Delay in COVID-19 Vaccinations: The Role of Travel Time to Vaccine Sites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Data
2.2. Regression Models
3. Results
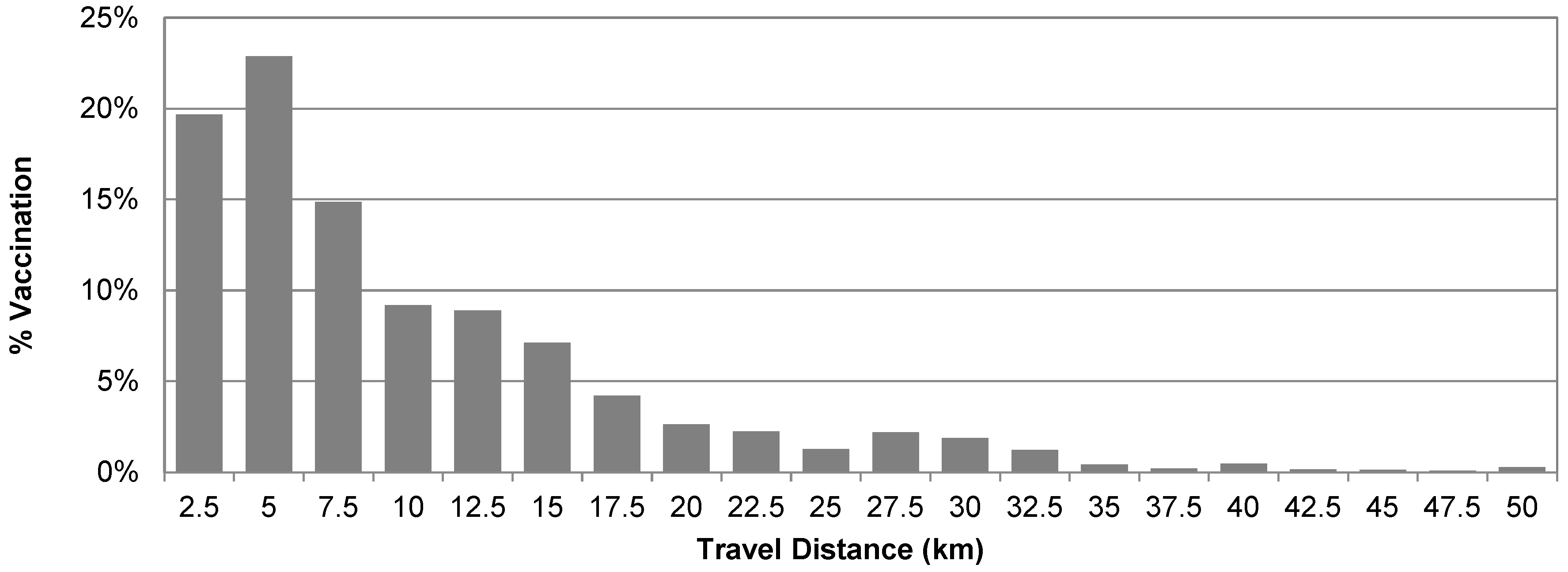
3.1. Descriptive Analysis
3.2. Model Estimation Results
3.3. Sensitivity Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Hazard-Based Duration Model
Appendix A.2. Sample Selection Bias
Appendix B

References
- Centers for Disease Control and Prevention (CDC). Stay Up to Date with COVID-19 Vaccines. Available online: https://www.cdc.gov/covid/vaccines/stay-up-to-date.html (accessed on 25 April 2025).
- Brownstein, N.C.; Reddy, H.; Whiting, J.; Kasting, M.L.; Head, K.J.; Vadaparampil, S.T.; Giuliano, A.R.; Gwede, C.K.; Meade, C.D.; Christy, S.M. COVID-19 vaccine behaviors and intentions among a national sample of United States adults ages 18-45. Prev. Med. 2022, 160, 107038. [Google Scholar] [CrossRef] [PubMed]
- Caspi, G.; Dayan, A.; Eshal, Y.; Liverant-Taub, S.; Twig, G.; Shalit, U.; Lewis, Y.; Shina, A.; Caspi, O. Socioeconomic disparities and COVID-19 vaccination acceptance: A nationwide ecologic study. Clin. Microbiol. Infect. 2021, 27, 1502–1506. [Google Scholar] [CrossRef]
- Frisco, M.L.; van Hook, J.; Thomas, K.J.A. Racial/ethnic and nativity disparities in U.S. COVID-19 vaccination hesitancy during vaccine rollout and factors that explain them. Soc. Sci. Med. 2022, 307, 115183. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Lee, J.; Jin, L.; Rahman, M.M.; Guerrero, A.; Zhao, M.; Lee, K. Sociodemographic and geographic disparities in COVID-19 booster vaccination in Nueces County, Texas, USA. Heliyon 2024, 10, e27763. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Huang, Y. COVID-19 vaccine hesitancy: The role of socioeconomic factors and spatial effects. Vaccines 2022, 10, 352. [Google Scholar] [CrossRef]
- Mondal, P.; Sinharoy, A.; Su, L. Sociodemographic predictors of COVID-19 vaccine acceptance: A nationwide US-based survey study. Public Health 2021, 198, 252–259. [Google Scholar] [CrossRef]
- Nino, M.; Hearne, B.; Cai, T. Trajectories of COVID-19 vaccine intentions among US adults: The role of race and ethnicity. SSM-Popul. Health 2021, 15, 100824. [Google Scholar] [CrossRef]
- Prickett, K.C.; Habibi, H.; Carr, P.A. COVID-19 vaccine hesitancy and acceptance in a cohort of diverse New Zealanders. Lancet Reg. Health West. Pac. 2021, 14, 100241. [Google Scholar]
- Chen, H.; Cao, Y.; Feng, L.; Chen, K.; Bei, H.; Murcio, R. Addressing the spatial disparity of COVID-19 vaccination services: A spatial optimization approach. Int. J. Appl. Earth Obs. Geoinf. 2024, 130, 103881. [Google Scholar] [CrossRef]
- MacDonald, N.E.; SAGE Working Group on Vaccine Hesitancy. Vaccine hesitancy: Definition, scope and determinants. Vaccine 2015, 33, 4161–4164. [Google Scholar] [CrossRef]
- Hardin, B.; Graboyes, M.; Kosty, D.; Cioffi, C. Vaccine decision making among people who inject drugs: Improving on the WHO’s 3C model of vaccine hesitancy. Preventive Med. Rep. 2023, 35, 102341. [Google Scholar] [CrossRef] [PubMed]
- Parisi, A.; Regazzi, L.; Spanaus, E.; Gris, A.; Cadeddu, C.; Moore, M.; Ricciardi, W.; Lomazzi, M. Impact of COVID-19 pandemic on vaccine hesitancy and sentiment changes: A survey of healthcare workers in 12 counties. Public Health 2025, 238, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Naderi, H.; Abbasian, Z.; Huang, Y. Measuring spatial accessibility of COVID-19 vaccination sites. Int. J. Manag. Appl. Sci. (IJMAS) 2023, 9, 111–114. [Google Scholar]
- Cochran, A.L.; Wang, J.; Wolfe, M.; Iacobucci, E.; Vinella-Brusher, E.; McDonald, N.C. Spatial and temporal trends in travel for COVID-19 vaccinations. AJPM Focus 2023, 2, 100122. [Google Scholar] [CrossRef]
- Cuadros, D.; Branscum, A.; Mukandavire, Z.; Miller, F.; MacKinnon, N. Dynamics of the COVID-19 epidemic in urban and rural areas in the United States. Ann. Epidemiol. 2021, 59, 16–20. [Google Scholar] [CrossRef]
- Bemanian, A.; Mosser, J.F. Investigating the spatial accessibility and coverage of the pediatric COVID-19 vaccine: An ecologic study of regional health data. Vaccines 2024, 12, 545. [Google Scholar] [CrossRef]
- Cao, Y.; Li, T.; Chen, H.; Zhao, Q.; Sun, J.; Grépin, K.A.; Kang, J. Evaluating geographic accessibility to COVID-19 vaccination across 54 countries/regions. BMJ Glob. Health 2025, 10, e017761. [Google Scholar] [CrossRef]
- Mazar, A.; Jaro, D.; Tomaino, G.; Carmon, Z.; Wood, W. Distance to vaccine sites is tied to decreased COVID-19 vaccine uptake. Proc. Natl. Acad. Sci. USA 2023, 2, pgad411. [Google Scholar] [CrossRef]
- Rader, B.; Astley, C.M.; Sewalk, K.; Delamater, P.L.; Cordiano, K.; Wronski, L.; Rivera, J.M.; Hallberg, K.; Pera, M.F.; Cantor, J.; et al. Spatial modeling of vaccine deserts as barriers to controlling SARS-CoV-2. Commun. Med. 2022, 2, 141. [Google Scholar] [CrossRef]
- Xu, Z.; Jiang, B. Effects of social vulnerability and spatial accessibility on COVID-19 vaccination coverage: A census-tract level study in Milwaukee County, USA. Int. J. Environ. Res. Public Health 2022, 19, 12304. [Google Scholar] [CrossRef]
- Chen, H.; Cao, Y.; Feng, L.; Zhao, Q. Measuring and Validating Spatial Accessibility to COVID-19 Vaccination Sites: A Case Study in England. 2022. Available online: https://osf.io/preprints/socarxiv/xvnps_v1 (accessed on 25 April 2025).
- Gligoric, K.; Kamath, C.; Weiss, D.J.; Bavadekar, S.; Liu, Y.; Shekel, T.; Schulman, K.; Gabrilovich, E. Revealed versus potential spatial accessibility of healthcare and changing patterns during the COVID-19 pandemic. Commun. Med. 2023, 3, 157. [Google Scholar] [CrossRef] [PubMed]
- Masters, N.B.; Zhou, T.; Meng, L.; Lu, P.J.; Kriss, J.L.; Black, C.; Omari, A.; Boone, K.; Weiss, D.; Carter, R.J.; et al. Geographic heterogeneity in behavioral and social drivers of COVID-19 vaccination. Am. J. Prev. Med. 2022, 63, 883–893. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Meyer, P.; Jin, L. Spatial access to health care and elderly ambulatory care sensitive hospitalizations. Public Health 2019, 169, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Mseke, E.P.; Jessup, B.; Barnett, T. Impact of distance and/or travel time on healthcare service access in rural and remote areas: A scoping review. J. Transp. Health 2024, 37, 101819. [Google Scholar] [CrossRef]
- Waller, A. Wondering Who Can Get a COVID-19 Vaccine Booster Shot and When? Here’s What Texans Need to Know. Texas Tribune. 2021. Available online: https://www.texastribune.org/2021/10/29/covid-19-vaccine-booster-shots/ (accessed on 25 April 2025).
- Neely, S.R.; Scacco, J.M. Receptiveness of American adults to COVID-19 vaccine boosters: A survey analysis. PEC Innov. 2022, 1, 100019. [Google Scholar] [CrossRef]
- Raman, S.; Kriner, D.; Ziebarth, N.; Simon, K.; Kreps, S. COVID-19 booster uptake among US adults: Assessing the impact of vaccine attributes, incentives, and context in a choice-based experiment. Soc. Sci. Med. 2022, 310, 115277. [Google Scholar] [CrossRef]
- Ioannou, G.N.; Green, P.; Locke, E.R.; Berry, K. Factors associated with early receipt of COVID-19 vaccination and adherence to second dose in the Veterans Affairs healthcare system. PLoS ONE 2021, 16, e0259696. [Google Scholar] [CrossRef]
- Bajema, K.L.; Rowneki, M.; Berry, K.; Bohnert, A.; Bowling, C.B.; Boyko, E.J.; Iwashyna, T.J.; Maciejewski, M.L.; O’Hare, A.M.; Osborne, T.F.; et al. Rates of and factors associated with primary and booster COVID-19 vaccine receipt by US veterans, December 2020 to June 2022. JAMA Netw. Open 2023, 6, e2254387. [Google Scholar] [CrossRef]
- Valckx, S.; Crevecoeur, J.; Verelst, F.; Vranckx, M.; Hendrickx, G.; Hens, N.; Van Damme, P.; Pepermans, K.; Beutels, P.; Neyens, T. Individual factors influencing COVID-19 vaccine acceptance in between and during pandemic waves (July–December 2020). Vaccine 2022, 40, 151–161. [Google Scholar] [CrossRef]
- Census Bureau. Urban and Rural. 2024. Available online: https://www.census.gov/programs-surveys/geography/guidance/geo-areas/urban-rural.html (accessed on 25 April 2025).
- Bennet, K.; Pumkam, C.; Probst, J. Rural-urban differences in the location of influenza vaccine administration. Vaccine 2011, 29, 5970–5977. [Google Scholar] [CrossRef]
- Boeing, G. Modeling and Analyzing Urban Networks and Amenities with OSMnx. 2024. Available online: https://geoffboeing.com/publications/osmnx-paper/ (accessed on 25 April 2025).
- Greene, W.H. Econometric Analysis, 8th ed.; Pearson: New York, NY, USA, 2018. [Google Scholar]
- Wooldridge, J.M. Econometric Analysis of Cross Section and Panel Data; MIT Press: Cambridge, MA, USA, 2002. [Google Scholar]
- Boehmke, F.J.; Morey, D.S.; Shannon, M. Selection bias and continuous-time duration models: Consequences and a proposed solution. Am. J. Political Sci. 2006, 50, 192–207. [Google Scholar] [CrossRef]
- Kelly, C.; Hulme, C.; Farragher, T.; Clarke, G. Are differences in travel time or distance to healthcare for adults in global north countries associated with an impact on health outcomes? A systematic review. BMJ Open 2016, 6, e013059. [Google Scholar] [CrossRef] [PubMed]
- Paul, E.; Fancourt, D. Predictors of uncertainty and unwillingness to receive the COVID-19 booster vaccine: An observational study of 22,139 fully vaccinated adults in the UK. Lancet Reg. Health Eur. 2022, 14, 100317. [Google Scholar] [CrossRef] [PubMed]
- Naderi, H.; Abbasian, Z.; Huang, Y. Disparity between expected spatial accessibility and actual travel time to vaccination sites: Implications for COVID-19 immunization delays. SSM-Population Health 2025, 30, 101804. [Google Scholar] [CrossRef]
- Chen, H.; Cao, Y.; Feng, L.; Zhao, Q.; Torres, J. Understanding the spatial heterogeneity of COVID-19 vaccine uptake in England. BMC Public Health 2023, 23, 895. [Google Scholar]
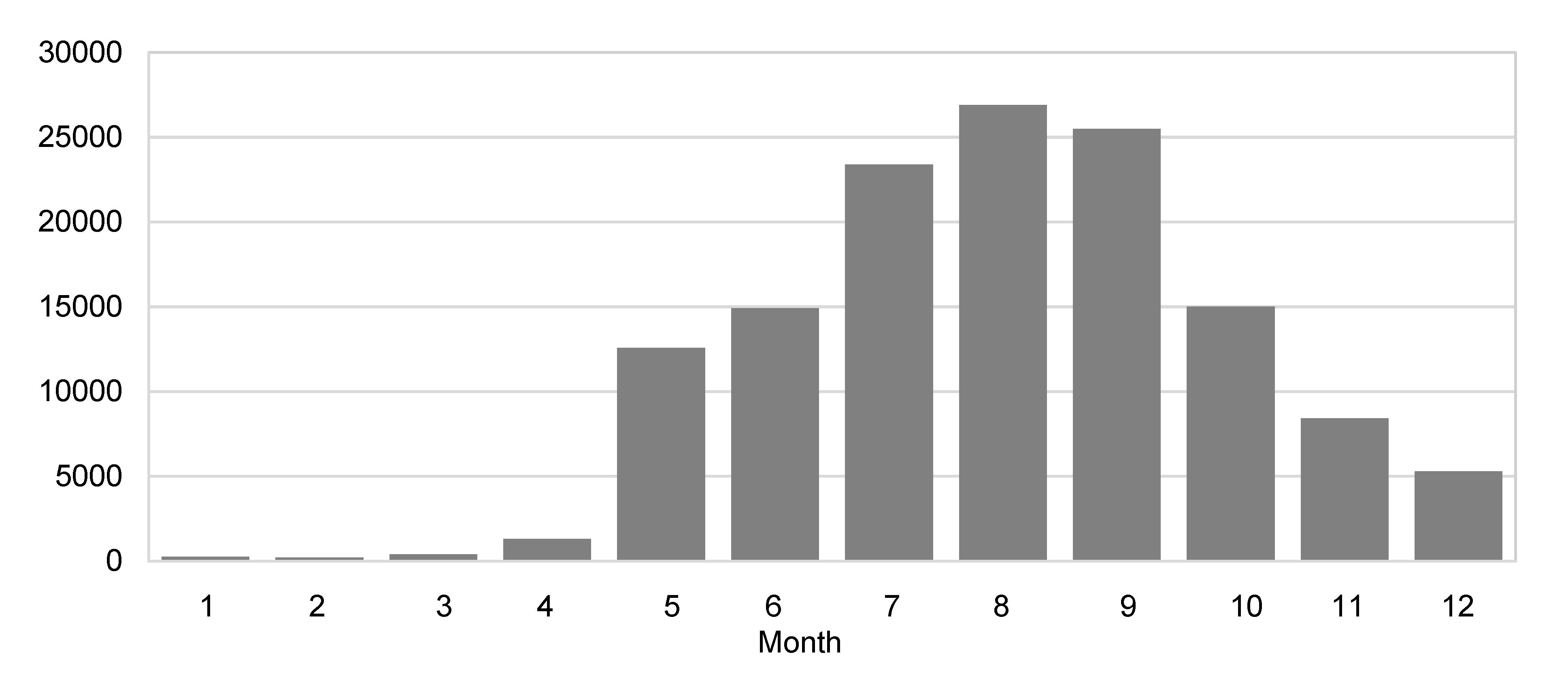

| Demographics (n = 142,712): | |
|---|---|
| Age [years], Median (IQR) | 54 (41–66) |
| Male, n (%) | 64,363 (45) |
| Non-Hispanic White, n (%) | 41,243 (29) |
| Hispanic, n (%) | 73,211 (51) |
| Black, n (%) | 4852 (3) |
| Asian, n (%) | 4709 (3) |
| Pacific Islander, n (%) | 285 (0) |
| Household income [USD], Median (IQR) | 61,485 (40,819–82,150) |
| Bachelor’s Degree, n (%) | 22,808 (16) |
| Urban Area, n (%) | 135,576 (95) |
| COVID-19 Infection, n (%) | 12,273 (9) |
| First Dose (n = 142,712): | |
| Travel Time [minutes], Median (IQR) | 7.648 (3.206–12.090) |
| Vaccine: Pfizer, n (%) | 50,234 (35) |
| Vaccine: Moderna, n (%) | 81,631 (57) |
| Vaccine: Johnson & Johnson, n (%) | 10,846 (8) |
| Second Dose (n = 135,548): | |
| Days between Vaccines, Median (IQR) | 28 (5–57) |
| Travel Time [minutes], Median (IQR) | 7.345 (2.934–11.756) |
| Vaccine: Pfizer, n (%) | 45,273 (33) |
| Vaccine: Moderna, n (%) | 74,144 (54) |
| First Booster (n = 74,352): | |
| Days between Vaccines, Median (IQR) | 240 (206–273) |
| Travel Time [minutes], Median (IQR) | 5.402 (1.456–9.348) |
| Vaccine: Pfizer, n (%) | 13,978 (18) |
| Vaccine: Moderna, n (%) | 23,495 (31) |
| Coeff. | 95% CI | Odds Ratio | |
|---|---|---|---|
| (A) Second Primary Dose | |||
| Constant | −2.053 | (−2.623, −1.483) * | 0.128 |
| Travel Time (log) | −0.023 | (−0.049, −0.003) | 0.977 |
| Age (log) | 0.719 | (0.671, 0.767) * | 2.053 |
| Male | −0.182 | (−0.222, −0.142) * | 0.834 |
| Hispanic | −0.115 | (−0.157, −0.073) * | 0.891 |
| Black | 0.234 | (0.114, 0.354) * | 1.263 |
| Asian | 0.056 | (−0.058, 0.170) | 1.058 |
| Pacific Islander | −0.351 | (−0.709, −0.007) * | 0.704 |
| Income (log) | 0.116 | (0.064, 0.168) * | 1.123 |
| Bachelor’s Degree (log) | 0.162 | (0.132, 0.192) * | 1.175 |
| Urban Area | 0.360 | (0.274, 0.446) * | 1.434 |
| COVID-19 Infection | 0.200 | (0.126, 0.274) * | 1.221 |
| Moderna | 0.208 | (0.16, 0.256) * | 1.231 |
| Pseudo R2 | 0.184 | (0.184, 0.184) * | |
| Log Likelihood | −35,592 | ||
| Observations (n) | 135,548 | ||
| (B) First Booster | |||
| Constant | −8.028 | (−8.364, −7.692) * | 0.000 |
| Travel Time (log) | −0.301 | (−0.315, −0.287) * | 0.740 |
| Age (log) | 1.198 | (1.168, 1.228) * | 3.314 |
| Male | −0.096 | (−0.12, −0.072) * | 0.908 |
| Hispanic | −0.065 | (−0.089, −0.041) * | 0.937 |
| Black | 0.221 | (0.155, 0.287) * | 1.248 |
| Asian | 0.433 | (0.367, 0.499) * | 1.542 |
| Pacific Islander | 0.138 | (−0.098, 0.374) | 1.148 |
| Income (log) | 0.324 | (0.294, 0.354) * | 1.382 |
| Bachelor’s Degree (log) | 0.094 | (0.076, 0.112) * | 1.098 |
| Urban Area | 0.035 | (−0.001, 0.071) | 1.036 |
| COVID-19 Infection | −0.142 | (−0.184, −0.101) * | 0.868 |
| Moderna (dose 2) | 0.308 | (0.282, 0.334) * | 1.360 |
| Johnson & Johnson (primary dose) | −0.539 | (−0.587, −0.491) * | 0.583 |
| Pseudo R2 | 0.101 | ||
| Log Likelihood | −85,919 | ||
| Observations (n) | 142,712 |
| Coeff. | 95% CI | Hazard Ratio | |
|---|---|---|---|
| Constant | −12.899 | (−12.989, −12.809) * | 0.000 |
| Travel Time (log) | −0.014 | (−0.020, −0.008) * | 0.986 |
| Age (log) | −0.785 | (−0.797, −0.773) * | 0.456 |
| Male | 0.096 | (0.082, 0.110) * | 1.100 |
| Hispanic | −0.251 | (−0.267, −0.235) * | 0.778 |
| Black | −1.017 | (−1.063, −0.971) * | 0.362 |
| Asian | 0.080 | (0.044, 0.116) * | 1.083 |
| Pacific Islander | 0.082 | (−0.06, 0.224) | 1.085 |
| Household Income (log) | −0.911 | (−0.917, −0.905) * | 0.402 |
| Bachelor’s Degree (log) | 0.162 | (0.154, 0.170) * | 1.175 |
| Urban Area | 0.174 | (0.132, 0.216) * | 1.190 |
| COVID-19 Infection | −0.020 | (−0.050, −0.012) * | 0.980 |
| Moderna (dose 2) | −0.078 | (−0.092, −0.064) * | 0.925 |
| Johnson & Johnson (primary dose) | 0.229 | (0.195, 0.263) * | 1.257 |
| Shape Parameter (α) | 4.612 | (4.586, 4.638) * | |
| Log Likelihood | −369,309 | ||
| Observations (n) | 74,352 |
| Coeff. | 95% CI | Hazard Ratio | |
|---|---|---|---|
| Constant | −31.944 | (−32.23, −31.658) * | 0.000 |
| Travel Time (log) | −0.015 | (−0.023, −0.007) * | 0.985 |
| Age (log) | 0.174 | (0.154, 0.194) * | 1.190 |
| Male | 0.112 | (0.098, 0.126) * | 1.118 |
| Hispanic | −0.067 | (−0.081, −0.053) * | 0.935 |
| Black | −0.121 | (−0.163, −0.079) * | 0.886 |
| Asian | −0.071 | (−0.107, −0.035) * | 0.931 |
| Pacific Islander | 0.247 | (0.111, 0.383) * | 1.280 |
| Household Income (log) | −0.016 | (−0.034, 0.002) * | 0.984 |
| Bachelor’s Degree (log) | −0.012 | (−0.022, −0.002) * | 0.988 |
| Urban Area | 0.087 | (0.055, 0.119) * | 1.091 |
| COVID-19 Infection | −0.060 | (−0.082, −0.038) * | 0.942 |
| Moderna (dose 2) | −0.107 | (−0.121, −0.093) * | 0.899 |
| Johnson & Johnson (primary dose) | 0.073 | (0.041, 0.105) * | 1.076 |
| Shape Parameter (α) | 5.671 | (5.639, 5.703) * | |
| Log Likelihood | −352,672 | ||
| Observations (n) | 135,548 |
| Coeff. | 95% CI | Hazard Ratio | |
|---|---|---|---|
| Constant | −9.351 | (−9.413, −9.289) * | 0.000 |
| Travel Time (log) | −0.055 | (−0.065, −0.045) * | 0.946 |
| Age (log) | −1.776 | (−1.790, −1.762) * | 0.169 |
| Male | 0.474 | (0.458, 0.490) * | 1.606 |
| Hispanic | −0.179 | (−0.193, −0.165) * | 0.836 |
| Black | −0.452 | (−0.492, −0.412) * | 0.636 |
| Asian | −0.323 | (−0.371, −0.275) * | 0.724 |
| Pacific Islander | 1.093 | (0.933, 1.253) * | 2.984 |
| Household Income (log) | −0.714 | (−0.718, −0.710) * | 0.490 |
| Bachelor’s Degree (log) | −0.247 | (−0.255, −0.239) * | 0.781 |
| Urban | −1.110 | (−1.146, −1.074) * | 0.330 |
| COVID-19 Infection | −0.574 | (−0.606, −0.542) * | 0.563 |
| Moderna (dose 2) | −0.527 | (−0.543, −0.511) * | 0.590 |
| Johnson & Johnson (primary dose) | 0.161 | (0.055, 0.267) * | 1.175 |
| Shape Parameter (α) | 8.180 | (8.058, 8.302) * | |
| Error Correlation (φ) | 0.215 | (0.187, 0.243) * | |
| Log Likelihood | −388,295 | ||
| Observations (n) | 74,352 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Lee, J. Delay in COVID-19 Vaccinations: The Role of Travel Time to Vaccine Sites. COVID 2025, 5, 70. https://doi.org/10.3390/covid5050070
Huang Y, Lee J. Delay in COVID-19 Vaccinations: The Role of Travel Time to Vaccine Sites. COVID. 2025; 5(5):70. https://doi.org/10.3390/covid5050070
Chicago/Turabian StyleHuang, Yuxia, and Jim Lee. 2025. "Delay in COVID-19 Vaccinations: The Role of Travel Time to Vaccine Sites" COVID 5, no. 5: 70. https://doi.org/10.3390/covid5050070
APA StyleHuang, Y., & Lee, J. (2025). Delay in COVID-19 Vaccinations: The Role of Travel Time to Vaccine Sites. COVID, 5(5), 70. https://doi.org/10.3390/covid5050070







