrossRef]
- Diego, J.G.-B.; Fernández-Soto, P.; Febrer-Sendra, B.; Crego-Vicente, B.; Muro, A. Loop-Mediated Isothermal Amplification in Schistosomiasis. J. Clin. Med. 2021, 10, 511. [Google Scholar] [CrossRef]
- Panzner, U.; Boissier, J. Natural intra- and intercalde human hybrid schostosomes in Africa with considerations on prevention through vaccination Microorganisms. Microorganism 2021, 9, 1465. [Google Scholar] [CrossRef]
- Nelwan, M.L. Schistosomiasis: Life Cycle, Diagnosis, and Control. Curr. Ther. Res. 2019, 91, 5–9. [Google Scholar] [CrossRef]
- Colley, D.G.; Bustinduy, A.L.; Secor, W.E.; King, C.H. Human schistosomiasis. Lancet 2014, 383, 2253–2264. [Google Scholar] [CrossRef]
- Patwary, F.K.; Archer, J.; Sturt, A.S.; Webb, E.L. Female Genital Schistosomiasis: Diagnostic Validation for Recombinant DNA-Polymerase-Amplification Assay using Cervicovaginal Lavage. Int. J. Obstet. Gynaecol. 2021, 128 (Suppl. S2), 248. [Google Scholar]
- Le, L.; Hsieh, M.H. Diagnosing Urogenital Schistosomiasis: Dealing with Diminishing Returns. Trends Parasitol. 2017, 33, 378–387. [Google Scholar] [CrossRef]
- Panzner, U.; Excler, J.L.; Kim, H.J. Recent advances and methodological considerations on vaccine candidates for human schistosomiasis Front. Trop. Dis. 2021, 2, 719369. [Google Scholar]
- Gandasegui, J.; Fernández-Soto, P.; Carranza-Rodríguez, C.; Perez-Arellano, J.-L.; Vicente, B.; López-Abán, J.; Muro, A. The Rapid-Heat LAMPellet Method: A Potential Diagnostic Method for Human Urogenital Schistosomiasis. PLoS Negl. Trop. Dis. 2015, 9, e0003963. [Google Scholar] [CrossRef]
- Rosser, A.; Rollinson, D.; Forrest, M.S.; Webster, B.L. Isothermal Recombinase Polymerase amplification (RPA) of Schistosoma haematobium DNA and oligochromatographic lateral flow detection. Parasites Vectors 2015, 8, 446. [Google Scholar] [CrossRef]
- Archer, J.; Barksby, R.; Pennance, T.; Rostron, P.; Bakar, F.; Knopp, S.; Allan, F.; Kabole, F.; Ali, S.M.; Ame, S.M.; et al. Analytical and Clinical Assessment of a Portable, Isothermal Recombinase Polymerase Amplification (RPA) Assay for the Molecular Diagnosis of Urogenital Schistosomiasis. Molecules 2020, 25, 4175. [Google Scholar] [CrossRef]
- Bayoumi, A.; Al-Refai, S.A.; Badir, M.S.; El-Aal, A.A.A.; El Akkad, D.M.H.; Saad, N.; Elesaily, K.M.; Aziz, I.Z.A. Loop-Mediated Isothermal Amplification (Lamp): Sensitive and Rapid Detection of Schistosoma Haematobium DNA in Urine Samples of Egyptian Suspected Cases. J. Egypt Soc. Parasitol. 2016, 46, 299–308. [Google Scholar]
- Eyoh, E.; McCallum, P.; Killick, J.; Amanfo, S.; Mutapi, F.; Astier, A.L. The anthelmintic drug praziquantel promotes human Tr1 differentiation. Immunol. Cell Biol. 2019, 97, 512–518. [Google Scholar] [CrossRef]
- Song, J.; Liu, C.; Mauk, M.G.; Rankin, S.C.; Lok, J.B.; Greenberg, R.M.; Bau, H.H. Two-Stage Isothermal Enzymatic Amplification for Concurrent Multiplex Molecular Detection. Clin. Chem. 2017, 63, 714–722. [Google Scholar] [CrossRef]
- Zhao, G.-H.; Li, J.; Blair, D.; Li, X.-Y.; Elsheikha, H.M.; Lin, R.-Q.; Zou, F.-C.; Zhu, X.-Q. Biotechnological advances in the diagnosis, species differentiation and phylogenetic analysis of Schistosoma spp. Biotechnol. Adv. 2012, 30, 1381–1389. [Google Scholar] [CrossRef]
- Mosquera-Romero, M.; Zuluaga-Idárraga, L.; Tobón-Castaño, A. Challenges for the diagnosis and treatment of malaria in low transmission settings in San Lorenzo, Esmeraldas, Ecuador. Malar. J. 2018, 17, 440. [Google Scholar] [CrossRef]
- Cavalcanti, M.G.; Cunha, A.F.A.; Peralta, J.M. The Advances in Molecular and New Point-of-Care (POC) Diagnosis of Schistosomiasis Pre- and Post-praziquantel Use: In the Pursuit of More Reliable Approaches for Low Endemic and Non-endemic Areas. Front. Immunol. 2019, 10, 858. [Google Scholar] [CrossRef]
- Wang, C.; Chen, L.; Yin, X.; Hua, W.; Hou, M.; Ji, M.; Yu, C.; Wu, G. Application of DNA-based diagnostics in detection of schistosomal DNA in early infection and after drug treatment. Parasites Vectors 2011, 4, 164. [Google Scholar] [CrossRef]
- Deng, M.-H.; Zhong, L.-Y.; Kamolnetr, O.; Limpanont, Y.; Lv, Z.-Y. Detection of helminths by loop-mediated isothermal amplification assay: A review of updated technology and future outlook. Infect. Dis. Poverty 2019, 8, 20. [Google Scholar] [CrossRef]
- Poulton, K.; Webster, B. Development of a lateral flow recombinase polymerase assay for the diagnosis of Schistosoma mansoni infections. Anal. Biochem. 2018, 546, 65–71. [Google Scholar] [CrossRef]
- Lobato, I.M.; O’Sullivan, C.K. Recombinase polymerase amplification: Basics, applications and recent advances. TrAC Trends Anal. Chem. 2018, 98, 19–35. [Google Scholar] [CrossRef]
- Li, H.-M.; Qin, Z.-Q.; Bergquist, R.; Qian, M.-B.; Xia, S.; Lv, S.; Xiao, N.; Utzinger, J.; Zhou, X.-N. Nucleic acid amplification techniques for the detection of Schistosoma mansoni infection in humans and the intermediate snail host: A structured review and meta-analysis of diagnostic accuracy. Int. J. Infect. Dis. 2021, 112, 152–164. [Google Scholar] [CrossRef]
- Song, J.; Liu, C.; Bais, S.; Mauk, M.G.; Bau, H.H.; Greenberg, R.M. Molecular Detection of Schistosome Infections with a Disposable Microfluidic Cassette. PLoS Negl. Trop. Dis. 2015, 9, e0004318. [Google Scholar] [CrossRef]
- Wong, Y.-P.; Othman, S.; Lau, Y.-L.; Radu, S.; Chee, H.-Y. Loop-mediated isothermal amplification (LAMP): A versatile technique for detection of micro-organisms. J. Appl. Microbiol. 2018, 124, 626–643. [Google Scholar] [CrossRef]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, E63. [Google Scholar] [CrossRef]
- Xu, J.; Rong, R.; Zhang, H.; Shi, C.; Zhu, X.; Xia, C. Sensitive and rapid detection of Schistosoma japonicum DNA by loop-mediated isothermal amplification (LAMP). Int. J. Parasitol. 2010, 40, 327–331. [Google Scholar] [CrossRef]
- Mwangi, I.N.; Agola, E.L.; Mugambi, R.M.; Shiraho, E.A.; Mkoji, G.M. Development and Evaluation of a Loop-Mediated Isothermal Amplification Assay for Diagnosis of Schistosoma mansoni Infection in Faecal Samples. J. Parasitol. Res. 2018, 2018, 1267826. [Google Scholar] [CrossRef]
- Gandasegui, J.; Fernández-Soto, P.; Muro, A.; Barbosa, C.S.; De Melo, F.L.; Loyo, R.; Gomes, E.C.D.S. A field survey using LAMP assay for detection of Schistosoma mansoni in a low-transmission area of schistosomiasis in Umbuzeiro, Brazil: Assessment in human and snail samples. PLoS Negl. Trop. Dis. 2018, 12, e0006314. [Google Scholar] [CrossRef]
- Yansouni, C.P.; Bottieau, E.; Lutumba, P.; Winkler, A.S.; Lynen, L.; Buscher, P.; Jacobs, J.; Gillet, P.; Lejon, V.; Alirol, E.; et al. Rapid diagnostic tests for neurological infections in central Africa. Lancet Infect. Dis. 2013, 13, 546–558. [Google Scholar] [CrossRef]
- Mori, Y.; Notomi, T. Loop-mediated isothermal amplification (LAMP): A rapid, accurate, and cost-effective diagnostic method for infectious diseases. J. Infect. Chemother. 2009, 15, 62–69. [Google Scholar] [CrossRef]
- Notomi, T.; Mori, Y.; Tomita, N.; Kanda, H. Loop-mediated isothermal amplification (LAMP): Principle, features, and future prospects. J. Microbiol. 2015, 53, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Wu, Z.; Li, G.; Huang, J.; Guo, Q.; Meng, X. A DNA molecular diagnostic technology with LAMP-like sensitivity based on one pair of hairpin primers-mediated isothermal polymerization amplification. Anal. Chim. Acta 2020, 1134, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Kaiglová, A.; Beňo, P.; Changoma, M.J.S. Detection of schistosomiasis applicable for primary health care facilities in endemic regions of Africa. Biologia 2017, 72, 1113–1120. [Google Scholar] [CrossRef]
- Nagamine, K.; Hase, T.; Notomi, T. Accelerated reaction by loop-mediated isothermal amplification using loop primers. Mol. Cell. Probes 2002, 16, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Nagamine, K.; Tomita, N.; Notomi, T. Detection of Loop-Mediated Isothermal Amplification Reaction by Turbidity Derived from Magnesium Pyrophosphate Formation. Biochem. Biophys. Res. Commun. 2001, 289, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Tomita, N.; Mori, Y.; Kanda, H.; Notomi, T. Loop-mediated isothermal amplification (LAMP) of gene sequences and simple visual detection of products. Nat. Protoc. 2008, 3, 877–882. [Google Scholar] [CrossRef]
- Zhang, X.; Lowe, S.B.; Gooding, J.J. Brief review of monitoring methods for loop-mediated isothermal amplification (LAMP). Biosens. Bioelectron. 2014, 61, 491–499. [Google Scholar] [CrossRef]
- Weerakoon, K.; Gobert, G.N.; Cai, P.; McManus, D.P. Advances in the Diagnosis of Human Schistosomiasis. Clin. Microbiol. Rev. 2015, 28, 939–967. [Google Scholar] [CrossRef]
- Kaneko, H.; Kawana, T.; Fukushima, E.; Suzutani, T. Tolerance of loop-mediated isothermal amplification to a culture medium and biological substances. J. Biochem. Biophys. Methods 2007, 70, 499–501. [Google Scholar] [CrossRef]
- Francois, P.; Tangomo, M.; Hibbs, J.; Bonetti, E.-J.; Boehme, C.C.; Notomi, T.; Perkins, M.D.; Schrenzel, J. Robustness of a loop-mediated isothermal amplification reaction for diagnostic applications. FEMS Immunol. Med. Microbiol. 2011, 62, 41–48. [Google Scholar] [CrossRef]
- Daher, R.K.; Stewart, G.; Boissinot, M.; Bergeron, M.G. Recombinase Polymerase Amplification for Diagnostic Applications. Clin. Chem. 2016, 62, 947–958. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Guo, Q.; Fu, Z.; Liu, J.; Lin, J.; Xiao, K.; Sun, P.; Cong, X.; Liu, R.; Hong, Y. Reviews and advances in diagnostic research on Schistosoma japonicum. Acta Trop. 2020, 213, 105743. [Google Scholar] [CrossRef] [PubMed]
- Tani, H.; Teramura, T.; Adachi, K.; Tsuneda, S.; Kurata, S.; Nakamura, K.; Kanagawa, T.; Noda, N. Technique for Quantitative Detection of Specific DNA Sequences Using Alternately Binding Quenching Probe Competitive Assay Combined with Loop-Mediated Isothermal Amplification. Anal. Chem. 2007, 79, 5608–5613. [Google Scholar] [CrossRef] [PubMed]
- Xing, W.; Yu, X.; Feng, J.; Sun, K.; Fu, W.; Wang, Y.; Zou, M.; Xia, W.; Luo, Z.; He, H.; et al. Field evaluation of a recombinase polymerase amplification assay for the diagnosis of Schistosoma japonicum infection in Hunan province of China. BMC Infect. Dis. 2017, 17, 164. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Song, L.-G.; Xie, H.; Liang, J.-Y.; Yuan, D.-Y.; Wu, Z.-D.; Lv, Z.-Y. Nucleic acid detection in the diagnosis and prevention of schistosomiasis. Infect. Dis. Poverty 2016, 5, 25. [Google Scholar] [CrossRef]
- Guo, J.-J.; Zheng, H.-J.; Xu, J.; Zhu, X.-Q.; Wang, S.-Y.; Xia, C.-M. Sensitive and Specific Target Sequences Selected from Retrotransposons of Schistosoma japonicum for the Diagnosis of Schistosomiasis. PLoS Negl. Trop. Dis. 2012, 6, e1579. [Google Scholar] [CrossRef]
- Xu, J.; Guan, Z.-X.; Zhao, B.; Wang, Y.-Y.; Cao, Y.; Zhang, H.-Q.; Zhu, X.-Q.; He, Y.-K.; Xia, C.-M. DNA Detection of Schistosoma japonicum: Diagnostic Validity of a LAMP Assay for Low-Intensity Infection and Effects of Chemotherapy in Humans. PLoS Negl. Trop. Dis. 2015, 9, e0003668. [Google Scholar] [CrossRef]
- WHO Expert Committee. Prevention and Control of Schistosomiasis and Soil-Transmitted Helminthiasis; World Health Organization: Geneva, Switzerland, 2002; pp. 1–57.
- Sun, K.; Xing, W.; Yuanyuan, W.; Fu, W.; Wang, Y.; Zou, M.; Luo, Z.; Xu, D. Recombinase polymerase amplification combined with a lateral flow dipstick for rapid and visual detection of Schistosoma japonicum. Parasites Vectors 2016, 9, 476. [Google Scholar] [CrossRef]
- Kane, R.A.; Rollinson, D. Comparison of the intergenic spacers and 3′ end regions of the large subunit (28S) ribosomal RNA gene from three species of Schistosoma. Parasitology 1998, 117, 235–242. [Google Scholar] [CrossRef]
- Gandasegui, J.; Fernández-Soto, P.; DaCal, E.; Rodríguez, E.; Saugar, J.M.; Yepes, E.; Aznar, M.L.; Espasa, M.; Ninda, A.; Bocanegra, C.; et al. Field and laboratory comparative evaluation of a LAMP assay for the diagnosis of urogenital schistosomiasis in Cubal, Central Angola. Trop. Med. Int. Health 2018, 23, 992–1001. [Google Scholar] [CrossRef]
- Fernández-Soto, P.; Gandasegui, J.; Rodríguez, C.C.; Pérez-Arellano, J.L.; Vicente, B.C.; Diego, J.G.-B.; López-Abán, J.; Vicente, B.; Muro, A. Detection of Schistosoma mansoni-derived DNA in human urine samples by loop-mediated isothermal amplification (LAMP). PLoS ONE 2019, 14, e0214125. [Google Scholar] [CrossRef] [PubMed]
- Wichmann, D.; Panning, M.; Quack, T.; Kramme, S.; Burchard, G.-D.; Grevelding, C.; Drosten, C. Diagnosing Schistosomiasis by Detection of Cell-Free Parasite DNA in Human Plasma. PLoS Negl. Trop. Dis. 2009, 3, e422. [Google Scholar] [CrossRef] [PubMed]
- Ibironke, O.A.; Shiff, C.; Garba, A.; Phillips, A.E.; Lamine, S.M. Diagnosis of Schistosoma haematobium by Detection of Specific DNA Fragments from Filtered Urine Samples. Am. J. Trop. Med. Hyg. 2011, 84, 998–1001. [Google Scholar] [CrossRef] [PubMed]
- Rostron, P.; Pennance, T.; Bakar, F.; Rollinson, D.; Knopp, S.; Allan, F.; Kabole, F.; Ali, S.M.; Ame, S.M.; Webster, B.L. Development of a recombinase polymerase amplification (RPA) fluorescence assay for the detection of Schistosoma haematobium. Parasites Vectors 2019, 12, 514. [Google Scholar] [CrossRef]
- Frimpong, M.; Kyei-Tuffuor, L.; Fondjo, L.A.; Ahor, H.S.; Adjei-Kusi, P.; Maiga-Ascofare, O.; Phillips, R.O. Evaluation of a real-time recombinase polymerase amplification assay for rapid detection of Schistosoma haematobium infection in resource-limited setting. Acta Trop. 2021, 216, 105847. [Google Scholar] [CrossRef]
- Jannotti-Passos, L.K.; Vidigal, T.H.D.A.; Dias-Neto, E.; Pena, S.D.J.; Simpson, A.J.G.; Dutra, W.O.; Souza, C.P.; Carvalho-Parra, J.F. PCR Amplification of the Mitochondrial DNA Minisatellite Region to Detect Schistosoma mansoni Infection in Biomphalaria glabrata Snails. J. Parasitol. 1997, 83, 395–399. [Google Scholar] [CrossRef]
- Fernández-Soto, P.; Arahuetes, J.G.; Hernández, A.S.; López-Abán, J.; Santiago, B.V.; Muro, A. A Loop-Mediated Isothermal Amplification (LAMP) Assay for Early Detection of Schistosoma mansoni in Stool Samples: A Diagnostic Approach in a Murine Model. PLoS Negl. Trop. Dis. 2014, 8, e3126. [Google Scholar] [CrossRef]
- García-Bernalt Diego, J.; Fernández-Soto, P.; Crego-Vicente, B.; Alonso-Castrillejo, S.; Febrer-Sendra, B.; Gómez-Sánchez, A.; Vicente, B.; López-Abán, J.; Muro, A. Progress in loop-mediated isothermal amplification assay for detection of Schistosoma mansoni DNA: Towards a ready-to-use test. Sci. Rep. 2019, 9, 14744. [Google Scholar] [CrossRef]
- Garcia-Bernalt, D.; Fernandez-Soto, P.; Alonso-Castrillejo, S.; Crego-Vicente, B.; Febrer, B.; Gomez, A.; Vicente, B.; Lopez-Aban, J.; Muro, A. Stabilization of SmMIT-LAMP reagents for application inn point-of-care diagnostic of schistosomiasis. Trans. R. Soc. Trop. Med. Hyg. 2019, 113 (Suppl. S1), S163. [Google Scholar]
- Camprubí, D.; Mendez, A.R.; Rodríguez, N.; Valls, M.E.; Garcia-Herrera, A.; Estrach, T.; Fustà, X.; Diego, J.G.-B.; Fernández, I.; Muñoz, J. A 38-year-old woman with zosteriform skin lesions. PLoS Negl. Trop. Dis. 2017, 11, e0005906. [Google Scholar] [CrossRef]
- Price, M.; Cyrs, A.; Sikasunge, C.S.; Mwansa, J.; Lodh, N. Testing the Infection Prevalence of Schistosoma mansoni after Mass Drug Administration by Comparing Sensitivity and Specificity of Species-Specific Repeat Fragment Amplification by PCR and Loop-Mediated Isothermal Amplification. Am. J. Trop. Med. Hyg. 2019, 101, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Lodh, N.; Mikita, K.; Bosompem, K.M.; Anyan, W.K.; Quartey, J.K.; Otchere, J.; Shiff, C.J. Point of care diagnosis of multiple schistosome parasites: Species-specific DNA detection in urine by loop-mediated isothermal amplification (LAMP). Acta Trop. 2017, 173, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Lodh, N.; Mikita, K.; Bosompem, K.M.; Anyan, W.K.; Quartey, J.K.; Otchere, J.; Shiff, C.J. Point of care diagnosis for multiple schistosome parasites: Species-specific DNA detection from single urine sample by lamp and PCR. Am. J. Trop. Med. Hyg. 2017, 97 (Suppl. S1), 233. [Google Scholar]
- Lodh, N.; Mikita, K.; Shiff, C. Lamp: Point-of -care diagnosis for Schistosoma mansoni and S. haematobium. Am. J. Trop. Med. Hyg. 2015, 93, 158–159. [Google Scholar]
- Lodh, N.; Mikita, K.; Naples, J.M.; Bosompem, K.M.; Quartey, J.; Shiff, C.J. Detecting multi schistosome species dna in single urine samples by lamp: A novel diagnostic test for schistosomiasis. Am. J. Trop. Med. Hyg. 2014, 91 (Suppl. S1), 348. [Google Scholar]
- Lodh, N.; Pulkkila, B.; Price, M.; Alhakimi, S.; Sikasunge, C.; Mwansa, J. Prevalence of due-schistosome infection among school children after MDA in Zambia: Comparison of six different diagnostics. Trop. Med. Int. Health 2021, 26 (Suppl. S1), 76–77. [Google Scholar]
- Pulkkila, B.; Sikasunge, C.S.; Mwansa, J.; Lodh, N. POC-LAMP for human schistosomes comparative cost and time analysis for variable arrangements. Am. J. Trop. Med. Hyg. 2019, 101, 213. [Google Scholar]
- Crego-Vicente, B.; Fernández-Soto, P.; Febrer-Sendra, B.; Diego, J.G.-B.; Boissier, J.; Angora, E.; Oleaga, A.; Muro, A. Application of a Genus-Specific LAMP Assay for Schistosome Species to Detect Schistosoma haematobium x Schistosoma bovis Hybrids. J. Clin. Med. 2021, 10, 1308. [Google Scholar] [CrossRef]
- Fernández-Soto, P.; Avendaño, C.; Sala-Vizcaíno, A.; Crego-Vicente, B.; Febrer-Sendra, B.; Diego, J.G.-B.; Oleaga, A.; López-Abán, J.; Vicente, B.; Patarroyo, M.A.; et al. Molecular Markers for Detecting Schistosoma Species by Loop-Mediated Isothermal Amplification. Dis. Markers 2020, 2020, 8042705. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panzner, U. Clinical Applications of Isothermal Diagnosis for Human Schistosomiasis. Encyclopedia 2022, 2, 690-704. https://doi.org/10.3390/encyclopedia2020048
Panzner U. Clinical Applications of Isothermal Diagnosis for Human Schistosomiasis. Encyclopedia. 2022; 2(2):690-704. https://doi.org/10.3390/encyclopedia2020048
Chicago/Turabian StylePanzner, Ursula. 2022. "Clinical Applications of Isothermal Diagnosis for Human Schistosomiasis" Encyclopedia 2, no. 2: 690-704. https://doi.org/10.3390/encyclopedia2020048





